-
PDF
- Split View
-
Views
-
Cite
Cite
D B Fofana, M d’Almeida, S Lambert-Niclot, G Peytavin, P M Girard, B Lafia, L Zohoun-Guidigbi, R K Keke, C Soulie, A G Marcelin, L Morand-Joubert, Resistance profile and treatment outcomes in HIV-infected children at virological failure in Benin, West Africa, Journal of Antimicrobial Chemotherapy, Volume 73, Issue 11, November 2018, Pages 3143–3147, https://doi.org/10.1093/jac/dky300
Close - Share Icon Share
Abstract
In Africa a high percentage of HIV-infected children continue to experience HIV treatment failure despite enormous progress. In Benin (West Africa), there are currently no data on HIV drug resistance at failure in paediatric populations.
To assess the frequency and patterns of HIV drug resistance among children with virological ART failures.
Dried blood spots from 62 HIV-infected children with virological failure were collected at the paediatric clinic of the National Hospital Center in Cotonou for genotyping and plasma drug concentration determination.
Characteristics of the population show a median age of 10 years (IQR 6–13) and a median duration on ART of 5 years (IQR 3–7). Viruses from 53 children were successfully amplified. Of these, 76% of patients were on an NNRTI-based regimen and 24% on a boosted PI-based regimen. NRTI, NNRTI and dual-class resistance was present in 71%, 84% and 65% of cases, respectively. Only 4% of the children had major resistance mutations to PIs and none had major resistance mutations to integrase inhibitors. Among the participants, 25% had undetectable antiretroviral concentrations.
Our results showed that the development of drug resistance could be one of the main consequences of high and continuous viral replication in HIV-infected children in Benin. Thus, inadequate attention to monitoring lifelong ART in children may prevent achievement of the goal of the United Nations Program on HIV and AIDS (UNAIDS) of 90% viral suppression among patients receiving ART.
Introduction
In Sub-Saharan Africa (SSA), access to paediatric ART has increased significantly in the past decade, which has reduced morbidity/mortality rates among HIV-infected children.1 Indeed, the 90–90–90 target of the United Nations Program on HIV and AIDS (UNAIDS) will ensure that treatment is extended to all populations, with a special focus on children and adolescents. However, ensuring long-term adherence to ART in order to minimize emergence of drug resistance mutations (DRMs) and to maintain the clinical benefits of ART into adulthood is the major challenge in the clinical management of HIV-infected children.2 In Benin, HIV prevalence was estimated to be 1.2%, with 67 000 infected patients in 2016.3 Among them, 6300 were children (0–14 years) and 2010 were on ART in 2016.4 Like most West African countries, Benin faces difficulties in the biological monitoring of patients, especially in children, including access to follow-up, viral load (VL) and drug resistance testing. WHO proposed a generic protocol for HIV drug resistance testing in developing countries consisting of using filter paper or dried blood spots (DBSs) as a good alternative. This study aimed to determine, for the first time, the frequency and patterns of HIV DRMs in Benin using DBSs among HIV-infected children and adolescents receiving ART in one of the largest public programmes in Benin.
Patients and methods
Ethics
This work was a pilot study and samples were taken initially for routine patient care at the Pediatric Clinic of the National Hospital Center. However, patients’ representatives were informed that samples and clinical data would be used for this research with oral consent obtained for each participant. In addition, the local Ethics Committee was informed of the use of the data for this research. It has to be noted that the results were first transmitted to the paediatricians for patient treatment before being analysed later for this study.
Study design
During routine biological monitoring, DBS specimens from 62 HIV-infected and treated children followed at the paediatric clinic of the National Hospital Center (Cotonou, Benin) were collected between 2015 and 2016. Children failing ongoing ART, defined as two consecutive VLs of >1000 copies/mL, were enrolled. The VL testing was performed on plasma using M2000 Abbott at Cotonou. The DBSs (Whatman 903 filter papers) were taken from EDTA blood tubes and prepared by putting 50 μL of blood on each of five concentric circles. When the VL was >1000 copies/mL, DBS specimens were stored at ambient temperature before being sent to the Virology Laboratory at Saint-Antoine Hospital (Paris, France), where they were stored at −80°C until use.
HIV-1 drug resistance genotyping
Genotypic resistance testing on the protease, reverse transcriptase and integrase regions of the HIV-1 pol gene was performed using the ANRS (French Agency for HIV Research and Hepatitis) technique (http://www.hivfrenchresistance.org/). Sequences were aligned and edited using SmartGene HIV Software (Innovation Park, Lausanne, Switzerland). Resistance to antiretrovirals (ARVs) was interpreted according to the Stanford algorithm version 7.0.
ARV concentration measurement
ARV exposure was assessed on DBS samples for 60 children at virological failure (VF) (lower limit of detection was <10 ng/mL). ARV concentrations were determined using LC-MS/MS (Waters Acquity UPLC® and Acquity TQD®, Waters Corp., Milford, MA, USA) in the Pharmacology department at the Bichat-Claude Bernard Hospital in Paris, France.
To assist easy interpretation in clinical practice, plasma ARV concentrations were calculated from DBS ARV concentrations using a mean haematocrit value according to gender as previously described.5 Calculated plasma ARV concentrations were categorized according to the international consensus on therapeutic drug monitoring of ARVs to show the applicability of DBS sampling (www.aidsinfo.nih.gov).
Data analysis
All analyses were performed using STATA, version 11 (Stata Corporation, College Station, TX, USA).
Results
Patient characteristics (Table 1)
The median age was 10 years (IQR 6–13) and 53% of the participants were male. The median duration on ART was 5 years (IQR 3–7); the median HIV-1 RNA concentration was 54 000 copies/mL (IQR 5543–170 000 copies/mL). Of the participants, 76% were on an NNRTI-based regimen, 24% on a boosted PI-based regimen and 13% were on second-line ARV treatment.
Characteristics of children and adolescents at virological failure (n = 62)
| Parameters . | Values . |
|---|---|
| Age, years, median (IQR) | 10 (6–13) |
| Male, n/N (%) | 33/62 (53) |
| Therapeutic line, n/N (%) | |
| first | 53/62 (85) |
| second | 9/62 (15) |
| Plasma HIV-1 RNA, copies/mL, median (IQR) | 54 000 (5543–170 000) |
| CD4 count, cells/mm3, median (IQR) | 362 (170–607) |
| Current treatment for children with successful amplification of HIV sequences, n/N (%) (n = 53) | |
| NNRTI-based regimens, first-line | |
| 3TC + ZDV + NVP | 12/53 (23) |
| 3TC + ZDV + EFV | 17/53 (32) |
| 3TC + ABC + NVP | 5/53 (9) |
| 3TC + ABC + EFV | 4/53 (8) |
| 3TC + TDF + EFV | 3/53 (6) |
| PI-based regimens, first-line | |
| 3TC + ZDV + LPV/r | 4/53 (8) |
| PI-based regimens, second-line | |
| 3TC + ABC or ZDV + LPV/r | 8/53 (15) |
| Duration of treatment, years, median, (IQR) | 5 (3–7) |
| Proportion of patients with one or more HIV DRM (%) | 92 |
| DRMs (%) | |
| NNRTI | 84 |
| NRTI | 71 |
| PI | 2 |
| INI | 0 |
| NNRTI and NRTI | 65 |
| NNRTI and NRTI and PI | 2 |
| Viral subtypes (%) | |
| CRF02_AG | 84 |
| A | 4 |
| B | 4 |
| Parameters . | Values . |
|---|---|
| Age, years, median (IQR) | 10 (6–13) |
| Male, n/N (%) | 33/62 (53) |
| Therapeutic line, n/N (%) | |
| first | 53/62 (85) |
| second | 9/62 (15) |
| Plasma HIV-1 RNA, copies/mL, median (IQR) | 54 000 (5543–170 000) |
| CD4 count, cells/mm3, median (IQR) | 362 (170–607) |
| Current treatment for children with successful amplification of HIV sequences, n/N (%) (n = 53) | |
| NNRTI-based regimens, first-line | |
| 3TC + ZDV + NVP | 12/53 (23) |
| 3TC + ZDV + EFV | 17/53 (32) |
| 3TC + ABC + NVP | 5/53 (9) |
| 3TC + ABC + EFV | 4/53 (8) |
| 3TC + TDF + EFV | 3/53 (6) |
| PI-based regimens, first-line | |
| 3TC + ZDV + LPV/r | 4/53 (8) |
| PI-based regimens, second-line | |
| 3TC + ABC or ZDV + LPV/r | 8/53 (15) |
| Duration of treatment, years, median, (IQR) | 5 (3–7) |
| Proportion of patients with one or more HIV DRM (%) | 92 |
| DRMs (%) | |
| NNRTI | 84 |
| NRTI | 71 |
| PI | 2 |
| INI | 0 |
| NNRTI and NRTI | 65 |
| NNRTI and NRTI and PI | 2 |
| Viral subtypes (%) | |
| CRF02_AG | 84 |
| A | 4 |
| B | 4 |
3TC, lamivudine; ZDV, zidovudine; NVP, nevirapine; EFV, efavirenz; ABC, abacavir; TDF, tenofovir; LPV/r, lopinavir/ritonavir.
Characteristics of children and adolescents at virological failure (n = 62)
| Parameters . | Values . |
|---|---|
| Age, years, median (IQR) | 10 (6–13) |
| Male, n/N (%) | 33/62 (53) |
| Therapeutic line, n/N (%) | |
| first | 53/62 (85) |
| second | 9/62 (15) |
| Plasma HIV-1 RNA, copies/mL, median (IQR) | 54 000 (5543–170 000) |
| CD4 count, cells/mm3, median (IQR) | 362 (170–607) |
| Current treatment for children with successful amplification of HIV sequences, n/N (%) (n = 53) | |
| NNRTI-based regimens, first-line | |
| 3TC + ZDV + NVP | 12/53 (23) |
| 3TC + ZDV + EFV | 17/53 (32) |
| 3TC + ABC + NVP | 5/53 (9) |
| 3TC + ABC + EFV | 4/53 (8) |
| 3TC + TDF + EFV | 3/53 (6) |
| PI-based regimens, first-line | |
| 3TC + ZDV + LPV/r | 4/53 (8) |
| PI-based regimens, second-line | |
| 3TC + ABC or ZDV + LPV/r | 8/53 (15) |
| Duration of treatment, years, median, (IQR) | 5 (3–7) |
| Proportion of patients with one or more HIV DRM (%) | 92 |
| DRMs (%) | |
| NNRTI | 84 |
| NRTI | 71 |
| PI | 2 |
| INI | 0 |
| NNRTI and NRTI | 65 |
| NNRTI and NRTI and PI | 2 |
| Viral subtypes (%) | |
| CRF02_AG | 84 |
| A | 4 |
| B | 4 |
| Parameters . | Values . |
|---|---|
| Age, years, median (IQR) | 10 (6–13) |
| Male, n/N (%) | 33/62 (53) |
| Therapeutic line, n/N (%) | |
| first | 53/62 (85) |
| second | 9/62 (15) |
| Plasma HIV-1 RNA, copies/mL, median (IQR) | 54 000 (5543–170 000) |
| CD4 count, cells/mm3, median (IQR) | 362 (170–607) |
| Current treatment for children with successful amplification of HIV sequences, n/N (%) (n = 53) | |
| NNRTI-based regimens, first-line | |
| 3TC + ZDV + NVP | 12/53 (23) |
| 3TC + ZDV + EFV | 17/53 (32) |
| 3TC + ABC + NVP | 5/53 (9) |
| 3TC + ABC + EFV | 4/53 (8) |
| 3TC + TDF + EFV | 3/53 (6) |
| PI-based regimens, first-line | |
| 3TC + ZDV + LPV/r | 4/53 (8) |
| PI-based regimens, second-line | |
| 3TC + ABC or ZDV + LPV/r | 8/53 (15) |
| Duration of treatment, years, median, (IQR) | 5 (3–7) |
| Proportion of patients with one or more HIV DRM (%) | 92 |
| DRMs (%) | |
| NNRTI | 84 |
| NRTI | 71 |
| PI | 2 |
| INI | 0 |
| NNRTI and NRTI | 65 |
| NNRTI and NRTI and PI | 2 |
| Viral subtypes (%) | |
| CRF02_AG | 84 |
| A | 4 |
| B | 4 |
3TC, lamivudine; ZDV, zidovudine; NVP, nevirapine; EFV, efavirenz; ABC, abacavir; TDF, tenofovir; LPV/r, lopinavir/ritonavir.
Resistance mutation patterns
The genotypic resistance test was successfully performed in 53 out of 62 patients (85%). Forty-nine (92%) harboured at least one major HIV DRM at the time of VF. The most common DRMs in the reverse transcriptase gene were M184V (47%), K103N (40%) and Y181C/V (16%). The presence of at least one thymidine analogue mutation (TAM), including M41L, D67N, K70R, T215F/Y, T210W and K219Q/E, was detected in 90% of patients and three or more were detected in 22% of patients.
Among children on a first-line regimen of ART, only minor PI mutations were observed. Two children on a second-line PI-based regimen (25%) harboured a major PI mutation such as L76V (25%), I54L (12.5%) or N88S (12.5%).
No major DRMs to integrase inhibitors (INIs) were identified for 29 integrase sequences successfully amplified. Some INI accessory DRMs, all polymorphic mutations, were observed, such as L74I/M (13%), T97A (3.4%) and E157Q (3.4%),
Drug resistance interpretation (Figure 1)
Only 8% of patients experiencing VF showed susceptibility to the three ARV classes. Resistance to at least one NRTI, one NNRTI and to two classes was present in 71%, 84% and 65% of cases, respectively. For the NRTIs, the prevalence of DRMs conferring high-level resistance was 53% for lamivudine/emtricitabine, 35% for zidovudine, 29% for abacavir and 13% for tenofovir. High-level resistance to a first-generation NNRTI was observed in 82% of patients for nevirapine and in 71% for efavirenz. For the second-generation NNRTIs, DRMs conferring high-level resistance to rilpivirine and etravirine were observed in 20% and 11% of patients, respectively. The prevalence of intermediate or possible resistance to tenofovir was 25% and to etravirine and rilpivirine was 42% and 36%, respectively. Full susceptibility was seen for tenofovir (62%), zidovudine (53%) and abacavir (38%).
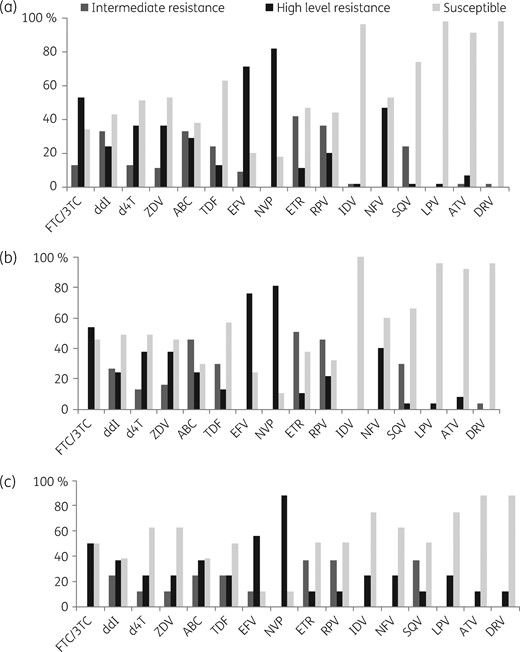
Frequency of susceptibility, intermediate resistance and full resistance to NRTIs, NNRTIs and PIs: (a) global frequency; (b) frequency in patients on first-line therapy; and (c) frequency in patients on second-line therapy. The resistance results were classified into three categories: (i) high level of resistance (presence of mutations associated with drug resistance); (ii) intermediate resistance (presence of mutations associated with low level of resistance); and (iii) susceptible (absence of resistance mutations). NVP, nevirapine; EFV, efavirenz; RPV, rilpivirine; 3TC, lamivudine; FTC, emtricitabine; ZDV, zidovudine; d4T, stavudine; TDF, tenofovir; ABC, abacavir; ddI, didanosine; ATV, atazanavir; DRV, darunavir; IDV, indinavir; LPV, lopinavir; SQV, saquinavir.
ARV concentration measurement
Overall, the median drug concentrations were: nevirapine (n = 16), 2952 ng/mL (IQR 543–8269); efavirenz (n = 18), 2637 ng/mL (IQR 1663–5442); lopinavir (n = 10), 2322 ng/mL (IQR 384–5119); and ritonavir (n = 10), 227 ng/mL (IQR 49–445). Of the participants, 25% (15/60) had undetectable ARV concentrations.
Discussion
This study reported for the first time the prevalence and patterns of HIV DRMs at VF in a Benin paediatric population. We observed a similar high frequency of resistance to at least one ARV in children on both first-line (39/45; 87%) and second-line (7/8; 88%) regimens. A lower number of children on a second-line regimen in this study may explain this similar frequency. Dual-class resistance was present in 65% of patients and a high rate of resistance or intermediate resistance to NRTIs and to NNRTIs could severely compromise future ART options. In addition, the presence of high-level resistance to NNRTIs at failure, even with PI-based regimens, in viruses of patients on first-line therapy supports the alarming results from other studies in children failing NNRTI-based regimens6–8 and PI-based ART in SSA.9,10 Furthermore, presence of at least three TAMs, conferring an intermediate to high level of resistance to NRTIs including tenofovir,11,12 was observed for 22% of cases, with no difference between first- and second-line failures. This prevalence was higher than that observed by Pillay et al.6 in a cohort of 89 HIV-infected children in South Africa despite a similar median treatment duration.
The impact of transmitted DRMs acquired via mother-to-child transmission was not evaluated in our work, but a recent study in children from Togo, a neighbouring country of Benin, showed a prevalence of transmitted DRMs of >10% among newly diagnosed HIV-infected children.8,13,14 In addition, a recent meta-analysis showed a high prevalence of pre-treatment HIV DRMs in children in SSA, both in ARV-exposed (42.7%) and ARV-unexposed children (12.7%).5,14–16 Results from our study suggest that current ART regimens based on NRTIs and NNRTIs could be ineffective for controlling HIV infection in the African paediatric population and the need to access higher genetic barrier ARV classes such as PIs such as darunavir and INIs is becoming urgent.
Major PI mutations at failure were not seen in viruses of patients on first-line therapy and were rare (4%) for those on second-line therapy. These results are in agreement with those reported in other studies in HIV-infected children failing first-7 and second-line therapies from resource-poor countries.10
The data on transmitted resistance to INI are rare in resource-limited countries. Here, we did not observe any major DRMs to INIs. However, we found some INI accessory DRMs at positions 74 (13%), 97 (3.4%) and 157 (3.4%). These polymorphisms (T97A and E157Q) were reported to be associated with resistance to INIs.17,18 Therefore, the presence of these polymorphisms should be carefully considered for treatment management in HIV-infected children in Benin and also in Africa.
Twenty-five percent of participants had undetectable ARV concentrations, which can partly explain VF but cannot explain totally a high rate of DRM selection in the studied population. This result raised another problem in the clinical management of HIV-infected children—adherence to long-term ART. Therefore, measuring ARV concentrations during the clinical follow-up seems to be an interesting tool to prevent treatment failure.
In conclusion, we observed a high prevalence of resistance to NNRTIs and NRTIs. However, the fact that tenofovir, zidovudine and abacavir are still active in some children failing treatment is encouraging. Nevertheless, intermediate resistance to both classes was not negligible in children failing first-line ART, which could reduce the efficacy of second- or third-line regimens. The low frequency of PI DRMs observed supports the preferred choice of PI over NNRTI-based combinations to initiate ART for HIV-infected children in resource-limited countries where drug resistance testing is mostly unavailable.
Acknowledgements
We thank all technicians from our hospital for technical assistance.
Funding
This work was supported by the Agence Nationale de Recherches sur le SIDA et les Hépatites Virales, Expertise France and IMEA (Institute of Medicine and Applied Epidemiology).
Transparency declarations
None to declare.
References
- hiv
- acquired immunodeficiency syndrome
- mutation
- africa
- africa, western
- anti-hiv agents
- child
- integrase inhibitors
- pediatrics
- treatment failure
- united nations
- benin republic
- treatment outcome
- virology
- viruses
- hiv infections
- anti-retroviral agents
- nucleoside reverse transcriptase inhibitors
- non-nucleoside reverse transcriptase inhibitors
- genotype determination
- blood spot specimen



