-
PDF
- Split View
-
Views
-
Cite
Cite
Anne-Genevieve Marcelin, Maxime Grude, Charlotte Charpentier, Pantxika Bellecave, Laura Le Guen, Coralie Pallier, Stéphanie Raymond, Audrey Mirand, Laurence Bocket, Djeneba Bocar Fofana, Constance Delaugerre, Thuy Nguyen, Brigitte Montès, Hélène Jeulin, Thomas Mourez, Samira Fafi-Kremer, Corinne Amiel, Catherine Roussel, Julia Dina, Mary-Anne Trabaud, Hélène Le Guillou-Guillemette, Sophie Vallet, Anne Signori-Schmuck, Anne Maillard, Virginie Ferre, Diane Descamps, Vincent Calvez, Philippe Flandre, Resistance to integrase inhibitors: a national study in HIV-1-infected treatment-naive and -experienced patients, Journal of Antimicrobial Chemotherapy, Volume 74, Issue 5, May 2019, Pages 1368–1375, https://doi.org/10.1093/jac/dkz021
Close - Share Icon Share
Abstract
To describe integrase strand transfer inhibitor (INSTI) resistance profiles and factors associated with resistance in antiretroviral-naive and -experienced patients failing an INSTI-based regimen in clinical practice.
Data were collected from patients failing an INSTI-containing regimen in a multicentre French study between 2014 and 2017. Failure was defined as two consecutive plasma viral loads (VL) >50 copies/mL. Reverse transcriptase, protease and integrase coding regions were sequenced at baseline and failure. INSTI resistance-associated mutations (RAMs) included in the Agence Nationale de Recherches sur le SIDA genotypic algorithm were investigated.
Among the 674 patients, 359 were failing on raltegravir, 154 on elvitegravir and 161 on dolutegravir therapy. Overall, 90% were experienced patients and 389 (58%) patients showed no INSTI RAMs at failure. The strongest factors associated with emergence of at least one INSTI mutation were high VL at failure (OR = 1.2 per 1 log10 copies/mL increase) and low genotypic sensitivity score (GSS) (OR = 0.08 for GSS ≥3 versus GSS = 0–0.5). Patients failing dolutegravir also had significantly fewer INSTI RAMs at failure than patients failing raltegravir (OR = 0.57, P = 0.02) or elvitegravir (OR = 0.45, P = 0.005). Among the 68 patients failing a first-line regimen, 11/41 (27%) patients on raltegravir, 7/18 (39%) on elvitegravir and 0/9 on dolutegravir had viruses with emergent INSTI RAMs at failure.
These results confirmed the robustness of dolutegravir regarding resistance selection in integrase in the case of virological failure in routine clinical care.
Introduction
Integrase strand transfer inhibitors (INSTIs), which actively block the integration of the HIV genome into the host DNA, represent the latest antiretroviral (ARV) class to be approved for treatment of HIV-infected individuals.1 There are currently four INSTIs approved for the treatment of HIV infection: raltegravir, elvitegravir, dolutegravir and, more recently, bictegravir. Although highly efficacious in the management of HIV, raltegravir, elvitegravir and dolutegravir are susceptible to the development of resistance mutations in the case of virological failure. Bictegravir is the only drug for which this has not yet been documented in vivo. The main resistance pathways that have been reported as selected both in vitro and in vivo with raltegravir are Y143, Q148 and N155.2 It is evident now that raltegravir and elvitegravir share both the Q148 and N155 major resistance pathways.3 However, the T66 and E92 pathways are predominantly selected by elvitegravir.4 In contrast to raltegravir and elvitegravir, which share a common resistance profile, dolutegravir has a markedly distinct resistance profile and appears to have a higher genetic barrier to resistance. Indeed, in clinical trials, it has not been shown to select for any resistance-associated mutations in treatment-naive patients when used in triple therapy.5–7 However, one case of emergence of an integrase (IN) resistance mutation (Q148K) and the M184V mutation in reverse transcriptase (RT) during virological failure in a treatment-naive man who initiated tenofovir disoproxil fumarate/emtricitabine plus dolutegravir has been recently published.8 An emergent dolutegravir resistance (T66I) in an ART-naive individual in a real-world setting has also been previously published.9 In addition, there have been some cases of treatment failure with resistance mutations in treatment-experienced but INSTI-naive patients, in particular with the emergence of the R263K mutation.10 Finally, in the particular setting of dolutegravir monotherapy in treatment-experienced patients, the selection of other substitutions at positions E92, Q148, N155 and S230 has been reported.11 Bictegravir is the most recent INSTI, and there is little information available with regard to resistance to this drug. Although bictegravir has a similar chemical structure to dolutegravir and has also selected R263K during in vitro passages, thus sharing common resistance profiles with dolutegravir, bictegravir retained potency against several of the INSTI-resistant mutants that caused a decrease in susceptibility to dolutegravir.12–14
Although INSTI mutation pathways have been studied extensively, most existing data arise from in vitro experiments or clinical trials with a limited number of patients and specific inclusion criteria. In this study, we focused on IN genotypic resistance tests performed in a real-world clinical setting by the French national Agence Nationale de Recherches sur le SIDA (ANRS) network in order to better characterize the profile of INSTI resistance among specimens obtained for clinical decision making and to identify factors associated with the selection of IN resistance mutations.
Patients and methods
Patients and ARV regimens
HIV-1-infected patients who experienced virological failure of an INSTI-containing regimen between 2014 and 2017 and with an available IN genotype were included in the study. Patients were treated with raltegravir, elvitegravir or dolutegravir, with a background regimen comprising mainly NRTIs, NNRTIs and/or PIs. Virological failure was defined as two consecutive HIV-1 viral loads (VLs) >50 copies/mL. Clinical data and treatment histories were collected for all patients recruited. Inclusion criteria and all data were checked by the study monitor. The 21 participating laboratories belong to the ANRS AC43 network and participate in the annual ANRS quality control assessment of HIV-1 drug resistance sequencing.15
Ethics
Individual ARV agents were recorded along with their dates of initiation and discontinuation, if applicable. All patients gave written informed consent that a de-identified, electronic version of their medical chart could be used for research purposes. The study was approved by the scientific committee of the ANRS AC43.
Genotypic resistance testing
The sequences of the protease (PR)-, RT- and IN-coding regions were determined at baseline and failure (on confirmation of plasma failure) in each laboratory using the ANRS consensus technique (http://www.hivfrenchresistance.org/), the Abbott ViroSeq kit or an in-house method. Although all patients have a genotype for IN, 13% failed to amplify the PR and 14% failed to amplify the RT. For resistance interpretation, we used RT, PR and IN mutations present in the ANRS algorithm (Version 28) to determine whether patients receiving a particular NRTI, NNRTI or PI had resistant, intermediate or susceptible virus strains (www.hivfrenchresistance.org). Resistance was also determined with the Stanford algorithm and defined as the accumulation of mutations giving a high resistance score (total score >60, Stanford HIV Drug Resistance Algorithm v.8.7).
Genotypic rules for interpretation of INSTI resistance according to the V28 ANRS algorithm are shown in Table S1 (available as Supplementary material at JAC Online). The INSTI-associated mutations used in the study are: T66AIK, L74FIM, V75I, E92Q, T97A, G118R, F121Y, E138AKT, G140ACS, Y143ACGHRS, P145S, S147G, Q148EGHKR, V151L, S153FY, N155HST, E157Q, S230R and R263K.
The genotypic susceptibility score (GSS) of the current regimen (without INSTI) was calculated according to the ANRS resistance algorithm. For each ARV drug, patients with drug-susceptible viruses were assigned a GSS of 1, and those with intermediate-level and high-level resistance were assigned scores of 0.5 and 0, respectively.
Statistical analysis
Quantitative variables are expressed as median and IQR, whereas categorical variables are in percentages. HIV-1 RNA at failure, viral subtype (B versus CRF02_AG and other non-B), baseline CD4 cell count, CD4 cell count at failure, nadir CD4, age, duration of infection, duration of INSTI treatment, the ongoing treatment (dual therapy, triple therapy and quadruple therapy or more) and GSS were investigated as potential factors in occurrence of INSTI mutations by the use of the Cochran–Armitage test. A logistic regression model was also used to investigate whether previous variables were independent predictors of occurrence of INSTI resistance-associated mutations (RAMs). All variables tested with a P value <0.10 in the univariate analysis were retained for the construction of the multivariate model. The latter only keeps the variables significantly associated with the occurrence of INSTI mutation with a P value <0.05.
Results
Overall, 674 patients failing an INSTI-containing regimen were included in the study from 21 French centres in the ANRS network. Patients were failing while receiving a raltegravir- (n = 359), elvitegravir- (n = 154) or dolutegravir- (n = 161) containing regimen and 10% were failing their first-line treatment. The main characteristics of the global study population are presented in Table 1. The average age was 48.5 years (IQR 39.9–55.4 years) and the majority (65%) of patients were male. Regarding HIV-1 subtypes, 55.8% harboured subtype B and the most frequent non-B subtype was CRF02_AG (18%). The most commonly prescribed combinations including an INSTI were two NRTIs (55%) and one NRTI + one PI (13%). The percentage of patients receiving one, two, three or more than three ARVs including an INSTI was 1%, 17%, 66% and 15%, respectively.
| Characteristic . | Value . |
|---|---|
| Male | 65 |
| Subtype B | 56 |
| Median time since HIV-1 diagnosis, years (IQR) | 15.7 (6.74–22.4) |
| Median duration of current INSTI regimen, months (IQR) | 10.7 (5.7–30) |
| Median baseline plasma HIV-1 RNA, log10 copies/mL (IQR) | 3.1 (1.9–4.9) |
| Pre-INSTI VL (copies/mL) | |
| <50 | 42.3 |
| 50–100 000 | 42.6 |
| >100 000 | 15.1 |
| Median baseline CD4 cell count/mm3 (IQR) | 371 (173–649) |
| CD4 baseline <200 cells | 32.3 |
| Median failure plasma HIV-1 RNA, log10 copies/mL (IQR) | 2.9 (2.3–4) |
| Median failure CD4 cell count/mm3 (IQR) | 418 (223–670) |
| INSTI co-treatment (%) | |
| NRTIs | 55.3 |
| NRTI + PI | 13.2 |
| NNRTIs | 7 |
| PIs | 5.6 |
| NNRTI + PI | 4.9 |
| NRTI + NNRTI | 3.8 |
| other | 8.7 |
| GSS (%) | |
| 0–0.5 | 16.11 |
| 1–1.5 | 27.22 |
| 2–2.5 | 44.07 |
| ≥3 | 12.59 |
| Characteristic . | Value . |
|---|---|
| Male | 65 |
| Subtype B | 56 |
| Median time since HIV-1 diagnosis, years (IQR) | 15.7 (6.74–22.4) |
| Median duration of current INSTI regimen, months (IQR) | 10.7 (5.7–30) |
| Median baseline plasma HIV-1 RNA, log10 copies/mL (IQR) | 3.1 (1.9–4.9) |
| Pre-INSTI VL (copies/mL) | |
| <50 | 42.3 |
| 50–100 000 | 42.6 |
| >100 000 | 15.1 |
| Median baseline CD4 cell count/mm3 (IQR) | 371 (173–649) |
| CD4 baseline <200 cells | 32.3 |
| Median failure plasma HIV-1 RNA, log10 copies/mL (IQR) | 2.9 (2.3–4) |
| Median failure CD4 cell count/mm3 (IQR) | 418 (223–670) |
| INSTI co-treatment (%) | |
| NRTIs | 55.3 |
| NRTI + PI | 13.2 |
| NNRTIs | 7 |
| PIs | 5.6 |
| NNRTI + PI | 4.9 |
| NRTI + NNRTI | 3.8 |
| other | 8.7 |
| GSS (%) | |
| 0–0.5 | 16.11 |
| 1–1.5 | 27.22 |
| 2–2.5 | 44.07 |
| ≥3 | 12.59 |
Values are expressed as percentages unless otherwise indicated.
| Characteristic . | Value . |
|---|---|
| Male | 65 |
| Subtype B | 56 |
| Median time since HIV-1 diagnosis, years (IQR) | 15.7 (6.74–22.4) |
| Median duration of current INSTI regimen, months (IQR) | 10.7 (5.7–30) |
| Median baseline plasma HIV-1 RNA, log10 copies/mL (IQR) | 3.1 (1.9–4.9) |
| Pre-INSTI VL (copies/mL) | |
| <50 | 42.3 |
| 50–100 000 | 42.6 |
| >100 000 | 15.1 |
| Median baseline CD4 cell count/mm3 (IQR) | 371 (173–649) |
| CD4 baseline <200 cells | 32.3 |
| Median failure plasma HIV-1 RNA, log10 copies/mL (IQR) | 2.9 (2.3–4) |
| Median failure CD4 cell count/mm3 (IQR) | 418 (223–670) |
| INSTI co-treatment (%) | |
| NRTIs | 55.3 |
| NRTI + PI | 13.2 |
| NNRTIs | 7 |
| PIs | 5.6 |
| NNRTI + PI | 4.9 |
| NRTI + NNRTI | 3.8 |
| other | 8.7 |
| GSS (%) | |
| 0–0.5 | 16.11 |
| 1–1.5 | 27.22 |
| 2–2.5 | 44.07 |
| ≥3 | 12.59 |
| Characteristic . | Value . |
|---|---|
| Male | 65 |
| Subtype B | 56 |
| Median time since HIV-1 diagnosis, years (IQR) | 15.7 (6.74–22.4) |
| Median duration of current INSTI regimen, months (IQR) | 10.7 (5.7–30) |
| Median baseline plasma HIV-1 RNA, log10 copies/mL (IQR) | 3.1 (1.9–4.9) |
| Pre-INSTI VL (copies/mL) | |
| <50 | 42.3 |
| 50–100 000 | 42.6 |
| >100 000 | 15.1 |
| Median baseline CD4 cell count/mm3 (IQR) | 371 (173–649) |
| CD4 baseline <200 cells | 32.3 |
| Median failure plasma HIV-1 RNA, log10 copies/mL (IQR) | 2.9 (2.3–4) |
| Median failure CD4 cell count/mm3 (IQR) | 418 (223–670) |
| INSTI co-treatment (%) | |
| NRTIs | 55.3 |
| NRTI + PI | 13.2 |
| NNRTIs | 7 |
| PIs | 5.6 |
| NNRTI + PI | 4.9 |
| NRTI + NNRTI | 3.8 |
| other | 8.7 |
| GSS (%) | |
| 0–0.5 | 16.11 |
| 1–1.5 | 27.22 |
| 2–2.5 | 44.07 |
| ≥3 | 12.59 |
Values are expressed as percentages unless otherwise indicated.
Virological failure occurred after a median time of 10.7 months (IQR 5.7–30) following administration of an INSTI-containing regimen. The median duration of INSTI treatment was significantly greater in patients taking raltegravir for 24.3 months (IQR 7.6–54.1) than in patients taking elvitegravir or dolutegravir for 8.15 months (IQR 4.7–14) or 6.9 months (IQR 3.4–12.3), respectively. At failure, median VL was 2.9 log10 copies/mL (IQR 2.3–4). Overall, viruses harboured no known INSTI RAMs and were thus considered to be fully genotypically susceptible to all INSTIs in 58% (n = 389) of cases. Thus, 42% of viruses harboured at least one INSTI RAM: one, two, three, four, and five and six mutations in 25% (n = 170), 10% (n = 71), 4% (n = 28), 2% (n = 13) and 0.45% (n = 3) of cases, respectively.
Regarding INSTI RAMs in our data set, the most frequently observed IN mutations were N155H/S/T (n = 112; 16.6%), L74F/I/M (n = 82; 12.1%), Q148H/K/R (n = 54; 8.0%) and T97A (n = 53; 7.9%). Other INSTI mutations detected were in <5% of cases: T66A/I/K (n = 15; 2.2%), V75I (n = 6; 0.9%), E92Q (n = 26; 3.9%), E138A/K/T (n = 22; 3.3%), G140A/C/S (n = 33; 4.9%), Y143A/C/G/H/R/S (n = 25; 4%), P145S (n = 3; 0.4%), S147G (n = 10; 1.5%), V151L (n = 1; 0.1%), S153F/Y (n = 2; 0.3%), E157Q (n = 22; 3.3%), S230G/R (n = 7; 1.0%) and R263K (n = 2; 0.3%). The two patients with R263K mutation were not failing dolutegravir. At the time of failure, one was receiving RAL + boosted darunavir and the second was receiving elvitegravir + tenofovir/emtricitabine. Q148H/K/R mutations were selected significantly more frequently in B subtypes than in non-B subtypes (P = 0.0135). In patients harbouring viruses with two or three INSTI RAMs, the most common combinations were G140S/Q148H (12%), T97A/G140S/Q148H (6%) and L74I/E92Q (5%). The detailed profiles of the 115 patients harbouring more than one INSTI RAM are shown in Table S2.
Interpretation of resistance to the different INSTIs is described in Figure 1. Using the ANRS algorithm, at failure, 36% of patients failing raltegravir exhibited plasma viruses considered genotypically resistant to raltegravir, 44% of patients failing elvitegravir exhibited plasma viruses considered resistant to elvitegravir, and 14% and 7% of patients failing dolutegravir exhibited plasma viruses considered resistant to dolutegravir once daily and twice daily, respectively (Figure 1a). In comparison, the interpretation of resistance using the Stanford algorithm is described in Figure 1(b) and showed similar results.
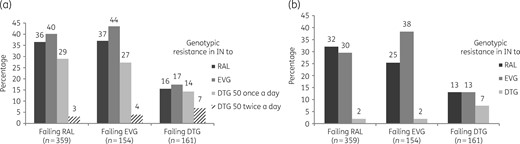
Genotypic interpretation of IN resistance to different INSTIs among the 674 patients failing an INSTI-containing regimen. Predicted resistance to raltegravir (RAL), elvitegravir (EVG) and dolutegravir (DTG) once daily or twice daily according to the IN sequence with the ANRS algorithm (a) or with the Stanford algorithm (b).
We aimed to characterize clinical and virological factors associated with the emergence of INSTI RAMs (Table 2). The final multivariate model shows a higher risk of occurrence of at least one INSTI RAM associated with a higher level of VL at failure (OR = 1.2 per 1 log10 copies/mL increase) and a lower risk of occurrence of at least one INSTI RAM with a higher level of GSS (OR = 0.29 for GSS = 1–1.5, OR = 0.12 for GSS = 2–2.5 and OR = 0.08 for GSS ≥ 3 versus GSS = 0–0.5). Figure 2 shows the association between the level of HIV VL at failure and the selection of INSTI RAMs in IN. In addition, patients failing dolutegravir harboured viruses with significantly fewer INSTI RAMs at failure than patients failing raltegravir (OR = 0.57, P = 0.02) and patients failing elvitegravir (OR = 0.45, P = 0.005).

Association between the level of HIV VL at failure and the selection of INSTI RAMs in IN.
Factors associated with the occurrence of INSTI resistance-associated mutations
| . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P value . | OR . | 95% CI . | P value . | |
| Age (per 10 year increase) | 1.115 | 0.977–1.273 | 0.1065 | |||
| Sex | 1.014 | 0.735–1.399 | 0.9316 | |||
| CD4 count at baseline (per 100 cells/mm3 increase) | 1.007 | 0.960–1.056 | 0.7764 | |||
| CD4 count at failure (per 100 cells/mm3 increase) | 0.988 | 0.941–1.038 | 0.6387 | |||
| Nadir CD4 count (per 100 cells/mm3 increase) | 0.99 | 0.902–1.087 | 0.8338 | |||
| Duration of infection (per 1 year increase) | 1.018 | 1.001–1.035 | 0.0393 | |||
| Duration of INSTI treatment (per 1 year increase) | 1.052 | 0.982–1.126 | 0.1519 | |||
| Log HIV RNA at baseline (per 1 log10 copies/mL increase) | 0.956 | 0.850–1.074 | 0.4478 | |||
| Log HIV RNA at failure (per 1 log10 copies/mL increase) | 1.345 | 1.165–1.553 | <0.0001 | 1.223 | 1.027–1.456 | 0.0242 |
| Viral subtype | ||||||
| B | 1 | |||||
| CFR02 | 0.869 | 0.572–1.319 | 0.5425 | |||
| non-B | 0.971 | 0.677–1.394 | 0.8239 | |||
| GSS | ||||||
| 0 or 0.5 | 1 | |||||
| 1 or 1.5 | 0.29 | 0.156–0.540 | 0.0715 | 0.293 | 0.156–0.551 | 0.1326 |
| 2 or 2.5 | 0.101 | 0.056–0.184 | <0.0001 | 0.116 | 0.063–0.213 | <0.0001 |
| ≥3 | 0.075 | 0.035–0.162 | <0.0001 | 0.079 | 0.036–0.174 | <0.0001 |
| Dual-therapy versus triple-therapy | 0.545 | 0.361–0.822 | 0.2545 | |||
| Dual-therapy versus four and more therapy | 0.437 | 0.253–0.754 | 0.0235 | |||
| DTG versus RAL | 0.406 | 0.270–0.610 | <0.0001 | 0.567 | 0.345–0.931 | 0.0251 |
| DTG versus EVG | 0.362 | 0.226–0.581 | <0.0001 | 0.448 | 0.254–0.789 | 0.0055 |
| . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P value . | OR . | 95% CI . | P value . | |
| Age (per 10 year increase) | 1.115 | 0.977–1.273 | 0.1065 | |||
| Sex | 1.014 | 0.735–1.399 | 0.9316 | |||
| CD4 count at baseline (per 100 cells/mm3 increase) | 1.007 | 0.960–1.056 | 0.7764 | |||
| CD4 count at failure (per 100 cells/mm3 increase) | 0.988 | 0.941–1.038 | 0.6387 | |||
| Nadir CD4 count (per 100 cells/mm3 increase) | 0.99 | 0.902–1.087 | 0.8338 | |||
| Duration of infection (per 1 year increase) | 1.018 | 1.001–1.035 | 0.0393 | |||
| Duration of INSTI treatment (per 1 year increase) | 1.052 | 0.982–1.126 | 0.1519 | |||
| Log HIV RNA at baseline (per 1 log10 copies/mL increase) | 0.956 | 0.850–1.074 | 0.4478 | |||
| Log HIV RNA at failure (per 1 log10 copies/mL increase) | 1.345 | 1.165–1.553 | <0.0001 | 1.223 | 1.027–1.456 | 0.0242 |
| Viral subtype | ||||||
| B | 1 | |||||
| CFR02 | 0.869 | 0.572–1.319 | 0.5425 | |||
| non-B | 0.971 | 0.677–1.394 | 0.8239 | |||
| GSS | ||||||
| 0 or 0.5 | 1 | |||||
| 1 or 1.5 | 0.29 | 0.156–0.540 | 0.0715 | 0.293 | 0.156–0.551 | 0.1326 |
| 2 or 2.5 | 0.101 | 0.056–0.184 | <0.0001 | 0.116 | 0.063–0.213 | <0.0001 |
| ≥3 | 0.075 | 0.035–0.162 | <0.0001 | 0.079 | 0.036–0.174 | <0.0001 |
| Dual-therapy versus triple-therapy | 0.545 | 0.361–0.822 | 0.2545 | |||
| Dual-therapy versus four and more therapy | 0.437 | 0.253–0.754 | 0.0235 | |||
| DTG versus RAL | 0.406 | 0.270–0.610 | <0.0001 | 0.567 | 0.345–0.931 | 0.0251 |
| DTG versus EVG | 0.362 | 0.226–0.581 | <0.0001 | 0.448 | 0.254–0.789 | 0.0055 |
DTG, dolutegravir; RAL, raltegravir; EVG, elvitegravir.
Factors associated with the occurrence of INSTI resistance-associated mutations
| . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P value . | OR . | 95% CI . | P value . | |
| Age (per 10 year increase) | 1.115 | 0.977–1.273 | 0.1065 | |||
| Sex | 1.014 | 0.735–1.399 | 0.9316 | |||
| CD4 count at baseline (per 100 cells/mm3 increase) | 1.007 | 0.960–1.056 | 0.7764 | |||
| CD4 count at failure (per 100 cells/mm3 increase) | 0.988 | 0.941–1.038 | 0.6387 | |||
| Nadir CD4 count (per 100 cells/mm3 increase) | 0.99 | 0.902–1.087 | 0.8338 | |||
| Duration of infection (per 1 year increase) | 1.018 | 1.001–1.035 | 0.0393 | |||
| Duration of INSTI treatment (per 1 year increase) | 1.052 | 0.982–1.126 | 0.1519 | |||
| Log HIV RNA at baseline (per 1 log10 copies/mL increase) | 0.956 | 0.850–1.074 | 0.4478 | |||
| Log HIV RNA at failure (per 1 log10 copies/mL increase) | 1.345 | 1.165–1.553 | <0.0001 | 1.223 | 1.027–1.456 | 0.0242 |
| Viral subtype | ||||||
| B | 1 | |||||
| CFR02 | 0.869 | 0.572–1.319 | 0.5425 | |||
| non-B | 0.971 | 0.677–1.394 | 0.8239 | |||
| GSS | ||||||
| 0 or 0.5 | 1 | |||||
| 1 or 1.5 | 0.29 | 0.156–0.540 | 0.0715 | 0.293 | 0.156–0.551 | 0.1326 |
| 2 or 2.5 | 0.101 | 0.056–0.184 | <0.0001 | 0.116 | 0.063–0.213 | <0.0001 |
| ≥3 | 0.075 | 0.035–0.162 | <0.0001 | 0.079 | 0.036–0.174 | <0.0001 |
| Dual-therapy versus triple-therapy | 0.545 | 0.361–0.822 | 0.2545 | |||
| Dual-therapy versus four and more therapy | 0.437 | 0.253–0.754 | 0.0235 | |||
| DTG versus RAL | 0.406 | 0.270–0.610 | <0.0001 | 0.567 | 0.345–0.931 | 0.0251 |
| DTG versus EVG | 0.362 | 0.226–0.581 | <0.0001 | 0.448 | 0.254–0.789 | 0.0055 |
| . | Univariate analysis . | Multivariate analysis . | ||||
|---|---|---|---|---|---|---|
| OR . | 95% CI . | P value . | OR . | 95% CI . | P value . | |
| Age (per 10 year increase) | 1.115 | 0.977–1.273 | 0.1065 | |||
| Sex | 1.014 | 0.735–1.399 | 0.9316 | |||
| CD4 count at baseline (per 100 cells/mm3 increase) | 1.007 | 0.960–1.056 | 0.7764 | |||
| CD4 count at failure (per 100 cells/mm3 increase) | 0.988 | 0.941–1.038 | 0.6387 | |||
| Nadir CD4 count (per 100 cells/mm3 increase) | 0.99 | 0.902–1.087 | 0.8338 | |||
| Duration of infection (per 1 year increase) | 1.018 | 1.001–1.035 | 0.0393 | |||
| Duration of INSTI treatment (per 1 year increase) | 1.052 | 0.982–1.126 | 0.1519 | |||
| Log HIV RNA at baseline (per 1 log10 copies/mL increase) | 0.956 | 0.850–1.074 | 0.4478 | |||
| Log HIV RNA at failure (per 1 log10 copies/mL increase) | 1.345 | 1.165–1.553 | <0.0001 | 1.223 | 1.027–1.456 | 0.0242 |
| Viral subtype | ||||||
| B | 1 | |||||
| CFR02 | 0.869 | 0.572–1.319 | 0.5425 | |||
| non-B | 0.971 | 0.677–1.394 | 0.8239 | |||
| GSS | ||||||
| 0 or 0.5 | 1 | |||||
| 1 or 1.5 | 0.29 | 0.156–0.540 | 0.0715 | 0.293 | 0.156–0.551 | 0.1326 |
| 2 or 2.5 | 0.101 | 0.056–0.184 | <0.0001 | 0.116 | 0.063–0.213 | <0.0001 |
| ≥3 | 0.075 | 0.035–0.162 | <0.0001 | 0.079 | 0.036–0.174 | <0.0001 |
| Dual-therapy versus triple-therapy | 0.545 | 0.361–0.822 | 0.2545 | |||
| Dual-therapy versus four and more therapy | 0.437 | 0.253–0.754 | 0.0235 | |||
| DTG versus RAL | 0.406 | 0.270–0.610 | <0.0001 | 0.567 | 0.345–0.931 | 0.0251 |
| DTG versus EVG | 0.362 | 0.226–0.581 | <0.0001 | 0.448 | 0.254–0.789 | 0.0055 |
DTG, dolutegravir; RAL, raltegravir; EVG, elvitegravir.
Among the 674 patients, 68 were failing a first-line INSTI-based regimen: 41 containing raltegravir, 18 elvitegravir and 9 dolutegravir. Among the 41 patients failing a raltegravir-based regimen, 11 (27%) harboured INSTI RAMs on their genotypic resistance test at failure: 4 with emergent mutations (1 L74I/M, 1 T97A, 1 Y143R and 1 V75I) and 7 for whom no baseline test was available (2 L74I, 1 T97A, 1 E138K, 1 N155H, 1 E92Q + N155H and 1 T97A + N155H + E157Q). Among the 18 patients failing an elvitegravir-based regimen, 7 (39%) harboured INSTI RAMs on their genotypic resistance test at failure: 5 with emergent mutations (1 T66I, 2 N155H, 1 E92Q + E157Q and 1 E92Q + S153Y + N155H) and 2 for whom no baseline test was available (1 L74I + P145S and 1 N155H + S230R). Among the nine patients failing a dolutegravir-based regimen, three harboured INSTI RAMs on genotypic resistance testing at failure, but none was considered emergent: two mutations were already present at baseline (one L74I and one E157Q) and one E138K for which no baseline test was available. Interestingly, 7/41 (17%) of the patients failing a first-line raltegravir-based regimen had plasma viruses with M184V (4 M184V alone and 3 with INSTI mutation). Among the 18 patients failing a first-line elvitegravir-based regimen, 7 (39%) had INSTI RAMs and all of them also displayed a M184V mutation, whereas it was displayed in 0/9 patients failing a dolutegravir first-line regimen. However, the Fisher test did not show a significant association between the emergence of the M184V mutation and INSTI treatment (P = 0.07).
Discussion
The development and expanding use of IN inhibitors in ARV-naive and ARV-experienced patients make it increasingly important to survey INSTI resistance in the context of large clinical settings.16 Here, we provide one of the largest data sets characterizing INSTI resistance among INSTI-failing patients obtained for clinical indications and in which collection of clinical and virological parameters was available.
Overall, our results show that in the case of virological failure with INSTI-containing regimens, 42% of viruses harboured at least one INSTI RAM. This rate is higher compared with that of a study that aimed to characterize INSTI resistance among IN resistance testing results obtained for clinical indications in the USA in which the investigators found that only 15.6% of viruses harboured INSTI major mutations.17 This difference could be related to the use of different lists of resistance mutations, as we take into account some INSTI mutations that may be considered polymorphisms by other interpretation algorithms. However, our results are similar to those of our previous study showing that 39% of patients’ viruses at the time of failure of raltegravir harbour at least one INSTI resistance mutation.18 Methodological differences between studies can be seen, as the patients included in both studies were not the same and the pre-defined list of INSTI RAMs has evolved with the inclusion of new mutations over time. In addition, in the present study, we have analysed failure of three different INSTIs and not only of raltegravir, compared with our previous study18 and with another study in which the laboratory did not obtain data on the patient’s treatment status (naive or experienced) or history of prior ARV exposures.17 This point is crucial as INSTIs have different resistance profiles and genetic barriers. Indeed, the second-generation INSTIs, including dolutegravir, display a more robust resistance profile than either raltegravir or elvitegravir, and offer a higher barrier to resistance compared with the first-generation class.19 The resistance profile of dolutegravir has been extensively characterized during the past few years, and high-level dolutegravir resistance requires double INSTI first-generation resistance mutations or specific patterns.3 This is supported by our results showing that at failure, only 14% and 7% of patients failing dolutegravir exhibited viruses considered genotypically resistant to dolutegravir once daily and twice daily, respectively, whereas 36% of patients failing raltegravir exhibited viruses considered resistant to raltegravir and 44% of patients failing elvitegravir exhibited viruses considered resistant to elvitegravir. Indeed, dolutegravir efficacy has been initially investigated in the VIKING Phase IIb study in which ARV-experienced patients with raltegravir- and/or elvitegravir-resistant viruses received 50 mg of dolutegravir either once daily (Cohort I) or twice daily (Cohort II).20 In spite of the positive results, the VIKING-3 study also highlighted how the dolutegravir response was most reduced in patients carrying viruses with resistance-associated mutations at positions G140 and Q148.21 This mutation complex is known to confer up to 10- to 20-fold reduced susceptibility to dolutegravir and, furthermore, patients harbouring viruses with Q148 + ≥2 mutations have 96% lower odds of achieving VL <50 copies/mL at week 24 compared with those with no Q148 mutations.22,23 In addition, our results reinforce the robustness of dolutegravir regarding selection of resistance in clinical practice as patients failing dolutegravir had significantly fewer INSTI resistance mutations at failure as compared with those failing raltegravir or elvitegravir.
The most common resistance pathways identified in the present study were N155H/S/T, L74F/I/M, Q148A/C/G/H/R/S and T97A. In addition, our findings corroborate previous observations indicating the unique propensity of subtype B for the development of the Q148 + G140 mutation pathway.24 A glycine to serine substitution at IN position 140 requires only one nucleotide change in subtype B and two nucleotide changes in many non-B clades, thus raising the genetic barrier to the emergence of G140 mutants. Indeed, we have previously published that CRF02_AG shows a higher genetic barrier to acquire mutations G140S and G140C, but not G140A.25 As mutations at codon 140 play a key role in restoring the fitness of Q148 mutants, their occurrence can also influence the emergence of Q148H/R/K, thus explaining the reduced prevalence of Q148 mutants observed in non-B subtypes. In the present study, some rare INSTI mutations have also been evidenced, such as the R263K mutation in two cases. The R263K mutation was the first mutation rarely selected at the time of virological failure in experienced patients failing a first-line dolutegravir-based treatment.10 Further in vitro studies on R263K mutants showed a moderate increase in the phenotypic resistance level and a drastic reduction in viral replicative capacity.13,26 More recently, it has been shown that bictegravir and cabotegravir, two more recent INSTIs, remained active against the R263K mutant.27 Other mutations (i.e. G118R and F121Y), rarely described in patients failing raltegravir,28 have also been shown to induce broad cross-resistance to dolutegravir in vitro.29 However, we did not see evidence of either G118R or F121Y in this study.
Another interesting mutation is the E157Q mutation. This mutation can be polymorphic with a natural prevalence between 1.7% and 5.6%, depending of the subtype, in viral sequences from ART-naive patients, but also an acquired mutation emerging at raltegravir failure as described in two case reports.30 Data on the phenotypic resistance level of E157Q mutants and virological response of patients harbouring an E157Q virus initiating an INSTI-based regimen showed that dolutegravir might be the most effective INSTI in such patients.31–33 However, in the present study, 1/9 patients who failed dolutegravir had a virus already harbouring an E157Q at baseline; thus, it is difficult to make strong recommendations.
In clinical practice, it has been shown that after previous exposure to first-generation INSTIs, treatment with dolutegravir showed long durability and that patients infected with a non-B HIV-1 subtype had a greater risk of having detectable VL at the last observation.34 It is also important to determine, in the case of virological failure, which factors are associated with the development of resistance mutations. In a previous study, we showed that a low GSS was associated with the presence of raltegravir-associated mutations and that a high HIV-1 VL level at failure (>1000 copies/mL) was associated with the presence of raltegravir-associated mutations.18 Here we reinforce this message, showing that patients with high VL (>3 log copies/mL) at failure and low GSS have a higher risk of selecting at least one INSTI RAM. This has clinical consequences suggesting that careful attention should be paid to patients with detectable VL under an INSTI regimen. A previous study by Lepik et al.9 retrospectively analysed a total of 985 individuals on INSTIs (raltegravir, elvitegravir and dolutegravir), including ART-naive and -experienced patients, in a Canadian cohort. Among the 985 patients, the number not virologically suppressed was 70 with raltegravir, 73 with elvitegravir and 65 with dolutegravir. The authors showed that CD4 <200 and <80% adherence were associated with an increased risk of emergent resistance to INSTIs and found no statistically significant difference in resistance rates between raltegravir, elvitegravir and dolutegravir during the first year, although drug resistance rates were numerically highest for raltegravir owing to more emergent RT mutations. It is difficult to compare the results of both studies because we do not have adherence data in our study and patients in the Lepik et al.9 study are more advanced patients than in ours. Indeed, in the Lepik et al.9 study, 70% of patients were INSTI naive (versus 27% in our study), with pre-INSTI CD4 <200/mm3 in 15% of patients (versus 32%) and with pre-INSTI HIV-1 RNA >100 000 copies/mL in 11% (versus 15%). However, the fact that among the 674 patients failing an INSTI regimen in our study, 58% did not harbour INSTI RAMs at failure suggests that lack of adherence could be a part of the explanation.
In this study we have focused particularly on failure in treatment-naive patients. At failure, 27% of patients receiving raltegravir had emergent or not previously evidenced INSTI RAMs, 39% with elvitegravir and none with dolutegravir. In addition, 17% of patients failing raltegravir had plasma viruses with an M184V mutation (four alone and three with an INSTI mutation) compared with 39% of patients failing elvitegravir (always associated with INSTI mutation) and none in patients failing dolutegravir. Our results corroborate data from clinical trials showing that raltegravir and elvitegravir have a relatively low genetic barrier to the development of resistance, with an overlapping resistance profile, and do not protect the NRTI backbone.35 In treatment-naive patients, data from a clinical trial showed resistance mutation neither to INSTIs nor to NRTIs in the rare patients experiencing virological failure in the dolutegravir arm up to 96 weeks.6 Thus our data corroborate the hypothesis that the use of dolutegravir as first-line therapy in clinical practice should also prevent the development of INSTI and associated NRTI drug resistance. This also could be related to the fact shown by in vitro data that M184I/V in RT and resistance to dolutegravir or the R263K substitution in IN are essentially incompatible.36,37 However, this should be carefully monitored because despite a high barrier to resistance, no ARV agent is impervious to resistance and, even though it is extremely rare to date, dolutegravir failure and resistance in treatment-naive patients is possible.8 Indeed, in their study, Lepik et al.9 described emergent dolutegravir resistance (T66I) in an ART-naive individual. Moreover, it has been shown recently that resistance against dolutegravir can emerge outside of the IN-coding region (in the 3′ PPT region) both in vitro38 and in patients.39 Indeed these results raise the possibility that some of the patients who fail dolutegravir-based regimens could in fact have acquired resistance mutations outside IN, in the 3′ PPT region.40,41 Thus, this region should also be sequenced to reach a firm conclusion regarding the robustness of dolutegravir in terms of resistance selection.
Overall, this paper describes one of the largest studies characterizing INSTI resistance among resistance testing obtained for clinical indications from naive and experienced patients failing raltegravir, elvitegravir and dolutegravir, and reveals factors associated with resistance to INSTIs that should be taken into consideration in clinical management. The results confirmed the robustness of dolutegravir regarding resistance selection in the IN-coding region in the case of virological failure in routine clinical care.
Acknowledgements
This work was presented in part as an oral presentation at the European AIDS Clinical Society Meeting, 2017, Milan, Italy (abstract PS3/1).
Members of the ANRS AC43 Resistance Study Group by location
Amiens, C. Roussel; Angers, H. Le Guillou-Guillemette, A. Ducancelle; Argenteuil, L. Courdavault; Avicenne, C. Alloui, P. Honore; Besançon, Q. Lepiller, D. Bettinger; Bordeaux, P. Bellecave, P. Pinson-Recordon, C. Tumiotto, S. Reigadas; Brest, S. Vallet, C. Payan, J. C. Duthe; Caen, M. Leroux, J. Dina, A. Vabret; Clermont-Ferrand, A. Mirand, C. Henquell; Créteil-Henri Mondor, M. Bouvier-Alias; Dijon, A. Simohamed; Fort de France, G. Dos Santos; Genève, S. Yerly, C. Gaille, W. Caveng, S. Chapalay, A. Calmy; Grenoble, A. Signori-Schmuck, P. Morand; HU Paris Sud, C. Pallier, M. Raho-Moussa, M. Mole, M.-J. Dulucq; Lille–Tourcoing, L. Bocket, K. Alidjinou; Limoges, S. Ranger-Rogez; Lyon, M. A. Trabaud, V. Icard, J. C. Tardy; Marseille, C. Tamalet; Metz/Thionville, C. Delamare; Montpellier, B. Montes; Nancy, E. Schvoerer, H. Fenaux; Nantes, A. Rodallec, E. André-Garnier, V. Ferré; Nice, A. De Monte; Orléans, A. Guigon, J. Guinard; Paris-Bichat Claude Bernard, D. Descamps, C. Charpentier, B. Visseaux, G. Peytavin; Paris-Necker, M. Fillion; Paris-Pitié-Salpêtrière, C. Soulié, I. Malet, M. Wirden, A. G. Marcelin, V. Calvez, P. Flandre, L. Assoumou, D. Costagliola; Paris-Saint Antoine, L. Morand-Joubert, S. Lambert-Niclot, D. Fofana; Paris-Saint Louis, C. Delaugerre, M. L. Chaix, N. Mahjoub; Paris-Tenon, C. Amiel; Poitiers, G. Giraudeau, A. Beby-Defaux, D. Plainchamp; Rennes, A. Maillard; Rouen, E. Alessandri-Gradt, M. Leoz, J. C. Plantier; Strasbourg, P. Gantner, S. Fafi-Kremer, P. Fischer; Toulouse, S. Raymond, J. Izopet, J Chiabrando; Tours, F. Barin, G. Fajole, O. Burgault; Versailles, S. Marque Juillet.
Members of the ANRS Clinical Centres by location
Angers, P. Abgueguen, V. Rabier, Y. M. Vandamme; Besançon, B. Hoen; Bordeaux, M. Dupon, P. Morlat, D. Neau; Brest, M. Garré, V. Bellein; Caen, R. Verdon, A. De la Blanchardière, S. Dargère, A. Martin, V. Noyou; Clermont-Ferrand, C. Jacomet; Créteil, J. D. Lelièvre, J. L. Lopez-Zaragoza; Dijon, B. Lorcerie; Fort de France, A. Cabié; Genève, S. Yerly; Grenoble, P. Leclercq, M. Blanc; Le Kremlin-Bicêtre, C. Goujard; Lille–Tourcoing, O. Robineau; Limoges, P. Weinbreck; Lyon, L. Cotte, D. Makhloufi; Marseille, I. Poizot-Martin, I. Ravaud; Montpellier, J. Reynes; Nancy, H. Fenaux; Nantes, F. Raffi; Nice, E. Cua, J. Durant, P. Pugliese; Orléans, L. Hocquelloux, T. Prazuck; Paris-Bichat Claude Bernard, Y. Yazdanpanah, R. Landman, S. Legac; Paris-HEGP, L. Weiss, M. Karmochkine; Paris-Jean-Verdier, S. Tassi; Paris-Necker-Enfants Malades, C. Duvivier; HU Paris-Sud, C. Bolliot, M. Malet, D. Vittecoq, M. Raho-Moussa, M. Mole; Paris-Pitié-Salpêtrière, C. Katlama, A. Simon; Paris-Saint Antoine, P. M. Girard, J. L. Meynard; Paris-Saint Louis, J. M. Molina; Paris-Tenon, V. Berrebi, G. Pialoux; Pointe à Pitre, I. Lamaury; Fort de France, A. Cabié; Poitiers, G. Le Moal, D. Plainchamp; Rennes, C. Michelet, J.-C. Duthe; Rouen, F. Caron, Y. Debab, G. Unal; Strasbourg, M. Partisani, D. Rey, P. Fischer; Toulouse, B. Marchou, P. Massip, P. Delobel; Tours, G. Gras, G. Fajole; Versailles, A. Greber Belan, Ruel, O. Beletry, F. Granier.
Funding
The research leading to these results has received funding from the Agence Nationale de Recherches sur le SIDA et les Hépatites virales (ANRS) and ViiV Healthcare.
Transparency declarations
None to declare.
References
Author notes
Members are listed in the Acknowledgement section.



