-
PDF
- Split View
-
Views
-
Cite
Cite
Véronique Joly, Charles Burdet, Roland Landman, Marie Vigan, Charlotte Charpentier, Christine Katlama, André Cabié, Aida Benalycherif, Gilles Peytavin, Patrick Yeni, France Mentre, Anne-Laure Argoud, Imane Amri, Diane Descamps, Yazdan Yazdanpanah, LAMIDOL Study Group , Dolutegravir and lamivudine maintenance therapy in HIV-1 virologically suppressed patients: results of the ANRS 167 trial (LAMIDOL), Journal of Antimicrobial Chemotherapy, Volume 74, Issue 3, March 2019, Pages 739–745, https://doi.org/10.1093/jac/dky467
Close - Share Icon Share
Abstract
To evaluate the dolutegravir+lamivudine combination in virologically suppressed patients living with HIV.
The ANRS 167 LAMIDOL trial was an open-label, single arm, multicentre trial assessing once-daily dolutegravir (50 mg)+lamivudine (300 mg) in virologically suppressed HIV-1 patients on first-line triple-drug regimens. The main criteria for inclusion in the trial were plasma viral load (pVL) ≤50 copies/mL for ≥2 years, CD4 nadir >200 cells/mm3 and WT HIV prior to treatment initiation. From week −8 (W−8) to day 0 (D0) (Phase 1), the current third agent was switched to dolutegravir. From D0 to W48 (Phase 2), patients received once-daily dolutegravir+lamivudine, except if intolerant or if pVL >50 copies/mL during Phase 1. Virological failure was defined as pVL >50 copies/mL in two consecutive samples. The study was designed to show that the strategy had an efficacy of ≥80%, assuming a 90% success rate with a type I error of 5% and a power of 90%.
In total, 104 of 110 patients enrolled in Phase 1 were included in Phase 2. These 104 patients were 86% male, 72% MSM and 87% CDC stage A. Their characteristics were (median): age 45 years, CD4 nadir 339 cells/mm3, baseline CD4 743 cells/mm3 and duration of viral suppression 4.5 years. The overall success rate at W48 was 97% (95% CI: 94%–100%), meeting the design expectation/assumption. Three therapeutic failures occurred: one virological failure at W4, one lost to follow-up at W32 and one interruption of therapeutic strategy at W40 after a blip (pVL 59 copies/mL but control pVL <50 copies/mL). Three viral blips occurred in two additional patients. Neither M184V nor integrase resistance mutations were detected after failure or blips.
Dolutegravir+lamivudine is a promising maintenance therapy in HIV-1-infected patients with controlled virological suppression.
Introduction
HIV treatment guidelines recommend combined antiretroviral therapy (cART) with two nucleoside/nucleotide reverse-transcriptase inhibitors (NRTIs) as a backbone combined with a third agent that is either an NNRTI, a PI or an integrase strand transfer inhibitor (INSTI). With the improved potency and the higher genetic barrier to resistance of recent drugs, interest has emerged in antiretroviral-sparing strategies including dual-therapy options. HIV has become a chronic disease and patients living with HIV need to be treated throughout their whole lifetime; however, the prevalence of comorbidities increases with ageing.1 Since drug-related adverse events associated with the long-term use of cART may contribute to comorbidities,2,3 reducing drug exposure is of interest. Dual regimens would also improve convenience and adherence, reduce drug–drug interactions and decrease costs. However, these regimens should be as effective as standard three-drug cART.
Dolutegravir is a potent INSTI with a high genetic barrier to resistance. Dolutegravir is well tolerated, given once daily, with or without food, and has few drug interactions. As a third agent of first-line cART, dolutegravir has shown non-inferiority compared with raltegravir, and superiority compared with efavirenz and darunavir in the randomized clinical trials SPRING-2, SINGLE and FLAMINGO, respectively.4–7
Lamivudine is a potent cytidine nucleoside analogue with a good safety profile, lack of drug interactions and convenient once-daily administration, with or without food. Low-cost generic versions of this drug are now available.
Dual-therapy studies have evaluated boosted PIs combined with lamivudine or raltegravir, demonstrating favourable results in both virologically suppressed and cART-naive patients, provided that the baseline plasma viral load (pVL) remains <100000 copies/mL.8 More recently, dual therapy combining an INSTI and an NNRTI was shown to be non-inferior to triple therapy when used as switch therapy in patients with virological suppression.9,10
Dolutegravir+lamivudine has been evaluated in both cART-naive and cART-treated virologically suppressed patients. In a recent pilot study in cART-naive patients, 90% of participants reached pVL <50 copies/mL at week 24, but lamivudine and dolutegravir resistance mutations emerged in one patient with virological failure, compromising the activity of both drugs.11 In virologically suppressed patients, Maggiolo et al.12 reported encouraging results from a prospective cohort and Taiwo et al.13 reported that dolutegravir+lamivudine was as effective as standard therapy, although in the latter trial, the chosen non-inferiority margin of 12% was higher than the 4% margin recommended by the FDA for switch trials.
The purpose of the present study (ANRS 167 Trial LAMIDOL) was to assess in a prospective non-comparative trial the efficacy, safety and tolerability of once-daily dolutegravir (50 mg) + lamivudine (300 mg) as a switch strategy in virologically suppressed patients on standard cART. Since few data were available at time of the trial initiation, we chose to design a single-arm study that needed a lower number of patients (rather than a comparative trial) that would rapidly provide information about the efficacy and safety of this unusual dual therapy. This non-comparative trial could be considered as a first step to obtain information useful for building a large randomized trial comparing this dual therapy with standard triple-drug therapy.
Patients and methods
Study design and participants
ANRS 167 LAMIDOL was a 56 week, single-arm, prospective multicentre trial in HIV-1-infected patients who were virologically suppressed on a first-line cART based on two NRTIs and a third agent consisting of a boosted PI, an NNRTI or an INSTI with no change in regimen in the past for virological failure. Previous change in cART for simplification and/or tolerance was allowed, except in the 6 months preceding screening. Inclusion criteria were age ≥18 years; pVL ≤50 copies/mL for at least 2 years, with at least three pVL determinations each year; nadir CD4 count >200 cells/mm3; pretreatment HIV genotype showing no resistance to any NRTI, NNRTI or PI and, when data were available, INSTI; acceptable laboratory values at screening [estimated glomerular filtration rate (eGFR) by modification of the diet in renal disease >60 mL/min, AST/ALT <3 × upper limit of normal, haemoglobin >10 g/dL, >100000 platelets/mm3]; negative pregnancy test at screening and use of contraception during the study for women of childbearing age; written informed consent. Breastfeeding women and individuals with chronic hepatitis B, severe liver disease or planned hepatitis C treatment during the study period were excluded.
Ethics
The trial was conducted in accordance with Good Clinical Practice and the ethical principles of the Helsinki declaration, and following ANRS recommendations for clinical research. The Ile-de-France Ethics Review Committee approved the study on 30 July 2015, and all participants gave their written informed consent before entering the study. The study was registered in ClinicalTrials.gov (NCT02527096) and EudraCT (2015-001492-44).
Procedures
The study was conducted in two consecutive phases. The screening visit at week −12 (W−12) was designed to obtain informed consent and to check the inclusion criteria. The HIV-RNA sequence of any previous drug-resistance genotype was collected and analysed (ANRS-AC11 resistance algorithm, version 254, 20154, http://www.hivfrenchresistance.org). Four weeks later, at the W−8 visit, the third agent was replaced by once-daily dolutegravir (50 mg) and patients were included in the 8 weeks of Phase 1. At D0 (baseline, end of Phase 1), once-daily lamivudine (300 mg) was substituted for the NRTI backbone, unless safety or virological events were observed. Thus, patients received dolutegravir+lamivudine once daily from baseline to W48. Patients were assessed clinically and biologically every 4 weeks from W−8 to W16, then every 8 weeks until W48.
Virological and immunological analyses
pVL [COBAS® AmpliPrep/COBAS® TaqMan® HIV-1 Test, version 2.0; Roche Molecular Systems, Branchburg, NJ, USA) or Abbott Real-Time HIV-1 (Abbott, Chicago, IL, USA] and T-cell subpopulations were measured at each visit in the local laboratories. A pVL value >50 copies/mL was confirmed on a new sample taken 2–4 weeks later, and an event was classified as a virological failure if the pVL at control testing was >50 copies/mL, and as a viral blip otherwise. An additional plasma sample was obtained and frozen at each visit for pVL assay, drug resistance genotyping and pharmacological assays. Resistance genotyping tests were performed by using Sanger direct sequencing procedures, and interpreted according to the ANRS-AC11 algorithm, version 25.
Pharmacological evaluation
The plasma concentrations of dolutegravir and lamivudine were determined in cases of virological failure using a validated LC-MS/MS method (Waters Acquity UPLC-TQD, Milford, MA, USA).14 Dolutegravir trough levels were interpreted according to different effective cut-offs of 1000 ng/mL at 24 h, based on the pharmacokinetic/pharmacodynamic relationship from the SAILING trial.15
Outcomes
The primary endpoint was therapeutic success, defined by the absence of both virological failure and strategy failure up to W48. Virological failure was defined as a first pVL >50 copies/mL, confirmed on a second sample 2–4 weeks later, as described above. Strategy failure was defined as discontinuation of the strategy for ≥30 days, because of adverse events, the patient’s or investigator’s decision, or loss to follow-up.
Secondary outcomes included the occurrence of serious adverse events; the occurrence of genotypic resistance in the case of failure; changes in the CD4 and CD8 cell counts and the CD4/CD8 ratio from D0 to W24 and W48; and changes in the eGFR from W−8 to W48.
Statistical considerations
The study was designed to show that the strategy had an efficacy of ≥80%, assuming a 90% success rate with a type I error of 5% and a power of 90%. Enrolment of 95 patients in Phase 2 was required to achieve this objective. This number was increased to a Phase 2 enrolment of 100 patients, and thus to 110 patients enrolled in Phase 1, to account for non-evaluable patients. The strategy was considered to be acceptable if ≤10 patients failed in Phase 2, including a maximum of 5 patients with virological failure.
The primary efficacy endpoint was analysed in all patients included in Phase 2. The observed proportion of patients achieving the efficacy endpoint was compared with the theoretical 80% success rate using a unilateral Wilcoxon test, and we computed its binomial 95% CI. The evolution of the CD4 cell count and CD4/CD8 ratio was analysed using a mixed-effects linear model. As a decrease in creatinine clearance had been previously reported in the first weeks after beginning dolutegravir treatment,13,16 the evolution of creatinine clearance was analysed using mixed-effects segmented regression analysis based on the hypothesis of slope change at the beginning of Phase 2 (D0).
Data were summarized as proportions for categorical variables, and median (minimum–maximum) for continuous variables. Analysis was performed in all patients included in Phase 2, and safety was evaluated in all patients included in Phase 1. Type I error was set at 0.05. All analyses were done with SAS version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
Between October 2015 and February 2016, 139 patients were screened and 110 patients from 17 sites were enrolled in the study. Of these 110 patients, 6 did not enter in Phase 2, including 3 patients because of adverse events and 3 patients because of the occurrence of a pVL >50 copies/mL during Phase 1 (Figure 1).
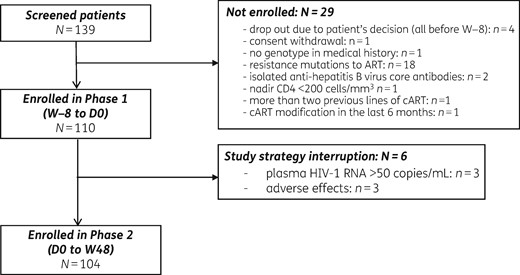
The baseline characteristics of patients included in Phase 2 are shown in Table 1. The median duration of viral suppression prior to enrolment in the study was 4.2 (2.0–9.1) years. Twenty-five patients (24%) had been previously exposed to an INSTI, either on current cART (22 patients) or on previous therapeutic lines (3 patients). Genotypic results for INSTIs were available in 21 patients (20%).
Demographic and baseline characteristics of the 104 patients included in Phase 2
| Characteristic . | Value . |
|---|---|
| Male sex, n (%) | 89 (85.6) |
| Age (years) | 45 (24–71) |
| Mode of transmission, n (%) | |
| MSM | 73 (70) |
| heterosexual | 29 (28) |
| PWID | 2 (2) |
| Time since HIV diagnosis (years) | 6.2 (2.3–24.5) |
| Nadir CD4 count (cells/mm3) | 339 (203–1155) |
| CDC stage (A/B/C), proportion of patients (%) | 87.5/8.7/3.8 |
| cART duration (years) | 4.5 (2.2–11.2) |
| Time on current cART (years) | 4.0 (0.5–11.3) |
| NRTI backbone (FTC/TDF or ABC/3TC) | 79/25 (76%/24%) |
| Duration (years) of suppressed plasma HIV RNAa | 4.2 (2.0–9.1) |
| CD4 count at enrolment (cells/mm3) | 743 (373–1571) |
| Third agent in cART at screening, n (%) | |
| NNRTI | 58 (55.8) |
| PI | 24 (23.1) |
| INSTI | 22 (21.2) |
| RAL/EVG/DTG (n) | 8/7/7 |
| Characteristic . | Value . |
|---|---|
| Male sex, n (%) | 89 (85.6) |
| Age (years) | 45 (24–71) |
| Mode of transmission, n (%) | |
| MSM | 73 (70) |
| heterosexual | 29 (28) |
| PWID | 2 (2) |
| Time since HIV diagnosis (years) | 6.2 (2.3–24.5) |
| Nadir CD4 count (cells/mm3) | 339 (203–1155) |
| CDC stage (A/B/C), proportion of patients (%) | 87.5/8.7/3.8 |
| cART duration (years) | 4.5 (2.2–11.2) |
| Time on current cART (years) | 4.0 (0.5–11.3) |
| NRTI backbone (FTC/TDF or ABC/3TC) | 79/25 (76%/24%) |
| Duration (years) of suppressed plasma HIV RNAa | 4.2 (2.0–9.1) |
| CD4 count at enrolment (cells/mm3) | 743 (373–1571) |
| Third agent in cART at screening, n (%) | |
| NNRTI | 58 (55.8) |
| PI | 24 (23.1) |
| INSTI | 22 (21.2) |
| RAL/EVG/DTG (n) | 8/7/7 |
Where not indicated otherwise, values given are median (IQR). 3TC, lamivudine; ABC, abacavir; DTG, dolutegravir; EVG, elvitegravir; FTC, emtricitabine; INSTI, integrase strand-transfer inhibitor; PWID, people who inject drugs; RAL, raltegravir; TDF, tenofovir disoproxil fumarate.
Detection limit: 50 copies/mL.
Demographic and baseline characteristics of the 104 patients included in Phase 2
| Characteristic . | Value . |
|---|---|
| Male sex, n (%) | 89 (85.6) |
| Age (years) | 45 (24–71) |
| Mode of transmission, n (%) | |
| MSM | 73 (70) |
| heterosexual | 29 (28) |
| PWID | 2 (2) |
| Time since HIV diagnosis (years) | 6.2 (2.3–24.5) |
| Nadir CD4 count (cells/mm3) | 339 (203–1155) |
| CDC stage (A/B/C), proportion of patients (%) | 87.5/8.7/3.8 |
| cART duration (years) | 4.5 (2.2–11.2) |
| Time on current cART (years) | 4.0 (0.5–11.3) |
| NRTI backbone (FTC/TDF or ABC/3TC) | 79/25 (76%/24%) |
| Duration (years) of suppressed plasma HIV RNAa | 4.2 (2.0–9.1) |
| CD4 count at enrolment (cells/mm3) | 743 (373–1571) |
| Third agent in cART at screening, n (%) | |
| NNRTI | 58 (55.8) |
| PI | 24 (23.1) |
| INSTI | 22 (21.2) |
| RAL/EVG/DTG (n) | 8/7/7 |
| Characteristic . | Value . |
|---|---|
| Male sex, n (%) | 89 (85.6) |
| Age (years) | 45 (24–71) |
| Mode of transmission, n (%) | |
| MSM | 73 (70) |
| heterosexual | 29 (28) |
| PWID | 2 (2) |
| Time since HIV diagnosis (years) | 6.2 (2.3–24.5) |
| Nadir CD4 count (cells/mm3) | 339 (203–1155) |
| CDC stage (A/B/C), proportion of patients (%) | 87.5/8.7/3.8 |
| cART duration (years) | 4.5 (2.2–11.2) |
| Time on current cART (years) | 4.0 (0.5–11.3) |
| NRTI backbone (FTC/TDF or ABC/3TC) | 79/25 (76%/24%) |
| Duration (years) of suppressed plasma HIV RNAa | 4.2 (2.0–9.1) |
| CD4 count at enrolment (cells/mm3) | 743 (373–1571) |
| Third agent in cART at screening, n (%) | |
| NNRTI | 58 (55.8) |
| PI | 24 (23.1) |
| INSTI | 22 (21.2) |
| RAL/EVG/DTG (n) | 8/7/7 |
Where not indicated otherwise, values given are median (IQR). 3TC, lamivudine; ABC, abacavir; DTG, dolutegravir; EVG, elvitegravir; FTC, emtricitabine; INSTI, integrase strand-transfer inhibitor; PWID, people who inject drugs; RAL, raltegravir; TDF, tenofovir disoproxil fumarate.
Detection limit: 50 copies/mL.
Treatment outcomes
The overall success rate at W48 was 97% (95% CI: 94%–100%), which was significantly higher than 80% (P < 10−6). Therapeutic failure occurred in three patients. One patient (Patient A) experienced a virological failure at W4 (pVL 84 copies/mL confirmed at 83 copies/mL 18 days later, see Table 2). He had received raltegravir as a third agent during first-line cART; baseline genotypic testing for INSTI was available and showed no resistance mutations. Trough dolutegravir and lamivudine plasma levels measured at time of failure were within the therapeutic range. Despite treatment intensification, and later treatment modification, pVL remained >50 copies/mL until W48. Another patient was lost to follow-up at W32. Finally, one patient (Patient B, see Table 2) was switched back to triple therapy at W40 after a blip (pVL 59 copies/mL), although the control viral load (44 copies/mL), obtained before intensification with tenofovir/emtricitabine/dolutegravir, failed to confirm virological failure; this patient was classified as interruption of therapeutic strategy. In this patient, trough dolutegravir plasma concentrations were below the efficacy threshold.
Therapeutic, virological and pharmacological characteristics of patients with at least one value of plasma viral load >50 copies/mL during Phase 2 (blips and failure)
| Patient . | Baseline . | Follow-up . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | previous cART . | INSTI RAM . | visit . | pVL (copies/mL) . | endpoint . | plasma drug (ng/mL)a . | HIV RAM . | cART modification . |
| Patient A | FTC/TDF+RAL then ABC/3TC +RAL | absence | W4 | 84 | virological failure at W24 |
| RNA: no RAM at W24 | ABC/3TC+DTG at W8 |
| W6 | 83 | |||||||
| W8 | 77 | |||||||
| W16 | 38 | |||||||
| W24 | 56 | |||||||
| W32 | 52 | RAL/ETR at W32 | ||||||
| W40 | 100 | |||||||
| W48 | 99 | |||||||
| Patient B | FTC/TDF +RPV then FTC/TDF+RPV | absence | W32 | 59 | therapeutic failure at W40 (interruption of strategy) |
| RNA: L74V/L DNA: M230I, V106I associated with stop codonsb | FTC/TDF +DTG at W40 (local investigator decision) |
| W40 | <50 | |||||||
| W48 | 55 | |||||||
| Patient C | ABC/3TC+fAPV then ABC/3TC+RPV | NA | W24 | 51 | blip | NA | not amplified | no |
| W28 to W48 | <50 | |||||||
| Patient D | FTC/TDF+EFV then FTC/TDF+RPV | NA | W40 | 67 | blip |
| RNA: no RAM in RT, not amplified for integrase | no |
| W43 | <50 | |||||||
| W48 | 130 | |||||||
| W52 | <50 | |||||||
| Patient . | Baseline . | Follow-up . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | previous cART . | INSTI RAM . | visit . | pVL (copies/mL) . | endpoint . | plasma drug (ng/mL)a . | HIV RAM . | cART modification . |
| Patient A | FTC/TDF+RAL then ABC/3TC +RAL | absence | W4 | 84 | virological failure at W24 |
| RNA: no RAM at W24 | ABC/3TC+DTG at W8 |
| W6 | 83 | |||||||
| W8 | 77 | |||||||
| W16 | 38 | |||||||
| W24 | 56 | |||||||
| W32 | 52 | RAL/ETR at W32 | ||||||
| W40 | 100 | |||||||
| W48 | 99 | |||||||
| Patient B | FTC/TDF +RPV then FTC/TDF+RPV | absence | W32 | 59 | therapeutic failure at W40 (interruption of strategy) |
| RNA: L74V/L DNA: M230I, V106I associated with stop codonsb | FTC/TDF +DTG at W40 (local investigator decision) |
| W40 | <50 | |||||||
| W48 | 55 | |||||||
| Patient C | ABC/3TC+fAPV then ABC/3TC+RPV | NA | W24 | 51 | blip | NA | not amplified | no |
| W28 to W48 | <50 | |||||||
| Patient D | FTC/TDF+EFV then FTC/TDF+RPV | NA | W40 | 67 | blip |
| RNA: no RAM in RT, not amplified for integrase | no |
| W43 | <50 | |||||||
| W48 | 130 | |||||||
| W52 | <50 | |||||||
3TC, lamivudine; ABC, abacavir; DTG, dolutegravir; EFV, efavirenz; ETR, etravirine; FTC, emtricitabine; fAPV, fosamprenavir; RAL, raltegravir; RAM, resistance-associated mutation; RPV, rilpivirine; RT, reverse transcriptase; TDF, tenofovir.
Interval between last drug intake and sampling. Expected trough drug levels (24 h after administration) were 20–60 ng/mL for lamivudine and 1000 ng/mL for dolutegravir.
The mutation M230I resulting from G to A APOBEC-induced mutations associated with stop codons favours defective proviruses.
Therapeutic, virological and pharmacological characteristics of patients with at least one value of plasma viral load >50 copies/mL during Phase 2 (blips and failure)
| Patient . | Baseline . | Follow-up . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | previous cART . | INSTI RAM . | visit . | pVL (copies/mL) . | endpoint . | plasma drug (ng/mL)a . | HIV RAM . | cART modification . |
| Patient A | FTC/TDF+RAL then ABC/3TC +RAL | absence | W4 | 84 | virological failure at W24 |
| RNA: no RAM at W24 | ABC/3TC+DTG at W8 |
| W6 | 83 | |||||||
| W8 | 77 | |||||||
| W16 | 38 | |||||||
| W24 | 56 | |||||||
| W32 | 52 | RAL/ETR at W32 | ||||||
| W40 | 100 | |||||||
| W48 | 99 | |||||||
| Patient B | FTC/TDF +RPV then FTC/TDF+RPV | absence | W32 | 59 | therapeutic failure at W40 (interruption of strategy) |
| RNA: L74V/L DNA: M230I, V106I associated with stop codonsb | FTC/TDF +DTG at W40 (local investigator decision) |
| W40 | <50 | |||||||
| W48 | 55 | |||||||
| Patient C | ABC/3TC+fAPV then ABC/3TC+RPV | NA | W24 | 51 | blip | NA | not amplified | no |
| W28 to W48 | <50 | |||||||
| Patient D | FTC/TDF+EFV then FTC/TDF+RPV | NA | W40 | 67 | blip |
| RNA: no RAM in RT, not amplified for integrase | no |
| W43 | <50 | |||||||
| W48 | 130 | |||||||
| W52 | <50 | |||||||
| Patient . | Baseline . | Follow-up . | ||||||
|---|---|---|---|---|---|---|---|---|
| . | previous cART . | INSTI RAM . | visit . | pVL (copies/mL) . | endpoint . | plasma drug (ng/mL)a . | HIV RAM . | cART modification . |
| Patient A | FTC/TDF+RAL then ABC/3TC +RAL | absence | W4 | 84 | virological failure at W24 |
| RNA: no RAM at W24 | ABC/3TC+DTG at W8 |
| W6 | 83 | |||||||
| W8 | 77 | |||||||
| W16 | 38 | |||||||
| W24 | 56 | |||||||
| W32 | 52 | RAL/ETR at W32 | ||||||
| W40 | 100 | |||||||
| W48 | 99 | |||||||
| Patient B | FTC/TDF +RPV then FTC/TDF+RPV | absence | W32 | 59 | therapeutic failure at W40 (interruption of strategy) |
| RNA: L74V/L DNA: M230I, V106I associated with stop codonsb | FTC/TDF +DTG at W40 (local investigator decision) |
| W40 | <50 | |||||||
| W48 | 55 | |||||||
| Patient C | ABC/3TC+fAPV then ABC/3TC+RPV | NA | W24 | 51 | blip | NA | not amplified | no |
| W28 to W48 | <50 | |||||||
| Patient D | FTC/TDF+EFV then FTC/TDF+RPV | NA | W40 | 67 | blip |
| RNA: no RAM in RT, not amplified for integrase | no |
| W43 | <50 | |||||||
| W48 | 130 | |||||||
| W52 | <50 | |||||||
3TC, lamivudine; ABC, abacavir; DTG, dolutegravir; EFV, efavirenz; ETR, etravirine; FTC, emtricitabine; fAPV, fosamprenavir; RAL, raltegravir; RAM, resistance-associated mutation; RPV, rilpivirine; RT, reverse transcriptase; TDF, tenofovir.
Interval between last drug intake and sampling. Expected trough drug levels (24 h after administration) were 20–60 ng/mL for lamivudine and 1000 ng/mL for dolutegravir.
The mutation M230I resulting from G to A APOBEC-induced mutations associated with stop codons favours defective proviruses.
Three viral blips were observed in two additional patients (Patients C and D, Table 2): at W24 (pVL 51 copies/mL, control <50 copies/mL 4 weeks later) (Patient C), and at W40 (pVL 67 copies/mL control <50 copies/mL 3 weeks later) and W48 (pVL 130 copies/mL, control <50 copies/mL 4 weeks later) (Patient D). One of these two patients (Patient D) had low trough dolutegravir plasma concentrations. When genotypic resistance was studied after failure or blips, resistance-associated mutations were not detected, with the exception of an L74V/L mutation that was unrelated to the patient history of cART and may result from random amplification of a clone in the context of very low pVL.
Biological outcomes
Over the 48 weeks of Phase 2, the CD4 cell count significantly increased by 54.7 cells/mm3 (95% CI: 21.2–88.2; P = 0.0016) and the CD4/CD8 ratio increased by 0.04 (95% CI: 0.007–0.07; P = 0.018). eGFR was reduced by 15.6 mL/min (95% CI: 12.8–18.5; P < 0.0001) in Phase 1, and was stable over the 48 weeks of follow up in Phase 2 (−0.22 mL/min; 95% CI: −1.8 to 1.4; P = 0.60).
Safety analyses
No HIV-related events occurred. One patient reported a serious adverse event during Phase 1 (grade 3 depression with suicidal ideation related to study treatment leading to dolutegravir interruption) and eight patients reported a serious adverse event during Phase 2: programmed hospitalization in three, viral polyarthritis in one, acute hepatitis C in one, grade 4 increase in creatine kinase in two concomitant to fitness activity, and depression leading to hospitalization in one patient with previous psychiatric disorders. Two out of these nine serious adverse events (two patients with depression) were considered to be related to dolutegravir by the investigator, but study drugs were interrupted in only one patient, during Phase 1.
Discussion
In this single-arm non-comparative trial, switching to dolutegravir+lamivudine in virologically controlled patients was well tolerated and maintained virological suppression at W48. Therapeutic success at W48 was achieved in 101 out of the 104 patients included in Phase 2 (97%; 95% CI: 94%–100%). Among the three patients with therapeutic failure, only one was related to virological failure. This patient had adequate dolutegravir plasma concentrations, but sporadic adherence lapses cannot be excluded. Viral blips were uncommon overall, an encouraging finding since frequent viral blips have been associated with suboptimal virological outcomes.17
The increase in CD4 cell count at W48 during Phase 2 was close to values reported in other switch trials using combinations with dolutegravir13,16 and probably results from the continuous increase observed under HIV treatment. The lack of a comparator arm precludes more precise analysis of these data.
When the LAMIDOL trial was designed, little information regarding dolutegravir+lamivudine18,19 was available. Although the emergence of resistance in cART-naive patients receiving dolutegravir-containing cART had not been reported,4–6 we could not assume that the high genetic barrier of dolutegravir would be sufficient to prevent resistance occurring in the context of dual therapy with detectable pVL at the time of treatment initiation. Since resistance to lamivudine and/or dolutegravir would reduce future therapeutic options, we favoured the evaluation of dolutegravir+lamivudine in patients with pVL <50 copies/mL in order to minimize this risk. In our study, patients with detectable pVL during follow-up (virological failure or blips) had no evidence of reverse transcriptase or integrase resistance mutations, confirming that future treatment options were not compromised. It must be stressed that the high proportion of patients (80%) without baseline INSTI genotype data did not affect the efficacy of the strategy, owing to the rare presence of INSTI resistance mutations in naive patients.20 It is noteworthy that in the ACTG A5353 trial, performed in a smaller number of cART-naive patients, there were three virological failures at W24, including one patient with emerging resistance to lamivudine and dolutegravir.11 Recent dolutegravir monotherapy studies have also shown dolutegravir not to be as robust as a PI in preventing resistance in the event of virological failure.21,22
Our results agree with other studies evaluating dolutegravir+lamivudine as switch therapy.12,13 Compared with these studies, our trial has the largest sample size, the longest follow-up and a high retention rate. In the study by Maggiolo et al.12 patients were followed prospectively but this cohort included only a fraction of the patients who switched therapy. Thus, tolerance might not have been adequately evaluated because patients who interrupted this dual therapy early were probably not included in the cohort. Follow-up visits were also less frequent, which could explain the absence of blips. Finally, although the median follow-up was 17 months, the proportion of patients evaluated at 12 months was not precisely reported. In the randomized pilot trial published by Taiwo et al.,13 although patients were followed until W48, the primary endpoint was evaluated at W24, the number of patients receiving dolutegravir+lamivudine was low (44), and the 12% non-inferiority margin did not allow a firm conclusion of non-inferiority compared with standard cART. Thus, it appears that the ANRS 167 LAMIDOL results bring additional important information about the therapeutic value of dolutegravir+lamvudine. Further virological substudies are ongoing to determine residual plasma viraemia using a single-copy assay and to measure the cellular HIV reservoir at baseline and at W48 of the trial.
In the LAMIDOL trial, in contrast to other studies, cART was modified in two steps: a first step during which the third agent of cART was switched to dolutegravir, and a second step during which patients received dual therapy. Adverse events are usually more frequent when switching to new medications compared with staying on a stable regimen;16,23 thus, it seemed important to be sure that the prescribed drugs were well tolerated before switching to a dual therapy that might have a lower effectiveness than standard cART, and thus be potentially more affected by poor adherence. This design may have overestimated the benefit of dual therapy by excluding patients with early intolerance, but appears appropriate for pragmatic use.
In our study, dolutegravir+lamivudine was generally safe and well tolerated, consistent with the known favourable toxicity profiles of these two agents. Among serious adverse events, only two were related to dolutegravir; both these events were depression, leading to dolutegravir interruption in one patient during Phase 1. Psychiatric symptoms have been reported with dolutegravir and occur usually in the first weeks following initiation of treatment.16 We observed that introduction of dolutegravir resulted in a limited decrease in eGFR, occurring soon after initiation of treatment and stabilizing thereafter. These changes were consistent with dolutegravir action as an inhibitor of the renal tubular organic cation transporter 224 and have been reported previously.12,16
The use of dolutegravir+lamivudine could offer economic benefits. Using a mathematical model, Girouard et al.25 demonstrated that an induction strategy for cART-naive patients with a triple-therapy regimen followed by a two-drug maintenance regimen of dolutegravir+lamivudine in virologically suppressed patients would likely be cost effective in the USA. This regimen might be the cheapest drug combination according to a recent WHO forecast that estimated the annual cost per patient at ∼US$46.26
This study has strengths and limitations. Strengths include the multicentre design, the sequential homogeneous 48 week follow-up with planned visits, the drug concentrations and virological assessments used to elucidate mechanisms of failure, and the large number of patients and high retention rate. Although there was no control arm, the 97% (95% CI: 94%–100%) success rate observed at W48 compared favourably with other triple-agent maintenance strategies.27 Limitations are the open-label non-comparative design, the highly selected population of patients with a high nadir and entry CD4 level, a long duration of prior effective HIV treatment without previous virological failure, and the absence of resistance mutations at baseline. Further studies are needed to explore this strategy in subjects with a shorter duration of viral suppression, a past history of virological failure and/or the presence of M184V resistance mutation in their historical genotype. A previous study suggests that dolutegravir+lamivudine remains effective despite a past history of M184V mutation.19
In summary, in HIV-1 patients with controlled pVL under cART, dolutegravir+lamivudine appears promising. Dolutegravir and lamivudine can be easily co-formulated, providing a single-pill therapeutic option. Further randomized studies with longer follow-up are needed to confirm the value of dolutegravir+lamivudine for lifelong maintenance therapy. The large Phase III TANGO trial comparing dolutegravir+lamivudine with tenofovir alafenamide-based triple therapy in virologically suppressed patients is ongoing.
Acknowledgements
We thank all the participants in the ANRS 167 trial, the patients’ association organizations and the TRT5 group for advice and support, the staff from the centres participating in the Lamidol trial and the members of the Data Safety Monitoring Board (Firouze Bani Sadr, Dominique Costagliola, Sabine Yerly and Jean Claude Alvarez).
Members of the ANRS 167 Study Group
Hôpital Avicenne, Bobigny: BOUCHAUD, Olivier; Hôpital Bicêtre, Le Kremlin Bicêtre: GOUJARD, Cécile; Hôpital Bichat, Paris: JOLY, Véronique, PHUNG, Bao; Hôpital Hotel Dieu, Paris: VIARD, Jean Paul; Hôpital Européen Georges Pompidou, Paris: WEISS, Laurence; Hôpital Necker, Paris: DUVIVIER, Claudine; Hôpital Pitié-Salpêtrière, Paris: KATLAMA, Christine; Hôpital Saint Antoine, Paris: GIRARD, Pierre Marie; Hôpital Saint Louis, Paris: MOLINA, Jean Michel; Hôpital Saint André, Bordeaux: MORLAT, Philippe; Hôpital Gabriel Montpied, Clermont Ferrand: JACOMET, Christine; Hôpital du Bocage, Dijon: PIROTH, Lionel; Hôpital Pierre Zobda-Quitman, Fort de France: CABIÉ, André; Hôpital Saint Marguerite, Marseille: POIZOT-MARTIN, Isabelle; Hôpital Gui de Chauliac, Montpellier: REYNES, Jacques; Hôpital Hotel Dieu, Nantes: ALLAVENA, Clotilde, BILLAUD, Eric, BOUTOUILLE, David, RAFFI, François, RELIQUET, Véronique; Hôpital de l’Archet, Nice: ROENTHAL, Eric; Hôpital de l’Archet, Nice: NAQVI, Alissa; Centre Hospitalier, Perpignan: AUMAITRE, Hughes; Hôpital Pontchaillou, Rennes: SOUALA, Faouzi; Hôpital Bretonneau, Tours: BERNARD, Louis; Hôpital Purpan, Toulouse: BIEZUNSKI, Noemie; Hôpital Gustave Dron, Tourcoing: AJANA, Faiza; Hôpital de la Croix Rousse, Lyon: MIAILHES, Patrick; Institut Médecine Epidémiologie Appliquée, Paris: AMAT, Karine, BENALICHERIF, Aida, SYLLA, Babacar.
Funding
The ANRS 167 Lamidol trial was supported and funded by the Agence Nationale de Recherche sur le Sida et les Hépatites Virales (ANRS), part of the Institut National de la Santé et de la Recherche Médicale (INSERM).
Transparency declarations
V. J. reports having received consultancy fees and/or travel grants from Janssen Pharmaceuticals and ViiV Healthcare. C. C., R. L. and D. D. report having received consultancy fees and/or travel grants from Janssen Pharmaceuticals, ViiV Healthcare, MSD and Gilead Sciences. G. P. received travel grants, consultancy fees, honoraria or study grants from various pharmaceutical companies, including Abbvie, Gilead Sciences, Janssen, Merck and ViiV Healthcare. Y. Y. reports having received consultancy honoraria from Abbvie, Bristol-Myers Squibb, Gilead, MSD, Pfizer, Tibotec and ViiV Healthcare. A. C. reports having received consultancy fees and/or travel grants from Janssen Pharmaceuticals, Gilead Sciences and ViiV Healthcare. All other authors: none to declare.
Author contributions
The Lamidol trial was designed by Véronique Joly, Roland Landman, France Mentre, Diane Descamps, Patrick Yeni and Yazdan Yazdanpanah; the methodology and analysis plan was constructed by France Mentre and Charles Burdet. Christine Katlama, André Cabié and Véronique Joly contributed to recruitment and follow-up of participants, along with all the staff members listed in the ANRS-167 study group. Aida Benalycherif carried out the data monitoring; coordination of assessment of virological and immunological data was conducted by Diane Descamps and Charlotte Charpentier, pharmacological data were collected by Gilles Peytavin. Charles Burdet, Marie Vigan and France Mentre undertook the statistical analysis. Anne-Laure Argoud ensured respect for the law and regulations. Imane Amri was responsible for pharmacovigilance. Véronique Joly, Roland Landman, Diane Descamps, Charles Burdet and Yazdan Yazdanpanah drafted the manuscript, which was critically revised by all the authors, who also approved the final version.
References
Author notes
Members of the ANRS 167 Study Group are listed in the Acknowledgements section.



