-
PDF
- Split View
-
Views
-
Cite
Cite
J Islam, D Ashiru-Oredope, E Budd, P Howard, A S Walker, S Hopkins, M J Llewelyn, A national quality incentive scheme to reduce antibiotic overuse in hospitals: evaluation of perceptions and impact, Journal of Antimicrobial Chemotherapy, Volume 73, Issue 6, June 2018, Pages 1708–1713, https://doi.org/10.1093/jac/dky041
Close - Share Icon Share
Abstract
In 2016/2017, a financially linked antibiotic prescribing quality improvement initiative Commissioning for Quality and Innovation (AMR-CQUIN) was introduced across acute hospitals in England. This aimed for >1% reductions in DDDs/1000 admissions of total antibiotics, piperacillin/tazobactam and carbapenems compared with 2013/2014 and improved review of empirical antibiotic prescriptions.
To assess perceptions of staff leading antimicrobial stewardship activity regarding the AMR-CQUIN, the investments made by hospitals to achieve it and how these related to achieving reductions in antibiotic use.
We invited antimicrobial stewardship leads at acute hospitals across England to complete a web-based survey. Antibiotic prescribing data were downloaded from the PHE Antimicrobial Resistance Local Indicators resource.
Responses were received from 116/155 (75%) acute hospitals. Owing to yearly increases in antibiotic use, most trusts needed to make >5% reductions in antibiotic consumption to achieve the AMR-CQUIN goal of 1% reduction. Additional funding was made available at 23/113 (20%) trusts and, in 18 (78%), this was <10% of the AMR-CQUIN value. Nationally, the annual trend for increased antibiotic use reversed in 2016/2017. In 2014/2015, year-on-year changes were +3.7% (IQR −0.8%, +8.4%), +9.4% (+0.2%, +19.5%) and +5.8% (−6.2%, +18.2%) for total antibiotics, piperacillin/tazobactam and carbapenems, respectively, and +0.1% (−5.4%, +4.0%), −4.8% (−16.9%, +3.2%) and −8.0% (−20.2%, +4.0%) in 2016/2017. Hospitals where staff believed they could reduce antibiotic use were more likely to do so (P < 0.001).
Introducing the AMR-CQUIN was associated with a reduction in antibiotic use. For individual hospitals, achieving the AMR-CQUIN was associated with favourable perceptions of staff and not availability of funding.
Introduction
Antimicrobial consumption is linked to antimicrobial resistance (AMR) both in populations and in individual people.1,2 As much as 50% of human antibiotic use may be unnecessary3 and reducing this overuse is a major priority in healthcare systems across the world.4,5
In the UK NHS, hospitals are responsible for a minority of total antibiotic use, but they are almost exclusively where the most broad-spectrum antibiotics, such as piperacillin/tazobactam and the carbapenems, are prescribed.6 Avoiding antibiotic overuse in hospitals is challenging because patients with clinically significant bacterial infections require prompt administration of effective antibiotics, almost always before definitive diagnostic information is available. Initiatives to prevent avoidable deaths from infection encourage rapid administration of reliably active antibiotics to patients who meet broad clinical criteria for sepsis; however, many of these antibiotics are subsequently deemed unnecessary.7 Despite a succession of initiatives in the NHS, such as increased funding for antimicrobial stewardship (AMS) (2003),8 a requirement in law for hospital trusts to ensure appropriate antimicrobial use (2008)9 and development of an Antimicrobial Toolkit for English hospitals called ‘Start Smart—Then Focus’ (SSTF) (2011),10 antibiotic use in NHS hospitals has increased year-on-year up until 2014.11
In 2015, NHS England required Clinical Commissioning Groups to submit their local baseline prescribing data to enable validation of prescribing patterns and antibiotic use.12 The following year saw the introduction of the first Commissioning for Quality and Innovation (CQUIN) for antibiotic prescribing (AMR-CQUIN).13 CQUINs are the main mechanism by which the NHS encourages hospitals to focus on the quality of care delivered by making a proportion of income conditional on achieving specific quality measures. The AMR-CQUIN was worth 0.25% of acute trust income (∼£650 000 for an average-size hospital based on the number of inpatient beds). Given the emphasis in SSTF on review and revision of antibiotic prescriptions as a key activity to control antibiotic use in hospitals, the four AMR-CQUIN components included empirical review of >90% of antibiotic prescriptions within 72 h along with reductions in antibiotic use (DDDs/1000 admissions) of ≥1% compared with baseline (2013/2014 data) for: (i) total antibiotics; (ii) piperacillin/tazobactam; and (iii) carbapenems.13 Hospital trusts were required by NHS England to submit antibiotic consumption data to PHE for the preceding years, 2014/2015 and 2016/2017, and received an additional payment for the submission of this data. All data submitted were published on the ‘Fingertips’ AMR Local Indicators data portal as part of the English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR).14 ‘Fingertips’ provides access to a wide range of local public health data presented as thematic profiles. It acts as an important national repository of data on antimicrobial use, AMR, infection prevention control and hospital-acquired infections.15 Following the introduction of the AMR-CQUIN, AMS leads at individual hospitals anecdotally reported varying success in securing financial investment to support achieving these quality improvements and expressed anxiety about achieving the antibiotic reductions needed to meet the AMR-CQUIN. The aim of this study was to establish how the AMR-CQUIN was perceived by the staff responsible for achieving it at individual hospitals, evaluate to what extent trusts made funding available to achieve it and finally, explore whether these factors had an impact on hospitals achieving reductions in antibiotic consumption.
Methods
A web-based survey (www.surveymonkey.com) was developed, piloted with three hospital AMS leads and refined. The full survey is available as Supplementary data at JAC Online. An email invitation to participate was sent to AMS leads at acute hospital NHS trusts on 8 December 2016. The names and contact details used were compiled by one of the investigators (D. A.-O.) as part of network development within ESPAUR. The people contacted had agreed to represent their organizations in communications with ESPAUR particularly related to the AMR-CQUIN activities. The survey was voluntary; two reminders were sent to recipients who didn’t respond initially. No incentives were offered and there was no advertising of the survey. The survey asked for information about: AMS team structures and activity, the reductions needed to achieve each AMR-CQUIN component, whether funding was in place to achieve this and perceptions about the AMR-CQUIN. The survey closed to respondents on 5 March 2017. When more than one survey was submitted from a hospital, the survey containing the greater number of completed fields was included. Any discordant answers were checked with the submitting hospital before removing the duplicate survey. In reporting the survey we have used the CHERRIES checklist for reporting results of Internet E-Surveys.16 A completed checklist is available as Supplementary data.
Hospital trust-level data on antibiotic consumption were downloaded from ESPAUR using data submitted by acute hospital NHS trusts since 2013 using a standardized spreadsheet provided to organizations. Data extracted from the survey were analysed using SPSS Version 24 (IBM®, UK) and GraphPad Prism™. Categorical variables were compared using Fisher’s exact tests and continuous variables using the Wilcoxon signed-rank test. According to current NHS Health Research Authority (HRA) guidance (available at www.hra.org.uk) ethical approval was not required for this study as this was a service evaluation of NHS staff. All antibiotic consumption data included were openly available.
Results
AMS at hospital trusts included in the survey
Responses were received from a total of 116/155 (75%) acute hospital trusts in England. The majority of surveys were completed by the lead antimicrobial pharmacist [64/116 (55%)], followed by another pharmacist [28/116 (24%)], the hospital lead antimicrobial clinician [22/116 (19%)] and in two cases the infection prevention control nurse [2/116 (2%)].
One hundred and eight out of 116 (93%) respondents reported their hospital had an AMS committee that met quarterly or more often. AMS committees always included a microbiologist and an antimicrobial pharmacist. Additional committee members varied with representation from acute medicine (78/116, 67%), surgery (64/116, 55%), paediatrics (49/116, 42%), intensive care (42/116, 36%), infection prevention directors (47/116, 41%) and clinical commissioners/General Practice representatives (55/116, 47%). Microbiology/Infection trainee doctors were on the AMS committee in only 27/116 (23%) trusts.
NICE guidance on AMS processes and systems had been considered at the AMS committee in 108/116 (93%) trusts and 94/116 (81%) respondents reported completing the NICE AMS baseline audit tool that helps identify areas to improve compliance with the guidance. A total of 72/116 (62%) trusts had formally reviewed SSTF, a further 27/116 (23%) trusts had informally reviewed SSTF and 64/116 (55.2%) had an action plan for its implementation. Most respondents (105/116, 91%) reported that their trust had accessed the AMR Local Indicators data on ‘Fingertips’ and these data have been shared with their AMS committee (82/116, 71%), immediate colleagues (79/116, 68%), trust boards (37/116, 32%) or commissioners (33/116, 28%). Only six respondents (5%) indicated data had been shared with front-line clinical staff.
Achieving the CQUIN measures
Although the AMR-CQUIN aimed to achieve reductions of ≥1% compared with baseline (2013/14), in many trusts antibiotic consumption had continued to rise between 2013/2014 and the introduction of the AMR-CQUIN in April 2016. Consequently, most trusts needed to achieve much larger reductions than ≥1% in 2016/2017 compared with 2015/2016 (Table 1).
| Antibiotic reductions needed to achieve AMR-CQUIN (%) . | Piperacillin/ tazobactam (N = 107), n (%) . | Carbapenems (N = 107), n (%) . | Total antibiotics (N = 108), n (%) . |
|---|---|---|---|
| <1 | 11 (10%) | 23 (21%) | 17 (16%) |
| 1–5 | 14 (13%) | 14 (13%) | 24 (22%) |
| 5–10 | 11 (10%) | 8 (7%) | 19 (18%) |
| 10–20 | 23 (21%) | 15 (14%) | 21 (19%) |
| >20 | 25 (23%) | 24 (22%) | 5 (5%) |
| Not known | 23 (21%) | 23 (21%) | 22 (20%) |
| Antibiotic reductions needed to achieve AMR-CQUIN (%) . | Piperacillin/ tazobactam (N = 107), n (%) . | Carbapenems (N = 107), n (%) . | Total antibiotics (N = 108), n (%) . |
|---|---|---|---|
| <1 | 11 (10%) | 23 (21%) | 17 (16%) |
| 1–5 | 14 (13%) | 14 (13%) | 24 (22%) |
| 5–10 | 11 (10%) | 8 (7%) | 19 (18%) |
| 10–20 | 23 (21%) | 15 (14%) | 21 (19%) |
| >20 | 25 (23%) | 24 (22%) | 5 (5%) |
| Not known | 23 (21%) | 23 (21%) | 22 (20%) |
Of those trusts surveyed, data were available for 107/116 trusts for piperacillin/tazobactam and carbapenems and for 108/116 trusts for total antibiotic consumption.
| Antibiotic reductions needed to achieve AMR-CQUIN (%) . | Piperacillin/ tazobactam (N = 107), n (%) . | Carbapenems (N = 107), n (%) . | Total antibiotics (N = 108), n (%) . |
|---|---|---|---|
| <1 | 11 (10%) | 23 (21%) | 17 (16%) |
| 1–5 | 14 (13%) | 14 (13%) | 24 (22%) |
| 5–10 | 11 (10%) | 8 (7%) | 19 (18%) |
| 10–20 | 23 (21%) | 15 (14%) | 21 (19%) |
| >20 | 25 (23%) | 24 (22%) | 5 (5%) |
| Not known | 23 (21%) | 23 (21%) | 22 (20%) |
| Antibiotic reductions needed to achieve AMR-CQUIN (%) . | Piperacillin/ tazobactam (N = 107), n (%) . | Carbapenems (N = 107), n (%) . | Total antibiotics (N = 108), n (%) . |
|---|---|---|---|
| <1 | 11 (10%) | 23 (21%) | 17 (16%) |
| 1–5 | 14 (13%) | 14 (13%) | 24 (22%) |
| 5–10 | 11 (10%) | 8 (7%) | 19 (18%) |
| 10–20 | 23 (21%) | 15 (14%) | 21 (19%) |
| >20 | 25 (23%) | 24 (22%) | 5 (5%) |
| Not known | 23 (21%) | 23 (21%) | 22 (20%) |
Of those trusts surveyed, data were available for 107/116 trusts for piperacillin/tazobactam and carbapenems and for 108/116 trusts for total antibiotic consumption.
Changes in antibiotic use after introduction of the AMR-CQUIN
Data gathered by ESPAUR from 130 acute hospital NHS trusts reporting annual data from 2013 onwards showed that increases in antibiotic use began to reverse in the 2015/2016 financial year, when trusts were first obliged to report usage data to ESPAUR (Figure 1). In that year, small, but statistically significant, year-on-year reductions were measured for total antibiotic use (P < 0.0001), piperacillin/tazobactam use (P < 0.0001) and carbapenem use (P = 0.05). In the AMR-CQUIN year (ending March 2017) there was no evidence that total antibiotic consumption changed compared with the previous year +0.1% (−5.4%, +4.0%; P = 0.05), but there were substantial and statistically significant reductions in piperacillin/tazobactam use of −4.8% (−16.9%, +3.2%; P < 0.0001) and carbapenem use of −8.0% (−20.2%, +4.0%; P < 0.001). However, there was striking variation between trusts. Changes in antibiotic consumption in 2016/2017 compared with a baseline of 2013/2014 ranged from −43% to +71% for total antibiotic use, −17% to +72% for piperacillin/tazobactam use and −79% to +44% for carbapenem use.
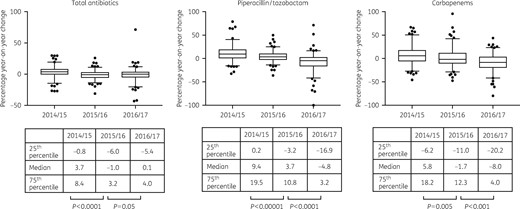
Year-on-year changes in antibiotic use at acute hospitals in England. Boxes show medians and quartiles, and whiskers show 5 and 95 percentiles. P values: Wilcoxon signed-rank test, two-tailed.
Of the surveyed trusts participating in the AMR-CQUIN, 41/111 (37%) achieved the quality measure for piperacillin/tazobactam, 61/111 (55%) for carbapenems and 48/111 (43%) for total antibiotic use (Table 2) based on information reported to PHE. The median reduction in antibiotic consumption compared with the 2013/2014 baseline achieved in those trusts surveyed was −0.2% (−11.9%, +10.1%) for total antibiotic use, +2.2% (−18.0%, +18.3%) for piperacillin/tazobactam and −7.8% (−29.4%, +12.1%) for carbapenems. The attitudes of AMS leaders to the AMR-CQUIN or availability of additional trust funding were not associated with achieving the CQUIN goals (P > 0.3; Table 2). Substantially more trusts achieved the AMR-CQUIN when the survey respondents were optimistic about meeting the CQUIN goals (P < 0.0001; Table 2).
| . | Piperacillin/tazobactam (N = 109) . | Carbapenems (N = 109) . | Total antibiotics (N = 108) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| achieved (N = 41), n (%) . | not achieved (N = 68), n (%) . | P . | achieved (N = 61), n (%) . | not achieved (N = 48), n (%) . | P . | achieved (N = 48), n (%) . | not achieved (N = 60), n (%) . | P . | |
| Funding availablea (n = 23) | 10 (24%) | 13 (19%) | 0.63 | 16 (26%) | 7 (15%) | 0.32 | 11 (23%) | 12 (20%) | 0.60 |
| Funding not available (n = 90) | 30 (73%) | 53 (78%) | 43 (70%) | 40 (83%) | 36 (75%) | 46 (77%) | |||
| CQUIN will help reduce antibiotic consumptionb (n = 35) | 16 (39%) | 16 (24%) | 0.34 | 18 (30%) | 15 (31%) | 0.94 | 14 (29%) | 18 (30%) | 0.96 |
| CQUIN will not help reduce antibiotic consumption (n = 29) | 11 (27%) | 16 (24%) | 15 (25%) | 12 (25%) | 10 (21%) | 17 (28%) | |||
| CQUIN will change AMSc | 21 (51%) | 32 (47%) | 0.84 | 22 (36%) | 30 (63%) | 0.42 | 30 (63%) | 23 (38%) | 0.53 |
| CQUIN will not change AMSc | 10 (24%) | 17 (25%) | 15 (25%) | 12 (25%) | 12 (25%) | 15 (25%) | |||
| CQUIN will safely reduce antibiotic consumptiond | 6 (15%) | 14 (21%) | 0.87 | 7 (11%) | 13 (27%) | 0.87 | 8 (17%) | 12 (20%) | 0.99 |
| CQUIN will not safely reduce antibiotic consumptiond | 11 (27%) | 13 (19%) | 10 (16%) | 14 (29%) | 10 (21%) | 14 (23%) | |||
| Trust predicted that they would achieve CQUINe | 26 (63%) | 2 (3%) | <0.0001* | 40 (66%) | 1 (2%) | <0.0001* | 31 (65%) | 1 (2%) | <0.0001* |
| Trust predicted that they would not achieve CQUINe | 6 (15%) | 53 (78%) | 4 (6%) | 42 (88%) | 5 (10%) | 48 (80%) | |||
| . | Piperacillin/tazobactam (N = 109) . | Carbapenems (N = 109) . | Total antibiotics (N = 108) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| achieved (N = 41), n (%) . | not achieved (N = 68), n (%) . | P . | achieved (N = 61), n (%) . | not achieved (N = 48), n (%) . | P . | achieved (N = 48), n (%) . | not achieved (N = 60), n (%) . | P . | |
| Funding availablea (n = 23) | 10 (24%) | 13 (19%) | 0.63 | 16 (26%) | 7 (15%) | 0.32 | 11 (23%) | 12 (20%) | 0.60 |
| Funding not available (n = 90) | 30 (73%) | 53 (78%) | 43 (70%) | 40 (83%) | 36 (75%) | 46 (77%) | |||
| CQUIN will help reduce antibiotic consumptionb (n = 35) | 16 (39%) | 16 (24%) | 0.34 | 18 (30%) | 15 (31%) | 0.94 | 14 (29%) | 18 (30%) | 0.96 |
| CQUIN will not help reduce antibiotic consumption (n = 29) | 11 (27%) | 16 (24%) | 15 (25%) | 12 (25%) | 10 (21%) | 17 (28%) | |||
| CQUIN will change AMSc | 21 (51%) | 32 (47%) | 0.84 | 22 (36%) | 30 (63%) | 0.42 | 30 (63%) | 23 (38%) | 0.53 |
| CQUIN will not change AMSc | 10 (24%) | 17 (25%) | 15 (25%) | 12 (25%) | 12 (25%) | 15 (25%) | |||
| CQUIN will safely reduce antibiotic consumptiond | 6 (15%) | 14 (21%) | 0.87 | 7 (11%) | 13 (27%) | 0.87 | 8 (17%) | 12 (20%) | 0.99 |
| CQUIN will not safely reduce antibiotic consumptiond | 11 (27%) | 13 (19%) | 10 (16%) | 14 (29%) | 10 (21%) | 14 (23%) | |||
| Trust predicted that they would achieve CQUINe | 26 (63%) | 2 (3%) | <0.0001* | 40 (66%) | 1 (2%) | <0.0001* | 31 (65%) | 1 (2%) | <0.0001* |
| Trust predicted that they would not achieve CQUINe | 6 (15%) | 53 (78%) | 4 (6%) | 42 (88%) | 5 (10%) | 48 (80%) | |||
Three respondents did not answer this question.
Thirty-nine respondents were unsure whether the CQUIN would reduce antibiotic consumption and 13 did not answer this question.
Nineteen respondents were unsure whether the CQUIN had changed AMS and 11 did not answer this question.
Fifty-seven respondents were unsure whether the CQUIN would safely reduce antibiotic consumption and 11 did not answer this question.
In total, 26, 28 and 29 respondents did not answer this question for piperacillin/tazobactam, carbapenems and total antibiotics, respectively.
| . | Piperacillin/tazobactam (N = 109) . | Carbapenems (N = 109) . | Total antibiotics (N = 108) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| achieved (N = 41), n (%) . | not achieved (N = 68), n (%) . | P . | achieved (N = 61), n (%) . | not achieved (N = 48), n (%) . | P . | achieved (N = 48), n (%) . | not achieved (N = 60), n (%) . | P . | |
| Funding availablea (n = 23) | 10 (24%) | 13 (19%) | 0.63 | 16 (26%) | 7 (15%) | 0.32 | 11 (23%) | 12 (20%) | 0.60 |
| Funding not available (n = 90) | 30 (73%) | 53 (78%) | 43 (70%) | 40 (83%) | 36 (75%) | 46 (77%) | |||
| CQUIN will help reduce antibiotic consumptionb (n = 35) | 16 (39%) | 16 (24%) | 0.34 | 18 (30%) | 15 (31%) | 0.94 | 14 (29%) | 18 (30%) | 0.96 |
| CQUIN will not help reduce antibiotic consumption (n = 29) | 11 (27%) | 16 (24%) | 15 (25%) | 12 (25%) | 10 (21%) | 17 (28%) | |||
| CQUIN will change AMSc | 21 (51%) | 32 (47%) | 0.84 | 22 (36%) | 30 (63%) | 0.42 | 30 (63%) | 23 (38%) | 0.53 |
| CQUIN will not change AMSc | 10 (24%) | 17 (25%) | 15 (25%) | 12 (25%) | 12 (25%) | 15 (25%) | |||
| CQUIN will safely reduce antibiotic consumptiond | 6 (15%) | 14 (21%) | 0.87 | 7 (11%) | 13 (27%) | 0.87 | 8 (17%) | 12 (20%) | 0.99 |
| CQUIN will not safely reduce antibiotic consumptiond | 11 (27%) | 13 (19%) | 10 (16%) | 14 (29%) | 10 (21%) | 14 (23%) | |||
| Trust predicted that they would achieve CQUINe | 26 (63%) | 2 (3%) | <0.0001* | 40 (66%) | 1 (2%) | <0.0001* | 31 (65%) | 1 (2%) | <0.0001* |
| Trust predicted that they would not achieve CQUINe | 6 (15%) | 53 (78%) | 4 (6%) | 42 (88%) | 5 (10%) | 48 (80%) | |||
| . | Piperacillin/tazobactam (N = 109) . | Carbapenems (N = 109) . | Total antibiotics (N = 108) . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| achieved (N = 41), n (%) . | not achieved (N = 68), n (%) . | P . | achieved (N = 61), n (%) . | not achieved (N = 48), n (%) . | P . | achieved (N = 48), n (%) . | not achieved (N = 60), n (%) . | P . | |
| Funding availablea (n = 23) | 10 (24%) | 13 (19%) | 0.63 | 16 (26%) | 7 (15%) | 0.32 | 11 (23%) | 12 (20%) | 0.60 |
| Funding not available (n = 90) | 30 (73%) | 53 (78%) | 43 (70%) | 40 (83%) | 36 (75%) | 46 (77%) | |||
| CQUIN will help reduce antibiotic consumptionb (n = 35) | 16 (39%) | 16 (24%) | 0.34 | 18 (30%) | 15 (31%) | 0.94 | 14 (29%) | 18 (30%) | 0.96 |
| CQUIN will not help reduce antibiotic consumption (n = 29) | 11 (27%) | 16 (24%) | 15 (25%) | 12 (25%) | 10 (21%) | 17 (28%) | |||
| CQUIN will change AMSc | 21 (51%) | 32 (47%) | 0.84 | 22 (36%) | 30 (63%) | 0.42 | 30 (63%) | 23 (38%) | 0.53 |
| CQUIN will not change AMSc | 10 (24%) | 17 (25%) | 15 (25%) | 12 (25%) | 12 (25%) | 15 (25%) | |||
| CQUIN will safely reduce antibiotic consumptiond | 6 (15%) | 14 (21%) | 0.87 | 7 (11%) | 13 (27%) | 0.87 | 8 (17%) | 12 (20%) | 0.99 |
| CQUIN will not safely reduce antibiotic consumptiond | 11 (27%) | 13 (19%) | 10 (16%) | 14 (29%) | 10 (21%) | 14 (23%) | |||
| Trust predicted that they would achieve CQUINe | 26 (63%) | 2 (3%) | <0.0001* | 40 (66%) | 1 (2%) | <0.0001* | 31 (65%) | 1 (2%) | <0.0001* |
| Trust predicted that they would not achieve CQUINe | 6 (15%) | 53 (78%) | 4 (6%) | 42 (88%) | 5 (10%) | 48 (80%) | |||
Three respondents did not answer this question.
Thirty-nine respondents were unsure whether the CQUIN would reduce antibiotic consumption and 13 did not answer this question.
Nineteen respondents were unsure whether the CQUIN had changed AMS and 11 did not answer this question.
Fifty-seven respondents were unsure whether the CQUIN would safely reduce antibiotic consumption and 11 did not answer this question.
In total, 26, 28 and 29 respondents did not answer this question for piperacillin/tazobactam, carbapenems and total antibiotics, respectively.
Funding towards achieving the CQUIN goals
Five of the trusts surveyed reported a decision had been taken not to participate in the AMR-CQUIN. A total of 68/116 (59%) trusts set out to meet the nationally set CQUIN reductions. Within the remaining trusts, 43/116 had negotiated local variations in some (18/116, 16%) or all (25/116, 22%) of the components. Funding had been made available to support achieving the AMR-CQUIN at only 23/113 (20%) participating trusts. Even when funding was made available in 18/23 (78%) trusts, the funding amount was <10% of the overall AMR-CQUIN value.
Perceptions and achieving the CQUIN goals
At the time the survey was conducted, the AMR-CQUIN had been in place for ∼6 months. Respondents were pessimistic about achieving the targets and only a minority felt their trust would achieve the necessary reductions for piperacillin/tazobactam (31/116, 27%), carbapenems (42/116, 36%) and total antibiotic use (34/116, 29%). Exactly half of the respondents (58/116, 50%) agreed with the statement that the AMR-CQUIN had changed AMS activity in their trust and 35/116 (30%) felt the AMR-CQUIN would reduce antibiotic consumption. However, only 22/116 (19%) felt that it would do so safely. Accordingly, 82/116 (71%) respondents were interested in the possibility of participating in research to evaluate how to safely optimize antibiotic ‘review and revise’.
Discussion
Reducing unnecessary antibiotic use among hospitalized patients is challenging because the need to ensure prompt effective empirical antibiotic treatment for patients with suspected life-threatening infection, coupled with fear of antibiotic resistance, drives increased use of the ‘ultra-broad-spectrum’ antibiotics that include piperacillin/tazobactam and carbapenems, which have been linked to AMR. In the UK, NHS SSTF attempts to address this challenge by asking prescribers to regularly review and revise empirical antibiotic prescriptions. Similar approaches are applied in other European countries17 and in the USA.18 Prescribers find ‘review and revise’ challenging.19 Without robust measures to support it ‘review and revise’ may not be effective in balancing drivers to increase use of broad-spectrum antibiotics in hospitalized patients.20 It is encouraging that this survey has demonstrated not only a high level of awareness of NICE guidance and SSTF, but also that over half the trusts now have an action plan for SSTF (55.3%) compared with 46% in 2014.21
Our findings that most hospitals have multidisciplinary AMS teams, meeting regularly, who had considered the relevant NICE guidance and assessed their activity against it, all illustrate how well AMS is embedded in NHS hospitals, as it is elsewhere in high-income settings where regulatory measures are in force.22 We did not gather detailed data on adequate staffing or specific action plans, which have been highlighted as key gaps in the strategic needs of AMS programmes.23 We focused on the impact of the specific AMR-CQUIN intervention, but our finding that so little new funding was made available, even at trusts that achieved their targets, suggests increased staffing was not a key factor.
A starting point for our study was concern that pharmacists and microbiologists responsible for AMS at individual hospitals doubted both the feasibility and safety of achieving the reductions required to achieve the AMR-CQUIN goals. By surveying the staff responsible for implementing AMS at acute trusts in England, we have determined that many staff did indeed have significant concerns about whether the AMR-CQUIN could be safely implemented. Although we did not explore the basis for concern, it is likely that prescribers are worried that efforts to reduce unnecessary antibiotic use may increase the risk of under-treatment of patients who need antibiotics. This is unsurprising given how few studies assessing antimicrobial reduction strategies in hospitalized patients have included meaningful clinical outcome data.24
Many hospitals were confronted with the need to make much greater reductions in antibiotic use in 2016/2017 to achieve a >1% reduction in antibiotic use, because of year-on-year increases in antibiotic use from a baseline (2013/2014) set two years prior to the introduction of the AMR-CQUIN. Consequently, several trusts either elected not to try and achieve the goals as set out or, when they did, they were unwilling to allocate even a small amount of the money they would lose for not achieving them, to improve stewardship activity. Nevertheless, while neither concern about the safety of the AMR-CQUIN nor lack of financial investment to achieve it appear to have impacted on the AMR-CQUIN being achieved, hospitals where staff were positive about its success were much more likely to achieve the reductions in antibiotic use required.
Our finding that only around half the hospitals surveyed achieved the antibiotic reduction targets is in striking contrast with the fact that ESPAUR data show that almost all trusts achieved the AMR-CQUIN target of 90% antibiotic prescriptions being reviewed.14 However, the explanation for this is likely to be that review more commonly results in continuing or changing treatment than discontinuation. ESPAUR data also report that only a small minority of antibiotic prescriptions are stopped at ‘review and revise’ (nationally an average of 7.8% during the last quarter of the financial year 2016–17).14 This is in keeping with how hard prescribers find it to stop antibiotic prescriptions that have already been written.19 Despite this, our data confirm that antimicrobial use in English hospitals started to decline in 2015/16, the year that commissioners were first required to report antibiotic prescribing data from acute hospitals. This may be a ‘Hawthorne effect’, explained by increased awareness and modification of prescribing habits by trust staff owing to an increase in data collection in the year prior to the introduction of the quality improvement itself. While we found no overall further reduction in antibiotic use in the year the AMR-CQUIN was introduced, the marked reductions seen in piperacillin/tazobactam and carbapenem use suggests a move away from these two ‘ultra-broad-spectrum’ agents to alternatives, potentially increasing total antibiotic DDDs by the use of more than one antibiotic.
Our study has limitations. Although the survey highlighted ongoing concern that the AMR-CQUIN may not be able to safely reduce antibiotic consumption we did not collect detailed information about the reasons for concerns. The survey was carried out 6 months after the AMR-CQUIN had started, which may have influenced respondents’ perceptions about whether they would achieve the CQUIN goals. We sought a single response from each hospital and did not quality control the responses, but respondents attested their leadership role in AMS at their trust and were asked to complete the survey in discussion with colleagues so we believe responses are likely to be reliable. Finally, we were not able to extract detailed information about the specific actions taken following introduction of the AMR-CQUIN, which may have allowed individual trusts to achieve reductions, or whether the total reductions resulted from shorter treatment durations or fewer patients being treated and whether overall appropriateness of treatment improved. There is a need for future work to understand these mechanisms better to support antibiotic optimization more widely.
Despite these limitations, our findings indicate that the AMR-CQUIN approach of setting goals backed up by robust data gathering and reporting has helped hospitals achieve reductions in antibiotic overuse. Positive staff attitudes rather than availability of new funding are likely to have been important at hospitals that achieved the reduction goals. Further efforts to improve ‘review and revise’ as a key element of hospital AMS practice will need both evidence and novel tools to support clinical decision and reassure staff that patient safety is not compromised when stopping unnecessary antibiotics.
Acknowledgements
We would like to thank Kieran Hand, Paul Wade and Stuart Brown for their feedback on the pilot survey.
Funding
This paper presents independent research funded by the National Institute for Health Research (NIHR) under its Programme Grants for Applied Research Programme (Reference Number RP-PG-0514-20015). A. S. W. and S. H. are supported by the NIHR Health Protection Research Unit (HPRU) in Healthcare Associated Infections and Antimicrobial Resistance at Oxford University in partnership with Public Health England (PHE) (grant HPRU-2012-10041). D. A.-O. and S. H. are affiliated with the NIHR HPRU in Healthcare Associated Infections and Antimicrobial Resistance at Imperial College London.
Transparency declarations
None to declare.
Disclaimer
The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research, the Department of Health or PHE.
Supplementary data
The full survey and completed checklist are available as Supplementary data at JAC Online.
References
G7
National Action Plan for Combating Antibiotic-Resistant Bacteria.
The Health and Social Care Act 2008: Code of Practice on the Prevention and Control of Infections and Related Guidance. https://www.gov.uk/government/publications/the-health-and-social-care-act-2008-code-of-practice-on-the-prevention-and-control-of-infections-and-related-guidance.
Quality Premium: 2015/16 Guidance for CCGs. https://www.england.nhs.uk/wp-content/uploads/2013/12/qual-prem-guid.pdf.



