-
PDF
- Split View
-
Views
-
Cite
Cite
Cécile Pouderoux, Agathe Becker, Sylvain Goutelle, Sébastien Lustig, Claire Triffault-Fillit, Fatiha Daoud, Michel Henry Fessy, Sabine Cohen, Frédéric Laurent, Christian Chidiac, Florent Valour, Tristan Ferry, Lyon Bone and Joint Infection Study Group , Subcutaneous suppressive antibiotic therapy for bone and joint infections: safety and outcome in a cohort of 10 patients, Journal of Antimicrobial Chemotherapy, Volume 74, Issue 7, July 2019, Pages 2060–2064, https://doi.org/10.1093/jac/dkz104
Close - Share Icon Share
Abstract
Optimal treatment of prosthetic joint infection and chronic osteomyelitis consists of surgical removal of biofilm-embedded bacteria, followed by a 6–12 week course of antimicrobial therapy. However, when optimal surgery is not feasible, oral prolonged suppressive antibiotic therapy (PSAT) is recommended to prevent prosthesis loosening and/or relapse of infection. Since 2010, we have used infection salvage therapy using off-label subcutaneous (sc) injection of a β-lactam as PSAT for patients in whom oral PSAT is not possible.
A single-centre prospective cohort study (2010–18) reporting treatment modalities, efficacy and safety in all patients receiving sc PSAT. NCT03403608.
The 10 included patients (median age 79 years) had polymicrobial (n = 5) or MDR bacterial (n = 4) prosthetic joint infection (knee, n = 4; hip, n = 3) or chronic osteomyelitis (n = 3). After initial intensive therapy, seven patients received ertapenem, three patients received ceftriaxone and one patient received ceftazidime by sc injection (one patient received 8 days of ceftriaxone before receiving ertapenem). In one patient, sc PSAT failed with recurrent signs of infection under treatment. In three patients, sc PSAT had to be discontinued due to side effects; in only one of these was the sc route implicated (skin necrosis following direct sc injection and not gravity infusion). Median treatment duration was 433 days. In six patients, sc PSAT was successful with favourable outcome at the time of writing. Interestingly, three patients with MDR bacterial carriage at baseline lost this under PSAT during follow-up.
As salvage therapy, sc PSAT delivered by gravity infusion is a safe and interesting alternative when an optimal surgical strategy is not feasible and no oral treatment is available.
Introduction
Treatment of prosthetic joint infection (PJI) and chronic osteomyelitis (CO) is an important challenge in the field of bone and joint infection (BJI), as they can impair functional prognosis and require prolonged antibiotic therapy that may have a significant impact on the patient’s gut microbiota ecology. Optimal treatment of chronic PJI and CO consists of surgical removal of the infected prosthesis, or debridement of infected tissues in CO, to remove bacteria embedded in biofilm at the implant surface and in sequestra. This is followed by 6–12 weeks of targeted antibiotic therapy.1
When optimal surgical management cannot be implemented (notably, in the elderly), treatment relies on debridement and implant retention (DAIR) for PJI, and surgical abstention or minimal bone debridement in CO. Because the risk of infection relapse is particularly high in these patients, prolonged suppressive antibiotic therapy (PSAT) using oral antibiotics is recommended,2,3 at least until resolution of BJI, or even life-long in some cases.
However, effective molecules for oral administration are not always available; subcutaneous (sc) PSAT can then be proposed to avoid the infectious and thrombotic complications of prolonged intravenous catheterization.4 This off-label sc administration is based on the experience that sc antimicrobial therapy, hydration and analgesia are well tolerated in geriatric patients and in palliative care.5–7 However, sc PSAT for BJI has been little evaluated to date.
Therefore, we conducted a prospective longitudinal efficacy and safety study in patients receiving PSAT.
Patients and methods
A single-centre prospective cohort study was performed in the Complex Bone and Joint Infection Reference Centre (Centre de Référence des Infections Ostéo-Articulaires Complexes) of Lyon (France), which is one of the nine regional reference centres for the management of complex BJI in France. The study was conducted from 2010 to 2018, including patients with chronic PJI or CO, defined as infection progression ≥4 weeks, ineligible for prosthesis removal or complete debridement, and consequently with indications for PSAT. When possible, patients underwent DAIR, or simple bone or soft-tissue abscess drainage, without sequestrum resection for CO. All patients received initial intensive therapy for 1–3 months, in line with BJI management guidelines.1 Subcutaneous administration was considered when the oral route was not feasible, due to the pathogen’s resistance profile and/or polymicrobial infection and/or history of drug-related adverse events. The antimicrobials used for PSAT were ertapenem and ceftriaxone, ceftazidime (which has a shorter half-life) being reserved for patients with impaired kidney function (glomerular filtration rate <60 mL/min) or Pseudomonas aeruginosa infection. For each antimicrobial, trough plasma concentrations (Cmin) were measured at PSAT equilibrium to ensure that the target of Cmin above the causative pathogen MIC was reached. The procedure for sc administration was 30–45 min gravity infusion of the antibiotic diluted in 50 mL of isotonic saline serum, using a disposable butterfly needle either in the anterior side of the thigh or in the abdominal flank. Patients were followed-up at least every month, and side effects and outcome were recorded. Failure was defined as recurrence of signs of local infection (pain, swelling, erythema, fistula or purulent discharge). Acquisition of MDR bacteria carriage in the gut microbiota [including carbapenemase-producing Gram-negative bacilli (GNB) and cephalosporin-resistant enterobacteria] was determined using rectal swabbing at each consultation.
Ethics
For this observational study, written information was given to all patients and their consent obtained. The study was registered as a clinical trial (no. NCT03403608) and was approved by the National Data Protection Commission (no. 17–283).
Results
The main results are reported in Table 1. Ten patients [four male and six female; median age 79 years (IQR 75–80)] requiring sc PSAT were included. Noteworthy comorbidities included two patients receiving long-term curative anticoagulation therapy; four others had chronic kidney failure and one had epilepsy under treatment. Seven patients had PJI (hip, n = 3; knee, n = 4; mainly large resection prosthesis without loosening) and three had CO (Figure 1). Five PJIs were recurrences after classic two-stage device exchange, with a median interval between reimplantation and relapse of 92 days (IQR 3–144). The other two patients with PJI were elderly and ineligible for explantation. The three remaining patients (with CO) encountered relapse after surgical treatment, at a median of 210 days (IQR 144–362).
Characteristics of BJI and outcome of sc PSAT following suboptimal surgical therapy
| Patient number [age (years)] . | Pathology . | Surgery/ microbiological diagnosis . | Pathogen . | Antibiotic administered subcutaneously . | Cmin (mg/L), mean ± SD . | Duration . | Side effect leading to treatment interruption . | Outcome at the last follow-up . | MDR carriage at baseline . | Carriage acquisition . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 (78) | knee PJI | DAIR | Escherichia coli, P. aeruginosa | ceftazidime 2 g/24 h | 5.6 ± 0.4 | 6 months | none | successa,b | E. coli | no |
| 2 (90) | hip PJI | DAIR | Streptococcus spp. (penicillin non-susceptible) | ceftriaxone 1 g/24 h | NP | 6 months | none | successb,c | Enterobacteriaceae | — |
| 3 (80) | pelvic CO | none/pelvic bone biopsy | E. coli ESBL | ertapenem 1 g/24 h | 22.9 ± 1.9 | 58 months | none | successa,b | E. coli | E. cloacae |
| 4 (73) | pelvic CO | none/deep sample | P. aeruginosa MDR, E. coli ESBL, Streptococcus agalactiae, Staphylococcus aureus, Proteus mirabilis | ertapenem 1 g/24 h | 14.8 ± 1.4 | 51 months | none | successd,e | P. aeruginosa | no |
| 5 (79) | hip PJI | DAIR | E. coli | ceftriaxone 1 g/24 h then | NP | 8 days | cholestatic hepatitis | failure before sc PSAT interruption | E. coli | K. pneumoniae |
| ertapenem 1 g/24 h | 10.5 ± 1.7 | 6 months | hypereosinophilia | |||||||
| 6 (67) | knee PJI | DAIR | S. aureus, P. mirabilis, Citrobacter koseri, Bacteroides fragilis, Finegoldia magna | ertapenem 1 g/12 h | 11.9 | 2 months | imbalanced epilepsy, drug rash and pruritus | failure after sc PSAT interruption | no | no |
| 7 (75) | hip PJI | DAIR | E. cloacae ESBL, Enterococcus faecalis, S. aureus | ertapenem 1 g/24 h | 3.3 + 0.8 | 13 months | none | successb,c | E. cloacae | Enterobacteriaceae, CRAB |
| 8 (85) | knee PJI | none/knee puncture | Morganella morganii | ceftriaxone 1 g/24 h | NP | 24 months | skin necrosis | lost to follow-up | E. coli, M. morganii | P. aeruginosa |
| 9 (80) | pelvic CO | none/pelvic bone biopsy | S. aureus, E. faecalis, Klebsiella pneumoniae, Staphylococcus epidermidis | ertapenem 1 g/12 h | 28.3 ± 8.8 | 5 months | hypereosinophilia | failure after sc PSAT interruption | S. aureus, K. pneumoniae, CRAB | no |
| 10 (75) | knee PJI | DAIR | E. coli ESBL | ertapenem 1 g/12 h | 2.88 ± 0.5 | 1 month | none | successb,c | E. coli, P. aeruginosa | no |
| Patient number [age (years)] . | Pathology . | Surgery/ microbiological diagnosis . | Pathogen . | Antibiotic administered subcutaneously . | Cmin (mg/L), mean ± SD . | Duration . | Side effect leading to treatment interruption . | Outcome at the last follow-up . | MDR carriage at baseline . | Carriage acquisition . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 (78) | knee PJI | DAIR | Escherichia coli, P. aeruginosa | ceftazidime 2 g/24 h | 5.6 ± 0.4 | 6 months | none | successa,b | E. coli | no |
| 2 (90) | hip PJI | DAIR | Streptococcus spp. (penicillin non-susceptible) | ceftriaxone 1 g/24 h | NP | 6 months | none | successb,c | Enterobacteriaceae | — |
| 3 (80) | pelvic CO | none/pelvic bone biopsy | E. coli ESBL | ertapenem 1 g/24 h | 22.9 ± 1.9 | 58 months | none | successa,b | E. coli | E. cloacae |
| 4 (73) | pelvic CO | none/deep sample | P. aeruginosa MDR, E. coli ESBL, Streptococcus agalactiae, Staphylococcus aureus, Proteus mirabilis | ertapenem 1 g/24 h | 14.8 ± 1.4 | 51 months | none | successd,e | P. aeruginosa | no |
| 5 (79) | hip PJI | DAIR | E. coli | ceftriaxone 1 g/24 h then | NP | 8 days | cholestatic hepatitis | failure before sc PSAT interruption | E. coli | K. pneumoniae |
| ertapenem 1 g/24 h | 10.5 ± 1.7 | 6 months | hypereosinophilia | |||||||
| 6 (67) | knee PJI | DAIR | S. aureus, P. mirabilis, Citrobacter koseri, Bacteroides fragilis, Finegoldia magna | ertapenem 1 g/12 h | 11.9 | 2 months | imbalanced epilepsy, drug rash and pruritus | failure after sc PSAT interruption | no | no |
| 7 (75) | hip PJI | DAIR | E. cloacae ESBL, Enterococcus faecalis, S. aureus | ertapenem 1 g/24 h | 3.3 + 0.8 | 13 months | none | successb,c | E. cloacae | Enterobacteriaceae, CRAB |
| 8 (85) | knee PJI | none/knee puncture | Morganella morganii | ceftriaxone 1 g/24 h | NP | 24 months | skin necrosis | lost to follow-up | E. coli, M. morganii | P. aeruginosa |
| 9 (80) | pelvic CO | none/pelvic bone biopsy | S. aureus, E. faecalis, Klebsiella pneumoniae, Staphylococcus epidermidis | ertapenem 1 g/12 h | 28.3 ± 8.8 | 5 months | hypereosinophilia | failure after sc PSAT interruption | S. aureus, K. pneumoniae, CRAB | no |
| 10 (75) | knee PJI | DAIR | E. coli ESBL | ertapenem 1 g/12 h | 2.88 ± 0.5 | 1 month | none | successb,c | E. coli, P. aeruginosa | no |
NP, not performed.
Walking without aid.
No pain.
Walking with canes/frame.
Confined to bed.
Need for non-morphine painkillers.
Characteristics of BJI and outcome of sc PSAT following suboptimal surgical therapy
| Patient number [age (years)] . | Pathology . | Surgery/ microbiological diagnosis . | Pathogen . | Antibiotic administered subcutaneously . | Cmin (mg/L), mean ± SD . | Duration . | Side effect leading to treatment interruption . | Outcome at the last follow-up . | MDR carriage at baseline . | Carriage acquisition . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 (78) | knee PJI | DAIR | Escherichia coli, P. aeruginosa | ceftazidime 2 g/24 h | 5.6 ± 0.4 | 6 months | none | successa,b | E. coli | no |
| 2 (90) | hip PJI | DAIR | Streptococcus spp. (penicillin non-susceptible) | ceftriaxone 1 g/24 h | NP | 6 months | none | successb,c | Enterobacteriaceae | — |
| 3 (80) | pelvic CO | none/pelvic bone biopsy | E. coli ESBL | ertapenem 1 g/24 h | 22.9 ± 1.9 | 58 months | none | successa,b | E. coli | E. cloacae |
| 4 (73) | pelvic CO | none/deep sample | P. aeruginosa MDR, E. coli ESBL, Streptococcus agalactiae, Staphylococcus aureus, Proteus mirabilis | ertapenem 1 g/24 h | 14.8 ± 1.4 | 51 months | none | successd,e | P. aeruginosa | no |
| 5 (79) | hip PJI | DAIR | E. coli | ceftriaxone 1 g/24 h then | NP | 8 days | cholestatic hepatitis | failure before sc PSAT interruption | E. coli | K. pneumoniae |
| ertapenem 1 g/24 h | 10.5 ± 1.7 | 6 months | hypereosinophilia | |||||||
| 6 (67) | knee PJI | DAIR | S. aureus, P. mirabilis, Citrobacter koseri, Bacteroides fragilis, Finegoldia magna | ertapenem 1 g/12 h | 11.9 | 2 months | imbalanced epilepsy, drug rash and pruritus | failure after sc PSAT interruption | no | no |
| 7 (75) | hip PJI | DAIR | E. cloacae ESBL, Enterococcus faecalis, S. aureus | ertapenem 1 g/24 h | 3.3 + 0.8 | 13 months | none | successb,c | E. cloacae | Enterobacteriaceae, CRAB |
| 8 (85) | knee PJI | none/knee puncture | Morganella morganii | ceftriaxone 1 g/24 h | NP | 24 months | skin necrosis | lost to follow-up | E. coli, M. morganii | P. aeruginosa |
| 9 (80) | pelvic CO | none/pelvic bone biopsy | S. aureus, E. faecalis, Klebsiella pneumoniae, Staphylococcus epidermidis | ertapenem 1 g/12 h | 28.3 ± 8.8 | 5 months | hypereosinophilia | failure after sc PSAT interruption | S. aureus, K. pneumoniae, CRAB | no |
| 10 (75) | knee PJI | DAIR | E. coli ESBL | ertapenem 1 g/12 h | 2.88 ± 0.5 | 1 month | none | successb,c | E. coli, P. aeruginosa | no |
| Patient number [age (years)] . | Pathology . | Surgery/ microbiological diagnosis . | Pathogen . | Antibiotic administered subcutaneously . | Cmin (mg/L), mean ± SD . | Duration . | Side effect leading to treatment interruption . | Outcome at the last follow-up . | MDR carriage at baseline . | Carriage acquisition . |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 (78) | knee PJI | DAIR | Escherichia coli, P. aeruginosa | ceftazidime 2 g/24 h | 5.6 ± 0.4 | 6 months | none | successa,b | E. coli | no |
| 2 (90) | hip PJI | DAIR | Streptococcus spp. (penicillin non-susceptible) | ceftriaxone 1 g/24 h | NP | 6 months | none | successb,c | Enterobacteriaceae | — |
| 3 (80) | pelvic CO | none/pelvic bone biopsy | E. coli ESBL | ertapenem 1 g/24 h | 22.9 ± 1.9 | 58 months | none | successa,b | E. coli | E. cloacae |
| 4 (73) | pelvic CO | none/deep sample | P. aeruginosa MDR, E. coli ESBL, Streptococcus agalactiae, Staphylococcus aureus, Proteus mirabilis | ertapenem 1 g/24 h | 14.8 ± 1.4 | 51 months | none | successd,e | P. aeruginosa | no |
| 5 (79) | hip PJI | DAIR | E. coli | ceftriaxone 1 g/24 h then | NP | 8 days | cholestatic hepatitis | failure before sc PSAT interruption | E. coli | K. pneumoniae |
| ertapenem 1 g/24 h | 10.5 ± 1.7 | 6 months | hypereosinophilia | |||||||
| 6 (67) | knee PJI | DAIR | S. aureus, P. mirabilis, Citrobacter koseri, Bacteroides fragilis, Finegoldia magna | ertapenem 1 g/12 h | 11.9 | 2 months | imbalanced epilepsy, drug rash and pruritus | failure after sc PSAT interruption | no | no |
| 7 (75) | hip PJI | DAIR | E. cloacae ESBL, Enterococcus faecalis, S. aureus | ertapenem 1 g/24 h | 3.3 + 0.8 | 13 months | none | successb,c | E. cloacae | Enterobacteriaceae, CRAB |
| 8 (85) | knee PJI | none/knee puncture | Morganella morganii | ceftriaxone 1 g/24 h | NP | 24 months | skin necrosis | lost to follow-up | E. coli, M. morganii | P. aeruginosa |
| 9 (80) | pelvic CO | none/pelvic bone biopsy | S. aureus, E. faecalis, Klebsiella pneumoniae, Staphylococcus epidermidis | ertapenem 1 g/12 h | 28.3 ± 8.8 | 5 months | hypereosinophilia | failure after sc PSAT interruption | S. aureus, K. pneumoniae, CRAB | no |
| 10 (75) | knee PJI | DAIR | E. coli ESBL | ertapenem 1 g/12 h | 2.88 ± 0.5 | 1 month | none | successb,c | E. coli, P. aeruginosa | no |
NP, not performed.
Walking without aid.
No pain.
Walking with canes/frame.
Confined to bed.
Need for non-morphine painkillers.
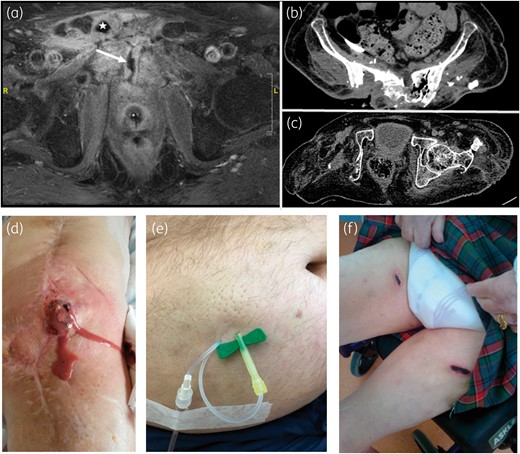
(a) Post-operative pubic CO on T1-weighted MRI after gadolinium injection and fat saturation, showing pubic symphysis abscess (star) with urethral fistula (arrow) in Patient 3, who was previously exposed to radiation and was treated with only antibiotics (no surgery). (b) Sacral CO with gas shown on a CT scan of Patient 4, who was previously exposed to radiation and was treated only with antibiotics (no surgery). (c) Relapsing right acetabular osteomyelitis on a CT scan, despite curettage and disarticulation following recurrence of native hip infection in a paraplegic patient (Patient 9, treated only with antibiotics). (d) Purulent discharge in Patient 10 with chronic relapsing prosthetic knee infection (previously treated by two-stage exchange with implantation of knee arthrodesis and soft tissue flap), treated here by debridement, implant retention and sc PSAT, with favourable outcome. (e) Orange-peel aspect of the skin without skin necrosis following gravity infusion (30–45 min) of 2 g of ceftazidime via a butterfly needle. (f) Necrosis after repeated direct (a few seconds) injections of 2 g of ceftriaxone (2–5 mL). This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Regarding surgical treatment, six patients underwent minimal surgery (DAIR), while no surgery was possible in the four remaining cases, including the three CO patients. Microbiological analyses of intraoperative samples, radiological bone biopsies, joint punctures or deep sampling identified five polymicrobial infections and four MDR GNB infections. Ertapenem, ceftriaxone and ceftazidime were used for sc PSAT in seven patients, three patients and one patient, respectively (one patient received 8 days of ceftriaxone before receiving ertapenem). Median sc treatment duration was 433 days (IQR 209.5–711.8), for a total of ∼6000 sc injections. Treatment was administered at home (n = 7) or in a nursing home (n = 3). Benign local reactions were observed in two patients following gravity infusion, such as an orange-peel skin aspect, with or without transient injection-site pruritus (Figure 1e). There were no significant haemorrhagic complications, even under curative anticoagulation therapy.
PSAT doses comprised: ceftriaxone, 1 g/day; ceftazidime, 2 g/day in a patient with chronic kidney injury; and ertapenem, 1 g/day in patients with chronic kidney failure or else 1 g/12 h. All patients (except those who were treated with ceftriaxone, for whom Cmin was not determined) presented a detectable Cmin higher than that for the targeted pathogen MIC. Suppressive sc treatment failed in one patient (relapse under ertapenem therapy) and was withdrawn for side effects in three other patients [respectively: skin necrosis (Figure 1f) following direct injection of ceftriaxone; non-controlled epilepsy, cutaneous rash and pruritus under ertapenem; and hypereosinophilia under ertapenem]. During follow-up, sc injections were switched to an intravenous route in one patient who developed terminal kidney failure unrelated to treatment, requiring chronic haemodialysis; one patient died of rectal cancer. In four patients, sc PSAT was ongoing at the time of writing (median follow-up: 14 months).
Concerning acquisition of MDR bacterial carriage in the gut microbiota, nine patients were already colonized with MDR GNB before sc PSAT: eight with third-generation cephalosporin-resistant Enterobacteriaceae and one with carbapenem-resistant Acinetobacter baumannii (CRAB). The only patient without prior colonization did not acquire MDR rectal pathogens during ertapenem sc PSAT. One patient with MDR Enterobacter cloacae at baseline acquired CRAB 1 month after beginning PSAT (ertapenem). Notably, three patients with MDR bacterial carriage at baseline lost this under PSAT during follow-up.
Discussion
This study reports our experience of sc PSAT, used exceptionally as an alternative to oral PSAT in patients with complex BJI. Although the risk/benefit ratio has not been clearly evaluated, it could be an easy-to-use option in the outpatient setting to maintain function and to avoid amputation in the elderly. This should be a useful strategy, given the rapid emergence of MDR bacteria in BJI, which reduces the choice of available oral antimicrobial treatments. In this series of 10 patients receiving sc PSAT totalling ∼6000 injections, no severe cutaneous reactions were observed when instructions for the 30–45 min gravity infusion were respected. However, cutaneous necrosis did occur in one patient receiving direct sc ceftriaxone injection, contrary to the prescription and recommendations for use of the product. Although it is difficult to draw any conclusions about the efficacy of sc PSAT based on the present study, only one failure (sc ertapenem treatment) was recorded in a population with a particularly high risk of relapse.
Pharmacokinetic data for sc administration of ertapenem8,9 and ceftriaxone10 show that the concentration peak is lower and the time to peak concentration longer for the sc route than for the intravenous route. However, the AUCs are identical, with satisfactory time above the MIC, which is the most important pharmacokinetic predictor of efficacy for such time-dependent antimicrobials.11 Ertapenem is now routinely used subcutaneously for urinary tract MDR infection.12 Subcutaneous ceftriaxone treatment is much more widely used in palliative care or for the elderly.13 In addition, the pharmacokinetics of ceftazidime via an sc route versus an intravenous route have been studied in dogs, with drug exposure results similar to those for ertapenem and ceftriaxone.14 However, optimal doses for sc PSAT remain to be defined, the objective being to maintain sufficient antibiotic concentrations in bone, while limiting the risk of drug-related adverse events and the acquisition of MDR bacteria in the gut microbiota. Antibiotic exposure and prolonged antibiotic therapy are risk factors for acquisition of MDR GNB carriage.15–18 Imipenem was shown to increase the risk of carbapenemase-producing GNB carriage.19 In the present study, one patient acquired a carbapenemase-producing GNB under ertapenem therapy. After acquisition, loss of MDR carriage is a major issue in patients receiving PSAT and it is noteworthy that three of our patients lost MDR carriage after discharge despite continuation of PSAT. Consequently, antibiotic pressure in the patient’s environment may be a crucial factor in eliminating MDR carriage. Carriage duration was recently studied in travellers who acquired MDR carriage; MDR carriage was lost within 3 months of their return in most cases, but the dynamics of carriage loss could be significantly disturbed by antibiotic intake.20
In conclusion, this prospective cohort study suggests that, when oral treatment is unavailable, sc PSAT is an effective and well-tolerated alternative when optimal surgery is not feasible.
Acknowledgements
This work was performed within the framework of the LABEX ECOFECT (ANR-11-LABX-0048) of Lyon University, as part of the ‘Investissements d'Avenir’ (ANR-11-IDEX-0007) programme of the French National Research Agency (ANR).
Members of the Lyon Bone and Joint Infection Study Group
Coordinator: Tristan Ferry. Infectious Diseases Specialists: Tristan Ferry, Florent Valour, Thomas Perpoint, Patrick Miailhes, Florence Ader, Agathe Becker, Sandrine Roux, Claire Triffault-Fillit, Anne Conrad, Marielle Perry, Cécile Pouderoux, Fatiha Daoud, Johanna Lippman, Evelyne Braun and Christian Chidiac. Surgeons: Sébastien Lustig, Elvire Servien Cécile Batailler, Romain Gaillard, Stanislas Gunst, Julien Roger, Charles Fiquet, Michel-Henry Fessy, Anthony Viste, Philippe Chaudier, Jean-Luc Besse, Lucie Louboutin, Gaël Gaudin, Tanguy Ledru, Andrien Van Haecke, Quentin Ode, Marcelle Mercier, Florie Alech-Tournier, Sébastien Martres, Franck Trouillet, Cédric Barrey, Emmanuel Jouanneau, Timothée Jacquesson, Brice Gerenton, Ali Mojallal, Fabien Boucher, Hristo Shipkov, Philippe Céruse, Carine Fuchsmann and Arnaud Gleizal. Anaesthesiologists: Frédéric Aubrun, Mikhail Dziadzko and Caroline Macabéo. Microbiologists: Frederic Laurent, Laetitia Beraut, Céline Dupieux, Camille Kolenda, Jérôme Josse and Claude-Alexandre Gustave. Imaging: Fabien Craighero, Loic Boussel and Jean-Baptiste Pialat. Nuclear Medicine: Isabelle Morelec. Pharmacokinetic/pharmacodynamic specialists: Michel Tod, Marie-Claude Gagnieu and Sylvain Goutelle. Clinical research assistant and database manager: Eugénie Mabrut.
Funding
This study was supported by Hospices Civils de Lyon.
Transparency declarations
None to declare.
References
Author notes
Members are listed in the Acknowledgements section.
- ceftriaxone
- biofilms
- ceftazidime
- disease transmission
- drug administration routes
- follow-up
- hip region
- hip joint
- subcutaneous injections
- knee region
- knee joint
- lactams
- prospective studies
- safety
- salvage therapy
- surgical procedures, operative
- infections
- bacteria
- skin necrosis
- antibiotic therapy
- antimicrobials
- ertapenem
- osteomyelitis, chronic
- prosthetic joint infections
- off-label use
- excision
- force of gravity
- duration of treatment
- infusion procedures
- joint infections
- prostheses



