-
PDF
- Split View
-
Views
-
Cite
Cite
Daniel Wüthrich, Aline Cuénod, Vladimira Hinic, Mario Morgenstern, Nina Khanna, Adrian Egli, Richard Kuehl, Genomic characterization of inpatient evolution of MRSA resistant to daptomycin, vancomycin and ceftaroline, Journal of Antimicrobial Chemotherapy, Volume 74, Issue 5, May 2019, Pages 1452–1454, https://doi.org/10.1093/jac/dkz003
Close - Share Icon Share
Sir,
MRSA infections represent a major public threat leading to an estimated 5400 and 11 000 deaths annually in Europe and the USA, respectively.1,2 In this study, we phenotypically and genomically characterized the evolution in a single patient of an MRSA isolate that finally became resistant to vancomycin, daptomycin and ceftaroline.
A patient in her fifties was transferred from Serbia to our hospital after a serious car accident causing fractures of the spine, legs and chest. Following surgical fracture stabilization, she developed bacteraemia with MRSA exhibiting resistance to ceftaroline, ceftobiprole and rifampicin. The patient was treated with high-dose daptomycin (10 mg/kg body weight intravenously). MRSA was detected in an implant-associated infection of the left tibia with concomitant septic arthritis of the knee. The implant was removed and the knee debrided. Bacteraemia resolved after 6 days. The remaining clinical course was aggravated by development of pleural empyema and adjacent lung abscesses with MRSA as well as a relapse of the septic arthritis. In both locations, newly daptomycin- and vancomycin-resistant MRSA was cultured and an hVISA phenotype was identified. Treatment change to co-trimoxazole and several surgical debridements finally cured the infection.
Five MRSA isolates, each representing a different location or time of the infection, were used for further characterization. The MICs were determined by Liofilchem® MIC test strips (Liofilchem, Italy). Screening for VISA/hVISA phenotype was performed by Glycopeptide Resistance Detection (GRD) Etest® strips (bioMérieux, France) and confirmed by population analysis profiling (PAP-AUC). WGS was performed using a MiSeq Illumina platform with 2 × 300 nt paired-end sequencing.3 Newly developed mutations were identified by comparing the reads of the sequenced strains against the de novo assembled genome of isolate 1 (SPAdes, version 3.11.1) using Pilon (version 1.22). Daptomycin-resistant isolates were passaged daily in antibiotic-free broth for 15 days to study MIC stability. ST and spa type were identified through Ridom SeqSphere (version 4.1.9). A literature search of previously reported mutations associated with ceftaroline, daptomycin, vancomycin or rifampicin resistance was performed (Table S1, available as Supplementary data at JAC Online) and the identified mutations were compared with sequences of MRSA isolates. The sequence data from this study have been submitted to GenBank under BioProject accession no. PRJNA488707. The patient gave written informed consent for publication.
Clinical, microbiological and genomic characteristics of the MRSA isolates (ST239, spa type t037, PVL negative) cultured from the same patient
 |
 |
spa, staphylococcal protein A; PVL, Panton-Valentine leucocidin.
Interpretation according to EUCAST breakpoint tables for interpretation of MICs and zone diameters (version 8.1, 2018) is given in parentheses. S, susceptible; R, resistant.
Mutations associated with resistance to multiple antibiotics are indicated with an asterisk.
Clinical, microbiological and genomic characteristics of the MRSA isolates (ST239, spa type t037, PVL negative) cultured from the same patient
 |
 |
spa, staphylococcal protein A; PVL, Panton-Valentine leucocidin.
Interpretation according to EUCAST breakpoint tables for interpretation of MICs and zone diameters (version 8.1, 2018) is given in parentheses. S, susceptible; R, resistant.
Mutations associated with resistance to multiple antibiotics are indicated with an asterisk.
Over the 2 month course of infection, three new single-nucleotide mutations (in mprF, asp3 and a non-coding region) developed between the first and last isolate. Interestingly, two isolates, each from a different site of infection, independently evolved mutations in the mprF locus (Table 1). Both mprF mutations have previously been associated with daptomycin and vancomycin resistance,4 which may explain the clinical failure under daptomycin therapy. Moreover, the first isolate already harboured several mutations reportedly associated with the VISA phenotype and with increased MICs of individual antibiotics (Table 1). We found the mutation N204K in mecA, which had separately been associated with ceftaroline resistance5 as well as vancomycin resistance6 but not both simultaneously until now. Further, the identified H481N substitution in rpoB leading to rifampicin resistance has been associated with cross-resistance to daptomycin and vancomycin.7 Interestingly, in vitro passaging reverted daptomycin resistance in both isolates after 15 days. This reversion went through a phenotypically heteroresistant state during passage days 9–13 (Figure 1). Both reverted isolates maintained the mprF mutation but developed as their only new mutation the same deletion of an IS5-like element upstream of a tRNA-coding locus (Table 1). This deletion was not yet present in a heteroresistant colony with increased MIC during passaging.
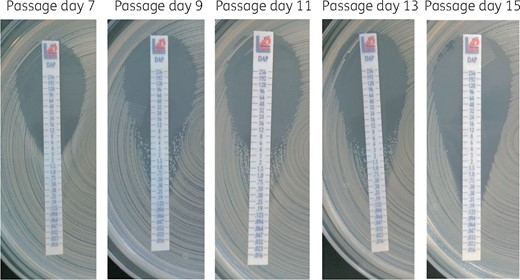
MIC test strips showing reversion of daptomycin resistance of isolate 5 passaged in vitro. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
In conclusion, we report, to the best of our knowledge, the first clinical case describing simultaneous resistance to vancomycin, daptomycin and ceftaroline in a single MRSA strain. The fact that two different single-nucleotide mutations in the mprF gene evolved in isolates from two separate anatomic locations underscores the risk of daptomycin resistance developing under selective pressure, especially in high-inoculum infections as present in our patient. Further, exposure to the in vivo milieu and innate immune system can drive evolution of resistance to daptomycin.8 This raises questions as to whether daptomycin should be avoided in high-inoculum infections or should be accompanied by a second antibiotic to avoid development of resistance, with β-lactams being promising candidates.9 Additionally, this case proves the risk of developing cross-resistance to vancomycin induced by daptomycin. However, daptomycin resistance seems to take a heavy toll on bacterial fitness since it completely reverted in both isolates after passaging in a non-selective environment. Further investigations will be necessary to determine whether the detected deletion accounts for the reversion or if epigenetic mechanisms were the cause.
With increasing knowledge about the effect of individual mutations and their corresponding phenotypes, WGS may represent a very useful clinical tool to predict the propensity of a strain to becoming resistant to a specific therapy, thereby fostering an individualized strain-specific therapeutic approach.
Funding
This study was supported by internal funding.
Transparency declarations
None to declare.
References
ECDC/EMA Joint Working Group. The Bacterial Challenge: Time to React. Stockholm, Sweden: ECDC,
US Department of Health and Human Services, CDC. Antibiotic Resistance Threats in the United States, 2013. Atlanta, GA, USA: US Department of Health and Human Services, CDC,



