-
PDF
- Split View
-
Views
-
Cite
Cite
Miaomiao Xie, Ruichao Li, Zhonghua Liu, Edward Wai Chi Chan, Sheng Chen, Recombination of plasmids in a carbapenem-resistant NDM-5-producing clinical Escherichia coli isolate, Journal of Antimicrobial Chemotherapy, Volume 73, Issue 5, May 2018, Pages 1230–1234, https://doi.org/10.1093/jac/dkx540
Close - Share Icon Share
Abstract
To investigate the genetic features of five plasmids recovered from an NDM-5-producing clinical Escherichia coli strain, BJ114, and to characterize the plasmid recombination event that occurred during the conjugation process.
The genetic profiles of the five plasmids were determined by PCR, conjugation, S1-PFGE, Southern hybridization and WGS analysis. Plasmid sequences were analysed with various bioinformatic tools.
Complete sequences of five plasmids were obtained. Two small plasmids, pBJ114-141 and pBJ114-46, were speculated to have recombined into a large fusion plasmid, pBJ114T-190. When conjugated to other E. coli strains, some of the fusion plasmids were able to be resolved into the original two single plasmids. A non-conjugative plasmid, pBJ114-96, exhibited a high degree of sequence identity with the phage P7-like plasmid as well as an mcr-1-bearing plasmid. Another plasmid, pBJ114-78, was found to contain multidrug resistance genes and various mobile elements.
The fusion plasmid recoverable from the transconjugant was found to be generated as a result of a recombination event that occurred upon interaction between a blaNDM-5-carrying plasmid and another plasmid present in the parental strain. Such recombination events presumably play a potential role in the dissemination of the blaNDM genes among different plasmids and pathogenic bacterial strains.
Introduction
The continuous emergence of novel carbapenem-resistant Enterobacteriaceae (CRE) strains in recent years has posed an increasing public health threat worldwide.1 Dissemination of mobile resistance elements, especially those carrying the New Delhi MBL (NDM) gene, has been regarded as a major mechanism responsible for causing a dramatic increase in the prevalence of CRE in hospital infections.2 Recently, we reported the widespread dissemination of carbapenem-resistant Escherichia coli in clinical settings.3 We showed that, in addition to the blaNDM genes, multiple β-lactamase genes and other important antimicrobial resistance genes such as fosA3 were often detectable in the same CRE strain. More interestingly, some of the CRE strains were found to carry blaNDM-bearing plasmids that were much smaller in size than the conjugative blaNDM-bearing plasmids detected in the corresponding transconjugants collected in the conjugation experiments, suggesting that plasmids harboured by CRE strains exhibit genetic plasticity. To investigate the genetic basis of such possible plasmid recombination events we selected one representative strain, carbapenem-resistant E. coli BJ114, to determine its complete plasmid profile through WGS, followed by comparative genetic analysis with the plasmid harboured by the transconjugant obtained from conjugation experiments. Our findings confirm that plasmid recombination events significantly enhance the accumulation of various resistance genes among bacterial pathogens.
Materials and methods
Identification of the E. coli strain carrying blaNDM
Clinical strain BJ114, whose carbapenem resistance phenotype was determined by the Kirby–Bauer disc diffusion method according to CLSI guidelines,4 was obtained from a hospital in Beijing, China in 2013 and identified as E.coli by MALDI-TOF MS. The strain was isolated from the urine sample of a paediatric patient suffering from a urinary tract infection.
Antimicrobial susceptibility testing against different antibiotics was conducted for this strain using the agar dilution method, and the results were interpreted according to CLSI guidelines.4,E. coli strain ATCC 25922 and Staphylococcus aureus ATCC 29213 were used as quality controls. The strain BJ114 was subjected to ST typing and screening of β-lactamase genes as described previously.5
Conjugation, S1-PFGE and Southern hybridization
Conjugation experiments were performed as previously described to test the transferability of the blaNDM-bearing plasmid,6 using a rifampicin-resistant E. coli EC600 (LacZ−, Nalr and Rifr) strain as recipient. Briefly, overnight culture of BJ114 and recipient strain E. coli EC600 were mixed (ratio of 1:1) in LB broth, which was subjected to overnight incubation on an LB agar plate. The mixture was then spread on a selective MacConkey agar plate containing meropenem (0.5 mg/L) and rifampicin (600 mg/L) to select transconjugants that had acquired the blaNDM-bearing plasmid. Carriage of such a plasmid in the parental strain and corresponding transconjugants was confirmed by PCR, S1-PFGE and Southern hybridization.
Plasmid sequencing and analysis
To obtain a comprehensive understanding of the genetic basis of the antibiotic resistance phenotypes bestowed by the plasmids in BJ114 and the corresponding transconjugant BJ114-EC600, the complete sequences of plasmids that they harboured were determined by whole-plasmid sequencing using the Illumina Nextseq 500 and single molecule real-time (SMRT) sequencing PacBio platforms. Paired-end Illumina reads (2 × 150 bp) and PacBio long reads were assembled with SPAdes 3.5.5 The complete sequences of the plasmids were annotated by the RAST tool,7 edited manually and submitted to the National Center for Biotechnology Information (NCBI) database with the following accession numbers: pBJ114-46 (MF679143), pBJ114-78 (MF679144), pBJ114-96 (MF679145), pBJ114-141 (MF679146) and pBJ114T-190 (MF679147). Easyfig8 and BRIG9 were used in comparative analysis and generation of plasmid maps.
Results and discussion
The E. coli strain BJ114 was found to be ST167 and highly resistant to a wide range of antibiotics, including imipenem, fosfomycin, ciprofloxacin and the cephalosporins, but remained susceptible to amikacin and polymyxin B (Table S1, available as Supplementary data at JAC Online). The carbapenem and cephalosporin resistance phenotypes of this strain could be transferred through conjugation. Both blaNDM-5 and blaCTX-M-65 were detectable in the parental strain and its transconjugant. S1-PFGE indicated that there were four plasmids of different sizes (∼141 kb, ∼96 kb, ∼78 kb and ∼46 kb) in BJ114, yet only one plasmid of ∼190 kb was detectable in the transconjugant BJ114-EC600 (Figure S1). The plasmid was named by combining strain name and plasmid size with kb as the unit. The fact that the plasmid found in the transconjugant BJ114-EC600 was larger than any of the four plasmids in the parental strain prompted us to investigate its structure and genetic content.3 Southern hybridization with the blaNDM probe indicated that the blaNDM-5 gene could be found in the smallest plasmid, with a size of ∼46 kb, in strain BJ114, and the ∼190 kb plasmid in the transconjugant BJ114-EC600 (Figure S1). To investigate how the blaNDM-5 gene could be transferred from a small plasmid to a larger one during the conjugation process, the complete sequences of five plasmids (pBJ114T-190, pBJ114-141, and pBJ114-46, together with another two plasmids pBJ114-96 and pBJ114-78 in the original strain) were obtained and subjected to further analysis.
The blaNDM-5-bearing plasmid pBJ114T-190 in the transconjugant BJ114-EC600 was found to be 190 564 bp in length, to exhibit a GC content of 50.7% and to comprise 253 predicted coding sequences. BLASTN results showed that it was 99% identical to the plasmid pETN48 (FQ482074, previously recovered from an E. coli strain) at 52% coverage (Figure S2). It was found to comprise the IncFIB and IncX3 replicons, the blaNDM-5, blaCTX-M-65, fosA, qnrS1, tet(A) and mph(A) genes, and some other resistance gene cassettes bounded by various insertion sequences. The largest plasmid harboured by the parental strain BJ114, pBJ114-141, was 141 555 bp in length with a GC content of 42.0%. BLASTN analysis showed that pBJ114-141 exhibited 99% identity at 70% coverage with the plasmid pETN48 of E. coli origin (NC_014615) in GenBank that carried a blaCTX-M-14 gene. pBJ114-141 was found to comprise the IncFIB replicon, the blaCTX-M-65, fosA and qnrS1 genes, and other resistance genes such as tet(A) and mph(A). The blaNDM-5-bearing plasmid pBJ114-46, harboured by the parental strain, was found to be 46 161 bp in length, to exhibit a GC content of 46.7% and to contain an IncX3 replicon and the blaNDM-5 gene. It should also be noted that plasmid pBJ114-46 was almost identical (99% identity with 100% coverage) to other conjugative IncX3 plasmids such as pNDM-QD28(KU167608.1) and pKW53T-NDM (KX214669.1), both harbouring the blaNDM-1 gene.3 Sequence analysis showed that these two plasmids, pBJ114-141 and pBJ114-46, which existed in the parental strain as single plasmids, formed a fusion plasmid, pBJ114T-190, upon being conjugated into the E. coli strain EC600 (Figures 1 and 2a). Detailed sequence analysis of these three plasmids enabled us to predict the possible mechanism of plasmid fusion. First, the ISKpn19 elements located in plasmid pBJ114-141 presumably attacked the hot spot (CCATTATCTA) of pBJ114-46 located between IS3000 and ISAba125 through intermolecular replicative transposition and resulted in ISKpn19 duplication (Figure 2b).10 These events resulted in the appearance of a 10 bp target site duplication (TSD) region, CCATTATCTA, in the fusion plasmid. To test if the fusion plasmid can be transferred to other host strains, a conjugation assay was performed with BJ114T-EC600 as the donor strain and azide-resistant E. coli J53 as the recipient strain. One hundred J53 transconjugants were randomly selected to screen for the presence of pBJ114T-190, pBJ114-141 and pBJ114-46 plasmids using specific primers (Table S2) with results showing that 98 transconjugants carried pBJ114-46, with 30 of them also carrying pBJ114-141 without integration status, whereas only two transconjugants carried pBJ114T-190. The data suggested that the cointegrate plasmid was not stable in E. coli J53 strain or not stable during the conjugation process. Further studies will be performed to investigate this conjugation event. Nevertheless, the fusion plasmid, pBJ114T-190, exhibits a significantly broadened resistance profile, covering several additional classes of antibiotics, including fosfomycin, another alternative therapeutic agent for treatment of infections caused by CRE. Apart from the two plasmids with the ability to cointegrate, another two plasmids pBJ114-96, a P1 phage-like plasmid, and pBJ114-78 carrying blaCTX-M-14, blaCMY-42 and other resistance genes, were also obtained. The detailed analysis is presented in Figures S3 to S5.
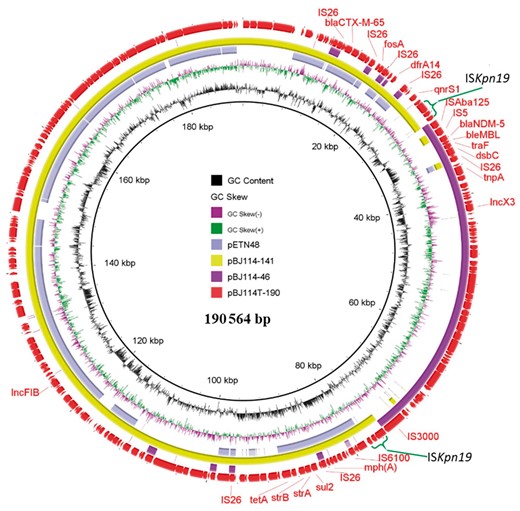
Circular comparison of four plasmids depicting the structural changes caused by plasmid recombination. The outmost red circle denotes the hybrid plasmid pBJ114T-190; the purple circle represents pBJ114-46; the light green and lilac circles, respectively, represent pBJ114-141 and a similar plasmid, pETN48, in the NCBI database. The two green braces around the fused region denote the duplicated region, which forms as a result of plasmid recombination. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
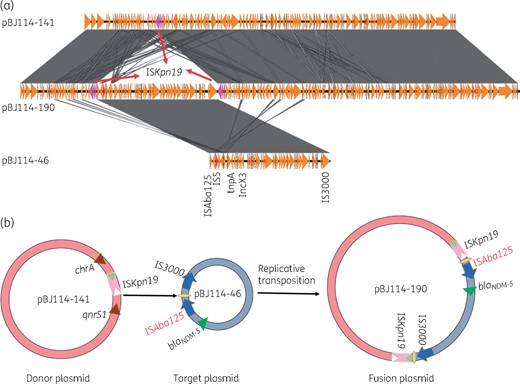
Mechanisms of plasmid fusion. (a) Structure alignment of three plasmids. Duplicated ISKpn19 elements are highlighted in pink. (b) Mechanisms of plasmid fusion. ISKpn19 in the donor plasmid initiates a replicative transposition event at the hot spot (CCATTATCTA) in pBJ114-46 located between IS3000 and ISAba125. The plasmid pBJ114-190 is the cointegrate resulting from the first stage of the replicative transposition event. This figure appears in colour in the online version of JAC and in black and white in the print version of JAC.
In this study, comprehensive analysis of the genetic content of a CRE strain enabled us to shed light on the resistance-encoding potential that a bacterial strain could exhibit. In addition to an IncX3 conjugative plasmid harbouring the blaNDM-5 gene, the test strain was found to harbour three other MDR plasmids that carried various resistance genes. Possession of these plasmids enabled this CRE strain to exhibit phenotypic resistance to almost all currently used antibiotics. Apart from transmission of a single plasmid, plasmid fusion between different plasmids may also occur at a low frequency. Fusion of two plasmids might help further extend the resistance profile and broaden the host spectrum of the fusion plasmid pBJ114-190. It has been demonstrated that plasmids are dynamic and ready to form hybrid plasmids through recombination.11 Plasmid cointegration is not rare in Gram-negative or Gram-positive bacteria and has been found to be associated with dissemination of antimicrobial resistance genes, including genes that code for β-lactamases.12,13 For example, Leelaporn et al.12 found that IS257 elements could mediate the generation of a plasmid cointegrate by unresolved replicative transposition. Lin et al.13 recently reported cointegration of a non-conjugative blaCTX-M-17-bearing plasmid, pIP843, with a ∼73 kb conjugative plasmid that may be responsible for the spread of the gene encoding CTX-M-17 and demonstrated that replication-inactivating insertions were potential means for stabilization of a cointegrate from two plasmids. For the fusion plasmid, pBJ114-190, the two replicon genes were intact, which may lead to the instability of the fusion plasmid during the conjugation process. The detailed mechanisms governing the stability of the fusion plasmid may need further study. Chen et al.14 also reported the identification of two blaKPC-bearing plasmids, pBK30683 (KF954760) and pBK30661 (KF954759), in two different clinical Klebsiella pneumoniae isolates, with plasmid pBK30661 (∼73 kb) being part of pBK30683 (∼160 kb). It was then speculated that plasmid pBK30661 might have integrated into pBK30683 or that pBK30661 might be excised from pBK30683. In addition, the presence of an IncY bacteriophage-like plasmid in the strain might also stimulate the plasmid fusion process under specific conditions since P1 and P7 bacteriophage plasmids were shown to mediate plasmid fusion events.15
In conclusion, this study characterized the complete genetic features of five plasmids by sequence analysis and depicted the high resistance development potential of bacterial pathogens. The presence of multiple mobile elements carrying various antibiotic resistance genes in different conjugative plasmids greatly challenges the development of effective novel antibiotics against MDR bacterial pathogens. In addition, fusion of plasmids that harbour different resistance genes likely plays a potential role in the development of new plasmids with extended resistance profile and bacterial host spectrum. The study warrants further investigation into factors affecting the frequency of forming and resolving the fusion plasmid and its role in MDR plasmid evolution.
Funding
This research was supported by the Collaborative Research Fund of Hong Kong Research Grant Council (C7038-15G and C5026-16G).
Transparency declarations
None to declare.
Supplementary data
Tables S1 and S2 and Figures S1 to S5 are available as Supplementary data at JAC Online.
References
Author notes
Miaomiao Xie and Ruichao Li contributed equally to this work.



