-
PDF
- Split View
-
Views
-
Cite
Cite
Rogelio de J Treviño-Rangel, Hiram Villanueva-Lozano, Karen S Méndez-Galomo, Elia M Solís-Villegas, Miguel A Becerril-García, Alexandra M Montoya, Efrén R Robledo-Leal, Gloria M González, In vivo evaluation of the antifungal activity of sertraline against Aspergillus fumigatus, Journal of Antimicrobial Chemotherapy, Volume 74, Issue 3, March 2019, Pages 663–666, https://doi.org/10.1093/jac/dky455
Close - Share Icon Share
Abstract
Invasive pulmonary aspergillosis is a life-threatening fungal disease principally caused by the ubiquitous mould Aspergillus fumigatus. This clinical entity is a major cause of morbidity and mortality (principally, but not restricted to, immunocompromised individuals). A few recent reports suggest in vitro fungicidal activity of sertraline against Aspergillus spp., but this activity has not yet been investigated in vivo.
To evaluate the antifungal activity of sertraline in two in vivo models of aspergillosis.
The antifungal activity of sertraline as monotherapy at three different doses (3, 10 and 15 mg/kg) was evaluated in Galleria mellonella and in a murine model of invasive pulmonary aspergillosis. Therapeutic efficacy parameters determined were larval survival and health index score for G. mellonella, whereas pulmonary fungal burden, galactomannan and lung histopathology were assessed in the murine model.
Sertraline treatments improved larval survival and health index score, especially at doses of 10 and 15 mg/kg. Moreover, 10 mg/kg sertraline was able to reduce pulmonary fungal burden with an efficacy comparable with that of 3 mg/kg amphotericin B and 10 mg/kg voriconazole.
To the best of our knowledge, this is the first in vivo study that evaluates the antifungal activity of sertraline against A. fumigatus, showing a possible promising option for the adjuvant treatment of pulmonary aspergillosis.
Introduction
Invasive pulmonary aspergillosis (IPA) is an opportunistic fungal infection mainly originated by Aspergillus fumigatus through conidia inhalation.1 The major risk factors are neutropenia and corticosteroid therapy,2 associated with mortality rates ranging from 35% to 80%.1,3 The antifungal armamentarium is still limited, expensive and inaccessible in many cases. Drug ‘repurposing’ represents a promising approach to discovering potential antifungal candidates.4
Sertraline is a selective serotonin reuptake inhibitor commonly used as an antidepressant,5 with some evidence of antifungal activity.6 Although there are reports of in vitro fungicidal activity of sertraline against Aspergillus,7–9 this property has not yet been corroborated in vivo, so the aim of this study was to evaluate this activity in two models of aspergillosis.
Materials and methods
Fungal strain
The strain utilized was A. fumigatus CRCEI-16007, which was isolated from a bronchoalveolar lavage from an elderly Mexican patient with pneumonia. Strain identity was confirmed by sequencing of hydrophobin (GenBank accession number: KX165404.1) and β-tubulin (GenBank accession number: KX165391.1) genes.10 Antifungal susceptibility of the isolate was determined according to the CLSI broth microdilution method,11 obtaining the following results: voriconazole MIC = 0.06 mg/L, amphotericin B MIC = 1 mg/L and sertraline MIC = 16 mg/L.
For the inocula preparations used in the in vivo studies the strain was grown on potato dextrose agar (PDA) at 37°C for 7 days. Conidia were harvested with 0.1% Tween 80 in saline and adjusted to the desired concentration.
Drugs
Voriconazole was purchased as Vfend (Pfizer, Mexico), amphotericin B was purchased as Amfotericina B (Pisa, Mexico) and sertraline was obtained as reagent-grade powder (TCI, Tokyo, Japan). Voriconazole and amphotericin B were reconstituted and diluted following the manufacturers’ instructions. Sertraline was dissolved in a 0.9 volume of sterile water and 0.1 volume of sterile 10× PBS stock solution. Drug solutions were freshly prepared before use.
Ethics
All experiments were performed with prior approval of the Ethics and Research Committee of the Faculty of Medicine, Universidad Autónoma de Nuevo León (registration number: MB16-001). Care, maintenance and handling of the animals were in accordance with Mexican regulations for animal experimentation (NOM-062-ZOO-1999) and were performed in a national certified animal facility (SAGARPA-SENASICA AUT-B-B-1216-029).
Galleria mellonella model of aspergillosis
G. mellonella caterpillars in the final instar larval stage of development (Cuautitlán Izcalli, Mexico) were used. Caterpillars (300 ± 100 mg weight) were randomly distributed in 10 larvae per group. Larvae were infected with 10 μL of an inoculum of 2 × 107 conidia/mL A. fumigatus CRCEI-16007 via the last left proleg.12 Two hours post-infection, 10 μL of drug was injected into the opposite proleg. Treatment groups were: voriconazole at 10 mg/kg, amphotericin B at 3 mg/kg and sertraline at 3, 10 and 15 mg/kg. After administration of treatments, larvae were maintained in Petri dishes at 37°C for 7 days. Infected larvae injected with sterile water were used as an untreated control group; untouched and pierced (sham) larvae were additional controls. Larval survival was monitored daily. Additionally, the G. mellonella health index score13 was recorded daily in order to evaluate the progression of the infection. Each experiment was performed at least three times and results are reported as mean values.
Murine model of IPA
A total of 90 BALB/c male mice, weighing 22–25 g (Harlan, Mexico), were used. Animals were housed in sterilized filter-top cages of five mice each under specific pathogen-free conditions. Sterile water and Purina rodent food were provided ad libitum. The murine model of IPA we adopted has been widely described previously.14 In brief, mice were immunocompromised as established by Sheppard et al.14 Animals were exposed for 1 h in an inhalation chamber to aerosolization of 12 mL of an inoculum of 1 × 109 conidia/mL A. fumigatus CRCEI-16007. In order to prevent opportunistic bacterial infections, mice received 5 mg/day ceftazidime (Pisa, Mexico) from days 1 to 6 post-infection. Animals were monitored daily for 7 days and sacrificed when moribund.
Infected mice were randomly divided into six groups of 15 animals per group: untreated controls, voriconazole at 10 mg/kg, amphotericin B at 3 mg/kg and sertraline at 3, 10 and 15 mg/kg. All treatments were intraperitoneally administered, started 24 h after infection once daily, with the exception of amphotericin B, which was given on days 1, 3 and 5 post-challenge. Invariably, all treatments concluded on day 6 after infection. Mice were euthanized on day 7 by cervical dislocation after controlled inhalation of 2% isoflurane (Boise, ID, USA).
The parameters of antifungal efficacy evaluated in this model were fungal burden, galactomannan (GM) antigenaemia and pulmonary histopathology. Tissue fungal burden was determined by quantitative culture on PDA of lung homogenates. GM was assayed in lung homogenates using a sandwich immunocapture ELISA (Platelia Aspergillus EIA; Bio-Rad, Marnes-la-Coquette, France) following the manufacturer’s instructions. GM index was calculated as the OD of each sample divided by the mean cut-off of the control OD; values ≥0.5 were considered positive. Finally, for histopathological evaluations, right lung lobes of all animals were collected, immediately fixed in 10% buffered formaldehyde, processed by microwave (Milestone, Italy) and paraffin embedded. Five micrometre sections were then cut and stained with Grocott’s methenamine silver stain in order to detect fungal elements.
Statistics
G. mellonella larval survival was plotted using the Kaplan–Meier method and differences between treatment groups were analysed by the log rank test (Mantel–Cox). Regarding the murine model, differences in fungal burden and GM index between the different groups were assessed for significance by ANOVA with Tukey’s post hoc test. Calculations and graphics were performed using Prism version 5.03 for Windows (La Jolla, CA, USA). A P value ≤0.05 was considered significant.
Results
Larval survival and health index score
Eighty percent of control larvae died between the third and fourth day post-challenge, accompanied by a progressive decrease in the health index score throughout the study (Figure 1). When treatments were administered, larval survival improved considerably dependent on both the drug and dose injected into the infected larvae, reaching a survival rate of >50% for 3 mg/kg amphotericin B and 10 mg/kg voriconazole (P < 0.0001) and a survival rate of 25% for 10 and 15 mg/kg sertraline (P = 0.0001) by day 5 post-infection. These findings were also reflected in the larval health index score, especially by day 3.
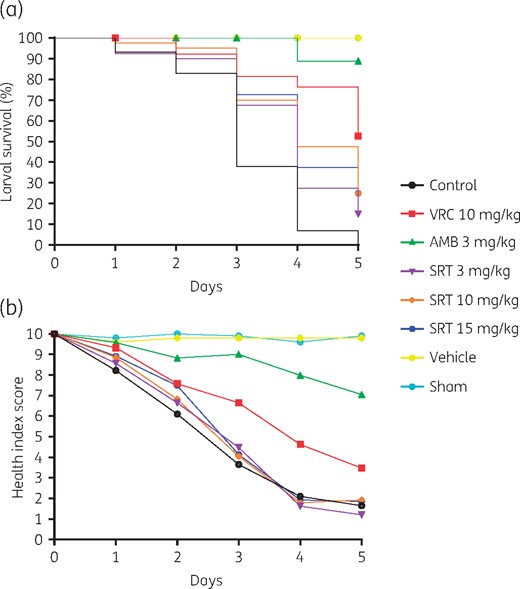
Kaplan–Meier survival curves (a) and health index scores (b) of G. mellonella infected with 2 × 107 conidia/mL A. fumigatus CRCEI-16007. Each experimental group included 10 larvae and the mean values of three independent experiments are shown. AMB, amphotericin B; SRT, sertraline; VRC, voriconazole. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
Fungal burden and GM antigenaemia
Initially, a preliminary pilot study was conducted in order to establish the infection kinetics of A. fumigatus CRCEI-16007 at different timepoints. One hour post-exposure, mice presented a median pulmonary fungal load of 4.74 log10 cfu/g, which decreased to 2.60 log10 cfu/g by day 3 and subsequently rebounded by day 7 post-challenge (data not shown). These findings were the starting point for the antifungal efficacy study. Results of pulmonary fungal burden are depicted in Table 1. There was a significant reduction in the pulmonary fungal burden in infected animals treated with 10 mg/kg voriconazole, 3 mg/kg amphotericin B and 10 mg/kg sertraline, as well as 15 mg/kg sertraline. On the other hand, GM indices were lower for the lung homogenates from animals that received any therapeutic scheme than for the samples of untreated controls (Table 1). This was especially evident in mice treated with 10 mg/kg voriconazole, 3 mg/kg amphotericin B and 15 mg/kg sertraline, along with 10 mg/kg sertraline.
Pulmonary fungal burden and GM index of mice exposed to 1 × 109 conidia/mL A. fumigatus CRCEI-16007 by day 7 post-challenge
| Group . | Fungal burden . | GM . | ||
|---|---|---|---|---|
| log10 cfu/g lung, median (range) . | P . | GM index, median (range) . | P . | |
| Control | 3.15 (2.49–3.63) | — | 2.34 (1.90–2.41) | — |
| Voriconazole at 10 mg/kg | 2.31 (1.91–3.00) | <0.0001 | 1.00 (0.47–1.66) | <0.0001 |
| Amphotericin B at 3 mg/kg | 2.29 (2.01–3.08) | <0.0001 | 0.87 (0.41–1.34) | <0.0001 |
| Sertraline at 3 mg/kg | 3.12 (2.06–3.68) | 0.6430 | 1.63 (1.01–2.35) | 0.0707 |
| Sertraline at 10 mg/kg | 2.27 (2.02–3.09) | <0.0001 | 1.70 (0.53–2.20) | 0.0081 |
| Sertraline at 15 mg/kg | 2.54 (1.98–3.36) | 0.0186 | 1.13 (0.75–2.08) | <0.0001 |
| Group . | Fungal burden . | GM . | ||
|---|---|---|---|---|
| log10 cfu/g lung, median (range) . | P . | GM index, median (range) . | P . | |
| Control | 3.15 (2.49–3.63) | — | 2.34 (1.90–2.41) | — |
| Voriconazole at 10 mg/kg | 2.31 (1.91–3.00) | <0.0001 | 1.00 (0.47–1.66) | <0.0001 |
| Amphotericin B at 3 mg/kg | 2.29 (2.01–3.08) | <0.0001 | 0.87 (0.41–1.34) | <0.0001 |
| Sertraline at 3 mg/kg | 3.12 (2.06–3.68) | 0.6430 | 1.63 (1.01–2.35) | 0.0707 |
| Sertraline at 10 mg/kg | 2.27 (2.02–3.09) | <0.0001 | 1.70 (0.53–2.20) | 0.0081 |
| Sertraline at 15 mg/kg | 2.54 (1.98–3.36) | 0.0186 | 1.13 (0.75–2.08) | <0.0001 |
Pulmonary fungal burden and GM index of mice exposed to 1 × 109 conidia/mL A. fumigatus CRCEI-16007 by day 7 post-challenge
| Group . | Fungal burden . | GM . | ||
|---|---|---|---|---|
| log10 cfu/g lung, median (range) . | P . | GM index, median (range) . | P . | |
| Control | 3.15 (2.49–3.63) | — | 2.34 (1.90–2.41) | — |
| Voriconazole at 10 mg/kg | 2.31 (1.91–3.00) | <0.0001 | 1.00 (0.47–1.66) | <0.0001 |
| Amphotericin B at 3 mg/kg | 2.29 (2.01–3.08) | <0.0001 | 0.87 (0.41–1.34) | <0.0001 |
| Sertraline at 3 mg/kg | 3.12 (2.06–3.68) | 0.6430 | 1.63 (1.01–2.35) | 0.0707 |
| Sertraline at 10 mg/kg | 2.27 (2.02–3.09) | <0.0001 | 1.70 (0.53–2.20) | 0.0081 |
| Sertraline at 15 mg/kg | 2.54 (1.98–3.36) | 0.0186 | 1.13 (0.75–2.08) | <0.0001 |
| Group . | Fungal burden . | GM . | ||
|---|---|---|---|---|
| log10 cfu/g lung, median (range) . | P . | GM index, median (range) . | P . | |
| Control | 3.15 (2.49–3.63) | — | 2.34 (1.90–2.41) | — |
| Voriconazole at 10 mg/kg | 2.31 (1.91–3.00) | <0.0001 | 1.00 (0.47–1.66) | <0.0001 |
| Amphotericin B at 3 mg/kg | 2.29 (2.01–3.08) | <0.0001 | 0.87 (0.41–1.34) | <0.0001 |
| Sertraline at 3 mg/kg | 3.12 (2.06–3.68) | 0.6430 | 1.63 (1.01–2.35) | 0.0707 |
| Sertraline at 10 mg/kg | 2.27 (2.02–3.09) | <0.0001 | 1.70 (0.53–2.20) | 0.0081 |
| Sertraline at 15 mg/kg | 2.54 (1.98–3.36) | 0.0186 | 1.13 (0.75–2.08) | <0.0001 |
Histopathological findings
Examination of tissues from untreated controls showed invasive fungal pneumonia with marked pulmonary lesions, where numerous elongated branching septate hyphae were present. Conversely, lungs of animals treated with 3 mg/kg amphotericin B presented ungerminated conidia exclusively, whereas fungi in tissue from the other experimental treatments exhibited degenerative reductions in hyphal length and swelling compared with what was observed in the pulmonary sections of controls.
Discussion
As both basic and clinical knowledge about IPA is still insufficient, in vivo models of disease have become increasingly necessary. The wax moth larva G. mellonella has been widely used to evaluate the virulence of human fungal pathogens, as well as to evaluate the efficacy of antifungal treatments.15 On the other hand, murine models of IPA have been developed to make the link between in vitro experiments and clinical trials. In this sense, inhalational models like the one performed in this study have been largely promoted for better recapitulating the course of natural exposure to the fungus.16 Pulmonary fungal loads obtained in the pilot study were similar to what other authors reported for the reference strain Af293.14,17 In order to evaluate disease progression, detection of GM and β-D-glucan are probably useful tools to correlate animal data with clinical results. The elevation of GM index we found in the control group and in those treated with amphotericin B was similar to that already reported by other authors.17,18 Nevertheless, GM interpretation is still complicated in rodents, since the positive cut-off values have been validated only in human specimens so far.19
Results of the present study are encouraging and could probably provide the rationale for the adjuvant treatment of IPA with sertraline. Thus, clinical trials are warranted in order to evaluate the potential role of this psychotropic agent for the clinical management of IPA.
Funding
This work was supported by the Instituto Científico Pfizer (Fondo de Investigación 2017) and the Consejo Nacional de Ciencia y Tecnología (CONACyT-INFRA-2015–01-251142).
Transparency declarations
None to declare.
References
Clinical and Laboratory Standards Institute.
Author notes
R. d. J. T.-R. and H. V.-L. contributed equally as first authors.
- lung
- amphotericin b
- antifungal agents
- aspergillosis
- bronchopulmonary aspergillosis
- mycoses
- immunologic adjuvants
- pharmaceutical adjuvants
- aspergillus
- aspergillus fumigatus
- immunocompromised host
- sertraline
- mice
- morbidity
- mortality
- voriconazole
- aspergillosis, invasive pulmonary
- mold
- galactomannan
- histopathology tests



