-
PDF
- Split View
-
Views
-
Cite
Cite
Ana Milinkovic, Paul Benn, Alejandro Arenas-Pinto, Nataliya Brima, Andrew Copas, Amanda Clarke, Martin Fisher, Gabriel Schembri, David Hawkins, Andy Williams, Richard Gilson, on behalf of the MiPEP Trial Team, Randomized controlled trial of the tolerability and completion of maraviroc compared with Kaletra® in combination with Truvada® for HIV post-exposure prophylaxis (MiPEP Trial), Journal of Antimicrobial Chemotherapy, Volume 72, Issue 6, June 2017, Pages 1760–1768, https://doi.org/10.1093/jac/dkx062
Close - Share Icon Share
Objectives: Post-exposure prophylaxis (PEP) for HIV is often poorly tolerated and not completed. Alternative PEP regimens may improve adherence and completion, aiding HIV prevention. We conducted a randomized controlled trial of a maraviroc-based PEP regimen compared with a standard-of-care regimen using ritonavir-boosted lopinavir.
Methods: Patients meeting criteria for PEP were randomized to tenofovir disoproxil/emtricitabine (200/245 mg) once daily plus ritonavir-boosted lopinavir (Kaletra® 400/100 mg) or maraviroc 300 mg twice daily. The composite primary endpoint was completion of 28 days of the allocated PEP regimen without grade 3 or 4 clinical or laboratory adverse events (AEs) related to the PEP medication.
Results: Two hundred and thirteen individuals were randomized (107 to maraviroc; 106 to Kaletra® arm). Follow-up rates were high in both groups. There was no difference in the primary endpoint; 70 (71%) in the maraviroc and 64 (65%) in the Kaletra® arm (P = 0.36) completed PEP without grade 3 or 4 AEs. Discontinuation of PEP was the same (18%) in both groups. There were no grade 3 or 4 clinical AEs in either arm, but more grade 1 or 2 clinical AEs in the Kaletra® arm (91% versus 70%; P < 0.001). Antidiarrhoeal medication use was higher in the Kaletra® arm (67% versus 25%; P < 0.001). There were no HIV seroconversions in the study period.
Conclusions: The completion rate in the absence of grade 3 or 4 AEs was similar with both regimens. Maraviroc-based PEP was better tolerated, supporting its use as an option for non-occupational PEP.
Introduction
HIV post-exposure prophylaxis (PEP) is a well-established prevention strategy in the UK and most of the developed world. The current UK National Guideline ‘Use of HIV Post-Exposure Prophylaxis Following Sexual Exposure’ (PEPSE) recommends triple combination therapy for 28 days, to be started as soon as possible after exposure, preferably within 24 h, but it can be offered up to 72 h after.1 PEP should be considered when other strategies for preventing HIV infection have not been used or failed, and requires a risk–benefit assessment to be undertaken for each individual presenting following an exposure event.
A prospective randomized controlled trial (RCT) to determine the efficacy of PEP following sexual exposure has been precluded due to the high number of participants that would be required for such a study. In addition, the evidence from observational studies in favour of efficacy has led to a lack of the necessary equipoise. A case–control study conducted in healthcare workers suggested that the use of zidovudine for PEP after percutaneous exposure to HIV-infected blood was associated with a significant decrease in the risk of HIV transmission.2 In addition, mother-to-child transmission studies where only the neonate received ART have also demonstrated a protective effect.3,4 Animal models mimicking sexual exposure either vaginally or rectally also show protective benefits of the use of ART and demonstrate that time to initiation and duration of PEP influence outcome of PEP, with delays and shorter courses reducing effectiveness.5
However, studies also suggest that PEP is often poorly tolerated, with individuals frequently reporting side effects and poor completion rates.6 As delayed initiation and non-completion of PEP are likely to reduce efficacy, it is important to manage actively the side effects and to choose regimens that are likely to be better tolerated.
At the time of initiation of our study, the UK PEPSE guideline for the use of PEP for HIV following sexual exposure,7 recommended tenofovir disoproxil/emtricitabine (200/245 mg) once daily as the fixed dose combination Truvada® and ritonavir-boosted lopinavir (Kaletra®) for 28 days as standard of care. In non-randomized comparisons, PEP regimens containing tenofovir disoproxil combined with lamivudine or emtricitabine were associated with improved completion rates and fewer treatment discontinuations due to adverse events (AEs), than regimens containing zidovudine.8,9 The combination of tenofovir disoproxil and emtricitabine has also been shown to prevent acquisition of HIV infection when used as pre-exposure prophylaxis (PrEP).10–12
The choice of third agent is less clear and depends on consideration of short-term tolerability. It is well recognized that ritonavir-boosted PIs boosted with ritonavir, including Kaletra®, are commonly associated with gastrointestinal side effects and elevations in blood lipids.13 In the ABT-730 study conducted in HIV-positive participants, 37% experienced grade 3 or 4 AEs and laboratory abnormalities in the Kaletra® arm.14 Kaletra® also inhibits cytochrome P450 CYP3A and therefore has the potential for drug–drug interactions.
Maraviroc, a CCR5 antagonist has been shown to be an effective antiretroviral agent in the MOTIVATE and MERIT studies.15–18 In MERIT, the percentage of patients achieving HIV-1 RNA <50 copies/mL was comparable to that in those receiving efavirenz where they had CCR5-tropic virus at baseline. In addition, maraviroc does not inhibit any of the major P450 enzymes at clinically relevant concentrations and appears to have fewer drug–drug interactions than Kaletra®. Furthermore, the observed frequency of grade 3 or 4 AEs was low (20%) in the MERIT study.
Maraviroc acts pre-integration, which may have theoretical advantages for use in both PrEP and PEP. Animal data demonstrate that the use of a CCR5 inhibitor reduced the likelihood of macaques acquiring simian immunodeficiency virus following vaginal exposure.19 Maraviroc has also been demonstrated to penetrate the male and female genital tract well and achieve high rectal tissue concentrations.20,21
We conducted an open-label RCT designed to determine whether a maraviroc-based PEP combination was superior to a Kaletra®-based combination. The comparison was based on the proportion of patients who completed a full PEP course in the absence of clinically important treatment-related toxicities.
Patients and methods
Study design and participants
We conducted a parallel group, open-label RCT to compare the tolerability of maraviroc-based PEP relative to a Kaletra®-based combination. We enrolled participants attending five sexual health clinics in England. Eligible participants were adults aged ≥18 years who were considered eligible for PEP for non-occupational exposure according to current UK national guidelines; participants had to report unprotected anal or vaginal intercourse with a known HIV-positive partner, or a partner at high risk for HIV. Patients with a positive HIV antibody test result at screening, currently receiving medication with known interactions with maraviroc or Kaletra®, pregnant or possibly pregnant were not eligible. If the source was known to have MDR HIV and therefore more likely to have CXCR4-tropic virus, these participants were also excluded.
Ethics
The protocol was reviewed and approved by the London-Riverside Research Ethics Committee (REC reference number: 11/LO/1333) and by the Medicines and Healthcare products Regulatory Agency. All participants provided written informed consent. The trial was registered with the International Standard Randomised Controlled Trial Number registry (number ISRCTN63350011).
Randomization
Randomization occurred on the day the patient attended the clinic requesting PEP. Participants were randomly assigned (1:1) to Truvada®, one tablet once daily in addition to either (i) maraviroc (300 mg), one tablet twice daily (experimental arm), or (ii) Kaletra® (lopinavir 200 mg, ritonavir 50 mg), two tablets twice daily (control arm) for 28 days. Block randomization was undertaken, with blocks of varying size, stratified by centre. Randomization was performed online; treatment allocation was open label.
Procedures
All trial participants started their allocated medication on the randomization day (baseline visit) and were followed according to the trial schedule, which included study visits at baseline, days 14 and 28 and month 4. Study medication was dispensed at baseline and again at the day 14 visit for an individual to complete the full course of 28 days of PEP, according to usual clinical practice. Adherence to the PEP regimen was measured by self-reported completion and a count of tablets remaining at day 14 and 28 visits. All clinical AEs (CAEs) and laboratory AEs were graded according to the Division of AIDS Table for Grading the Severity of Adult and Paediatric AEs (Version 2.0, November 2014) by investigators and reported to the co-ordinating centre following standard ICH GCP Guidance. The review of any serious AEs was carried out by an independent clinical reviewer who was blinded to the study allocation.
Switching between study arms was not allowed, but participants could be switched to alternative PEP regiments for safety and tolerability reasons.
Outcomes
The primary outcome was a composite endpoint of completion of 28 days of the allocated PEP regimen without grade 3 or 4 CAEs or laboratory AEs (excluding lipid abnormalities) related to PEP. The secondary outcomes included completion rates of 28 days of allocated PEP regimen, rates of grade 1, 2, 3 or 4 CAEs and laboratory abnormalities; adherence to the allocated PEP regimen; number of doses of antidiarrhoeal and/or antiemetic medication taken; rates of HIV seroconversion at month 4 after exposure; number of sexual partners and unprotected anal/vaginal intercourse (i) while receiving PEP, and (ii) in the 3 months after completion of PEP with a potentially serodiscordant partner. Rates of sexually transmitted infections (gonorrhoea, chlamydia, lymphogranuloma venereum, syphilis, hepatitis B and C) were also examined at baseline and follow-up. For testing, CAEs were grouped by organ and laboratory AEs by system.
For the purposes of this study, completion of allocated PEP combination to day 28 (or 14) was defined as not stopping the PEP combination by day 28 (or 14), irrespective of whether some doses were missed.
Sample size
We calculated that with 140 patients recruited per arm, allowing for a 75% follow-up rate, 105 patients would provide the primary outcome in each arm. This sample size provided 80% power to demonstrate the superiority of maraviroc-based PEP relative to Kaletra®-based PEP if in the experimental arm the prevalence of the primary outcome (completion of 28 days of PEP without grade 3 or 4 toxicity) was 20% higher (70%) than the estimated rate in the control arm (50%). The sample size also provided 80% power if under maraviroc the prevalence was 79% and in the Kaletra® arm it was 60%. Though this was not formally designed as a non-inferiority trial and no choice of non-inferiority margin was made, we also calculated that this sample size would provide over 80% power to demonstrate the non-inferiority of maraviroc relative to Kaletra® if the prevalence of the primary outcome was 60% under maraviroc and 50% under Kaletra®, or 69% under maraviroc and 60% under Kaletra®, if non-inferiority is defined as a prevalence not more than 10% lower than in the Kaletra® arm.
Primary and secondary outcome analyses
All primary comparisons of the two PEP treatments were made according to the randomization arm (ITT). All effect measures are presented with 95% CIs, with P values based on two-sided tests; a 5% significance level was used.
Adjustment was made for recruiting centre and for any factors for which an imbalance between arms was seen at baseline or which were seen linked to differential loss to follow-up between arms. Regression analysis was used; standard logistic regression for binary outcomes and ordinal logistic regression for ordinal outcomes. The effect measures are ORs, presented with 95% CIs; primary comparisons of arms are based on the adjusted ORs (aORs).
All analyses were conducted using Stata SE version 12.0 (Stata Corporation, College Station, TX, USA).
Missing data
All analyses are presented for complete cases only, except for analysis of the primary outcome measure, which was repeated after multiple imputing missing outcome values. Missing data in the primary outcome were anticipated, arising if a patient attended the 14 day visit and reported PEP compliance to that time and no grade 3 or 4 laboratory AEs or CAEs attributable to PEP, but then drops out. Imputation was based on data from similar patients who do not drop out after the 14 day visit, and conducted separately by randomization arm. Imputation was also conducted separately for the two components of the primary outcome (completion of PEP, absence of grade 3 or 4 AEs attributable to PEP) based on logistic regression models including age as a predictor, as it was seen to be related to the primary outcome. Age (as a continuous variable) and site (three categories) were also then adjusted for in all analyses.
Results
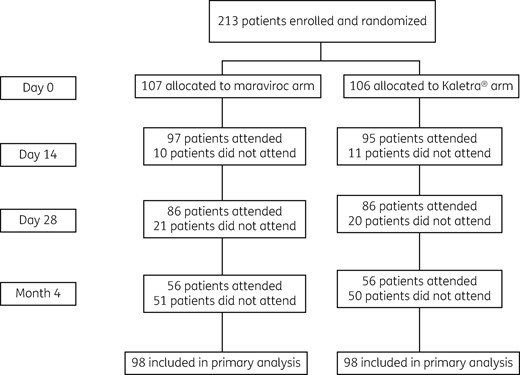
Between August 2012 and December 2014, 213 participants were recruited; 107 were randomized to Truvada®/maraviroc and 106 to Truvada®/Kaletra®. Recruitment was discontinued early because the national recommended standard of care regimen for PEP was changed to Truvada®/raltegravir.1
| Characteristic . | Maraviroc arm (N = 107) . | Kaletra® arm (N = 106) . |
|---|---|---|
| Age in years | 33.6 (9.15) | 34.4 (10.0) |
| Sex | ||
| Female | 4 (4) | 1 (1) |
| Male | 103 (96) | 105 (99) |
| Ethnicitya | ||
| Black | 4 (4) | 3 (3) |
| South Asian | 1 (1) | 4 (4) |
| Other/mixed | 10 (10) | 10 (10) |
| White | 86 (85) | 85 (83) |
| Sexual orientationa | ||
| Bisexual | 12 (12) | 10 (10) |
| Heterosexual | 6 (6) | 4 (4) |
| Homosexual | 83 (82) | 89 (86) |
| Number of sex partners in the last 3 months | ||
| 1 | 9 (9) | 20 (20) |
| 2 | 17 (17) | 19 (19) |
| 3–9 | 42 (43) | 34 (34) |
| 10+ | 30 (31) | 26 (27) |
| Previous PEP | ||
| No | 70 (69) | 62 (60) |
| Yes | 32 (31) | 41 (40) |
| Number of times had PEP previously | ||
| 1 | 16 (50) | 25 (61) |
| 2+ | 16 (50) | 16 (39) |
| Had STI screen at baseline | ||
| No | 71 (68) | 57 (54) |
| Yes | 33 (32) | 48 (46) |
| Any STIs at baseline visit if screened | ||
| No | 29 (88) | 42 (88) |
| Yes | 4 (12) | 6 (13) |
| Syphilis at baseline if tested | ||
| No | 29 (100) | 28 (97) |
| Yes | 0 (0) | 1 (3) |
| Characteristic . | Maraviroc arm (N = 107) . | Kaletra® arm (N = 106) . |
|---|---|---|
| Age in years | 33.6 (9.15) | 34.4 (10.0) |
| Sex | ||
| Female | 4 (4) | 1 (1) |
| Male | 103 (96) | 105 (99) |
| Ethnicitya | ||
| Black | 4 (4) | 3 (3) |
| South Asian | 1 (1) | 4 (4) |
| Other/mixed | 10 (10) | 10 (10) |
| White | 86 (85) | 85 (83) |
| Sexual orientationa | ||
| Bisexual | 12 (12) | 10 (10) |
| Heterosexual | 6 (6) | 4 (4) |
| Homosexual | 83 (82) | 89 (86) |
| Number of sex partners in the last 3 months | ||
| 1 | 9 (9) | 20 (20) |
| 2 | 17 (17) | 19 (19) |
| 3–9 | 42 (43) | 34 (34) |
| 10+ | 30 (31) | 26 (27) |
| Previous PEP | ||
| No | 70 (69) | 62 (60) |
| Yes | 32 (31) | 41 (40) |
| Number of times had PEP previously | ||
| 1 | 16 (50) | 25 (61) |
| 2+ | 16 (50) | 16 (39) |
| Had STI screen at baseline | ||
| No | 71 (68) | 57 (54) |
| Yes | 33 (32) | 48 (46) |
| Any STIs at baseline visit if screened | ||
| No | 29 (88) | 42 (88) |
| Yes | 4 (12) | 6 (13) |
| Syphilis at baseline if tested | ||
| No | 29 (100) | 28 (97) |
| Yes | 0 (0) | 1 (3) |
Data are mean (SD), or n (%).
aPercentages exclude missing data.
| Characteristic . | Maraviroc arm (N = 107) . | Kaletra® arm (N = 106) . |
|---|---|---|
| Age in years | 33.6 (9.15) | 34.4 (10.0) |
| Sex | ||
| Female | 4 (4) | 1 (1) |
| Male | 103 (96) | 105 (99) |
| Ethnicitya | ||
| Black | 4 (4) | 3 (3) |
| South Asian | 1 (1) | 4 (4) |
| Other/mixed | 10 (10) | 10 (10) |
| White | 86 (85) | 85 (83) |
| Sexual orientationa | ||
| Bisexual | 12 (12) | 10 (10) |
| Heterosexual | 6 (6) | 4 (4) |
| Homosexual | 83 (82) | 89 (86) |
| Number of sex partners in the last 3 months | ||
| 1 | 9 (9) | 20 (20) |
| 2 | 17 (17) | 19 (19) |
| 3–9 | 42 (43) | 34 (34) |
| 10+ | 30 (31) | 26 (27) |
| Previous PEP | ||
| No | 70 (69) | 62 (60) |
| Yes | 32 (31) | 41 (40) |
| Number of times had PEP previously | ||
| 1 | 16 (50) | 25 (61) |
| 2+ | 16 (50) | 16 (39) |
| Had STI screen at baseline | ||
| No | 71 (68) | 57 (54) |
| Yes | 33 (32) | 48 (46) |
| Any STIs at baseline visit if screened | ||
| No | 29 (88) | 42 (88) |
| Yes | 4 (12) | 6 (13) |
| Syphilis at baseline if tested | ||
| No | 29 (100) | 28 (97) |
| Yes | 0 (0) | 1 (3) |
| Characteristic . | Maraviroc arm (N = 107) . | Kaletra® arm (N = 106) . |
|---|---|---|
| Age in years | 33.6 (9.15) | 34.4 (10.0) |
| Sex | ||
| Female | 4 (4) | 1 (1) |
| Male | 103 (96) | 105 (99) |
| Ethnicitya | ||
| Black | 4 (4) | 3 (3) |
| South Asian | 1 (1) | 4 (4) |
| Other/mixed | 10 (10) | 10 (10) |
| White | 86 (85) | 85 (83) |
| Sexual orientationa | ||
| Bisexual | 12 (12) | 10 (10) |
| Heterosexual | 6 (6) | 4 (4) |
| Homosexual | 83 (82) | 89 (86) |
| Number of sex partners in the last 3 months | ||
| 1 | 9 (9) | 20 (20) |
| 2 | 17 (17) | 19 (19) |
| 3–9 | 42 (43) | 34 (34) |
| 10+ | 30 (31) | 26 (27) |
| Previous PEP | ||
| No | 70 (69) | 62 (60) |
| Yes | 32 (31) | 41 (40) |
| Number of times had PEP previously | ||
| 1 | 16 (50) | 25 (61) |
| 2+ | 16 (50) | 16 (39) |
| Had STI screen at baseline | ||
| No | 71 (68) | 57 (54) |
| Yes | 33 (32) | 48 (46) |
| Any STIs at baseline visit if screened | ||
| No | 29 (88) | 42 (88) |
| Yes | 4 (12) | 6 (13) |
| Syphilis at baseline if tested | ||
| No | 29 (100) | 28 (97) |
| Yes | 0 (0) | 1 (3) |
Data are mean (SD), or n (%).
aPercentages exclude missing data.
There was no difference in the combined primary endpoint between study arms: 71% and 65% in the maraviroc and Kaletra® arms, respectively, at day 28 (P = 0.357). Multiple imputation of the primary outcome was conducted because of missing data and this provided very similar results (73% and 67%; P = 0.330). The completion rate of PEP in the maraviroc arm was 82% compared with 77% in the Kaletra® arm (P = 0.350) (Table 2). By day 28 of follow-up, there were no serious AEs or grade 3 or 4 CAEs in the study population. However, there were 123 grade 1 or 2 CAEs in the maraviroc arm and 175 in the Kaletra® arm. Participants randomized to maraviroc had a significantly lower rate of gastrointestinal AEs (OR = 0.32; 95% CI = 0.18–0.56; P < 0.0001) (Table 3). Therefore, a lower proportion of participants in the maraviroc arm were prescribed antidiarrhoeal medication (OR = 0.15; 95% CI= 0.08–0.28; P < 0.001) and there was somewhat lower use of antiemetic medication (OR = 0.68; 95% CI = 0.39–1.16; P = 0.158) (Table 2).
Outcome measures, summary statistics and OR comparing maraviroc with Kaletra® arm
| Outcome measure (N = denominator across arms) . | Maraviroc arm n (%), N = 107 . | Kaletra® arm n (%), N = 106 . | P valuea . | P value, OR (95% CI) . | |
|---|---|---|---|---|---|
| unadjustedb . | adjustedb,c . | ||||
| Primary: completion of 28 days of allocated PEP regimen without grade 3 or 4 CAEs or laboratory AEs related to PEP, N = 196 | |||||
| No | 28 (29) | 34 (35) | 0.357 | P = 0.357 | P = 0.254 |
| Yes | 70 (71) | 64 (65) | 1.33 (0.73–2.43) | 1.44 (0.77–2.70) | |
| Primary after imputation: completion of 28 days of allocated PEP regimen without grade 3 or 4 CAEs or laboratory AEs related to PEP, N = 213 | |||||
| No | 27% | 33% | 0.350 | P = 0.330 | P = 0.262 |
| Yes | 73% | 67% | 1.35 (0.74–2.46) | 1.43 (0.77–2.65) | |
| Completion of 28 days of allocated PEP regimen, N = 193 | |||||
| No | 17 (18) | 22 (23) | 0.351 | P = 0.352 | P = 0.309 |
| Yes | 80 (82) | 74 (77) | 1.40 (0.69–2.84) | 1.46 (0.70–3.04) | |
| Laboratory abnormalities, highest grade reported, N = 196 | |||||
| 0 | 37 (37) | 23 (24) | 0.108 | P = 0.056 | P = 0.079 |
| 1 | 29 (29) | 30 (31) | 0.61 (0.37–1.01) | 0.63 (0.38–1.05) | |
| 2 | 20 (20) | 31 (33) | |||
| 3 | 13 (13) | 13 (12) | |||
| Grade 3+ laboratory abnormality related to PEP, N = 196 | |||||
| No | 87 (88) | 85 (88) | 0.957 | P = 0.957 | P = 0.911 |
| Yes | 12 (12) | 12 (12) | 0.98 (0.42–2.30) | 0.95 (0.39–2.29) | |
| CAEs, highest grade reported, N = 196 | |||||
| 0 | 30 (30) | 9 (9) | 0.001 | P < 0.001 | P < 0.001 |
| 1 | 59 (60) | 69 (71) | 0.32 (0.17–0.59) | 0.32 (0.17–0.59) | |
| 2 | 10 (10) | 19 (20) | |||
| Number of missed doses of Truvada® over 14 days, if completed 14 days of allocated PEP regimen, N = 184 | |||||
| 0 | 89 (95) | 83 (92) | 0.493 | P = 0.502 | P = 0.514 |
| 1+ | 5 (5) | 7 (8) | 0.67 (0.20–2.18) | 0.67 (0.20–2.22) | |
| Number of missed doses of Truvada® over 28 days, if completed 28 days of allocated PEP regimen, N = 154 | |||||
| 0 | 68 (85) | 68 (92) | 0.178 | P = 0.190 | P = 0.123 |
| 1+ | 12 (15) | 6 (8) | 2.00 (0.71–5.64) | 2.31 (0.80–6.68) | |
| Number of missed doses of maraviroc or Kaletra® over 14 days if completed 14 days of allocated PEP regimen, N = 184 | |||||
| 0 | 80 (85) | 73 (81) | 0.865 | P = 0.409 | P = 0.384 |
| 1 | 12 (13) | 11 (12) | 0.72 (0.33–1.56) | 0.71 (0.33–1.54) | |
| 2+ | 2 (2) | 6 (7) | |||
| Number of missed doses of maraviroc or Kaletra® over 28 days, if completed 28 days of allocated PEP regimen, N = 154 | |||||
| 0 | 62 (78) | 58 (78) | 0.853 | P = 0.829 | P = 0.867 |
| 1 | 8 (10) | 9 (12) | 1.09 (0.51–2.32) | 1.07 (0.50–2.28) | |
| 2+ | 10 (12) | 7 (10) | |||
| Number of doses of antidiarrhoeal medication taken over 28 days, N = 196 | |||||
| 0 | 74 (75) | 32 (33) | P < 0.001 | P < 0.001 | P < 0.001 |
| 1–5 | 18 (18) | 28 (29) | |||
| 6+ | 7 (7) | 37 (38) | 0.16 (0.09–0.28) | 0.15 (0.08–0.28) | |
| Number of doses of antiemetic taken over 28 days, N = 196 | |||||
| 0 | 58 (59) | 49 (51) | 0.059 | P = 0.157 | P = 0.158 |
| 1–5 | 22 (22) | 20 (21) | 0.68 (0.39–1.16) | 0.68 (0.39–1.16) | |
| 6+ | 19 (19) | 28 (29) | |||
| Number of days absent from work or college over 28 days (not including days absent for clinic visits), N = 173 | |||||
| 0 | 77 (87) | 73 (87) | 0.843 | P = 0.905 | P = 0.863 |
| 1–5 | 5 (6) | 6 (7) | 1.05 (0.44–2.53) | 1.08 (0.45–2.61) | |
| 6+ | 7 (8) | 5 (6) | |||
| HIV seroconversion 4 months after exposure, N = 119 | |||||
| No | 66 (100) | 53 (100) | 1.000 | – | – |
| Yes | 0 (0) | 0 (0) | |||
| Additional visits, N = 196 | |||||
| No | 88 (90) | 91 (93) | 0.446 | P = 0.449 | P = 0.338 |
| Yes | 10 (10) | 7 (7) | 1.48 (0.54–4.05) | 1.67 (0.58–4.90) | |
| Outcome measure (N = denominator across arms) . | Maraviroc arm n (%), N = 107 . | Kaletra® arm n (%), N = 106 . | P valuea . | P value, OR (95% CI) . | |
|---|---|---|---|---|---|
| unadjustedb . | adjustedb,c . | ||||
| Primary: completion of 28 days of allocated PEP regimen without grade 3 or 4 CAEs or laboratory AEs related to PEP, N = 196 | |||||
| No | 28 (29) | 34 (35) | 0.357 | P = 0.357 | P = 0.254 |
| Yes | 70 (71) | 64 (65) | 1.33 (0.73–2.43) | 1.44 (0.77–2.70) | |
| Primary after imputation: completion of 28 days of allocated PEP regimen without grade 3 or 4 CAEs or laboratory AEs related to PEP, N = 213 | |||||
| No | 27% | 33% | 0.350 | P = 0.330 | P = 0.262 |
| Yes | 73% | 67% | 1.35 (0.74–2.46) | 1.43 (0.77–2.65) | |
| Completion of 28 days of allocated PEP regimen, N = 193 | |||||
| No | 17 (18) | 22 (23) | 0.351 | P = 0.352 | P = 0.309 |
| Yes | 80 (82) | 74 (77) | 1.40 (0.69–2.84) | 1.46 (0.70–3.04) | |
| Laboratory abnormalities, highest grade reported, N = 196 | |||||
| 0 | 37 (37) | 23 (24) | 0.108 | P = 0.056 | P = 0.079 |
| 1 | 29 (29) | 30 (31) | 0.61 (0.37–1.01) | 0.63 (0.38–1.05) | |
| 2 | 20 (20) | 31 (33) | |||
| 3 | 13 (13) | 13 (12) | |||
| Grade 3+ laboratory abnormality related to PEP, N = 196 | |||||
| No | 87 (88) | 85 (88) | 0.957 | P = 0.957 | P = 0.911 |
| Yes | 12 (12) | 12 (12) | 0.98 (0.42–2.30) | 0.95 (0.39–2.29) | |
| CAEs, highest grade reported, N = 196 | |||||
| 0 | 30 (30) | 9 (9) | 0.001 | P < 0.001 | P < 0.001 |
| 1 | 59 (60) | 69 (71) | 0.32 (0.17–0.59) | 0.32 (0.17–0.59) | |
| 2 | 10 (10) | 19 (20) | |||
| Number of missed doses of Truvada® over 14 days, if completed 14 days of allocated PEP regimen, N = 184 | |||||
| 0 | 89 (95) | 83 (92) | 0.493 | P = 0.502 | P = 0.514 |
| 1+ | 5 (5) | 7 (8) | 0.67 (0.20–2.18) | 0.67 (0.20–2.22) | |
| Number of missed doses of Truvada® over 28 days, if completed 28 days of allocated PEP regimen, N = 154 | |||||
| 0 | 68 (85) | 68 (92) | 0.178 | P = 0.190 | P = 0.123 |
| 1+ | 12 (15) | 6 (8) | 2.00 (0.71–5.64) | 2.31 (0.80–6.68) | |
| Number of missed doses of maraviroc or Kaletra® over 14 days if completed 14 days of allocated PEP regimen, N = 184 | |||||
| 0 | 80 (85) | 73 (81) | 0.865 | P = 0.409 | P = 0.384 |
| 1 | 12 (13) | 11 (12) | 0.72 (0.33–1.56) | 0.71 (0.33–1.54) | |
| 2+ | 2 (2) | 6 (7) | |||
| Number of missed doses of maraviroc or Kaletra® over 28 days, if completed 28 days of allocated PEP regimen, N = 154 | |||||
| 0 | 62 (78) | 58 (78) | 0.853 | P = 0.829 | P = 0.867 |
| 1 | 8 (10) | 9 (12) | 1.09 (0.51–2.32) | 1.07 (0.50–2.28) | |
| 2+ | 10 (12) | 7 (10) | |||
| Number of doses of antidiarrhoeal medication taken over 28 days, N = 196 | |||||
| 0 | 74 (75) | 32 (33) | P < 0.001 | P < 0.001 | P < 0.001 |
| 1–5 | 18 (18) | 28 (29) | |||
| 6+ | 7 (7) | 37 (38) | 0.16 (0.09–0.28) | 0.15 (0.08–0.28) | |
| Number of doses of antiemetic taken over 28 days, N = 196 | |||||
| 0 | 58 (59) | 49 (51) | 0.059 | P = 0.157 | P = 0.158 |
| 1–5 | 22 (22) | 20 (21) | 0.68 (0.39–1.16) | 0.68 (0.39–1.16) | |
| 6+ | 19 (19) | 28 (29) | |||
| Number of days absent from work or college over 28 days (not including days absent for clinic visits), N = 173 | |||||
| 0 | 77 (87) | 73 (87) | 0.843 | P = 0.905 | P = 0.863 |
| 1–5 | 5 (6) | 6 (7) | 1.05 (0.44–2.53) | 1.08 (0.45–2.61) | |
| 6+ | 7 (8) | 5 (6) | |||
| HIV seroconversion 4 months after exposure, N = 119 | |||||
| No | 66 (100) | 53 (100) | 1.000 | – | – |
| Yes | 0 (0) | 0 (0) | |||
| Additional visits, N = 196 | |||||
| No | 88 (90) | 91 (93) | 0.446 | P = 0.449 | P = 0.338 |
| Yes | 10 (10) | 7 (7) | 1.48 (0.54–4.05) | 1.67 (0.58–4.90) | |
χ2, Fisher's exact and Mann–Whitney tests were used as appropriate.
ORs based on logistic regression for binary outcome or ordinal logistic regression assuming proportional odds for ordinal outcome.
Adjusted for age (continuous) and site.
Outcome measures, summary statistics and OR comparing maraviroc with Kaletra® arm
| Outcome measure (N = denominator across arms) . | Maraviroc arm n (%), N = 107 . | Kaletra® arm n (%), N = 106 . | P valuea . | P value, OR (95% CI) . | |
|---|---|---|---|---|---|
| unadjustedb . | adjustedb,c . | ||||
| Primary: completion of 28 days of allocated PEP regimen without grade 3 or 4 CAEs or laboratory AEs related to PEP, N = 196 | |||||
| No | 28 (29) | 34 (35) | 0.357 | P = 0.357 | P = 0.254 |
| Yes | 70 (71) | 64 (65) | 1.33 (0.73–2.43) | 1.44 (0.77–2.70) | |
| Primary after imputation: completion of 28 days of allocated PEP regimen without grade 3 or 4 CAEs or laboratory AEs related to PEP, N = 213 | |||||
| No | 27% | 33% | 0.350 | P = 0.330 | P = 0.262 |
| Yes | 73% | 67% | 1.35 (0.74–2.46) | 1.43 (0.77–2.65) | |
| Completion of 28 days of allocated PEP regimen, N = 193 | |||||
| No | 17 (18) | 22 (23) | 0.351 | P = 0.352 | P = 0.309 |
| Yes | 80 (82) | 74 (77) | 1.40 (0.69–2.84) | 1.46 (0.70–3.04) | |
| Laboratory abnormalities, highest grade reported, N = 196 | |||||
| 0 | 37 (37) | 23 (24) | 0.108 | P = 0.056 | P = 0.079 |
| 1 | 29 (29) | 30 (31) | 0.61 (0.37–1.01) | 0.63 (0.38–1.05) | |
| 2 | 20 (20) | 31 (33) | |||
| 3 | 13 (13) | 13 (12) | |||
| Grade 3+ laboratory abnormality related to PEP, N = 196 | |||||
| No | 87 (88) | 85 (88) | 0.957 | P = 0.957 | P = 0.911 |
| Yes | 12 (12) | 12 (12) | 0.98 (0.42–2.30) | 0.95 (0.39–2.29) | |
| CAEs, highest grade reported, N = 196 | |||||
| 0 | 30 (30) | 9 (9) | 0.001 | P < 0.001 | P < 0.001 |
| 1 | 59 (60) | 69 (71) | 0.32 (0.17–0.59) | 0.32 (0.17–0.59) | |
| 2 | 10 (10) | 19 (20) | |||
| Number of missed doses of Truvada® over 14 days, if completed 14 days of allocated PEP regimen, N = 184 | |||||
| 0 | 89 (95) | 83 (92) | 0.493 | P = 0.502 | P = 0.514 |
| 1+ | 5 (5) | 7 (8) | 0.67 (0.20–2.18) | 0.67 (0.20–2.22) | |
| Number of missed doses of Truvada® over 28 days, if completed 28 days of allocated PEP regimen, N = 154 | |||||
| 0 | 68 (85) | 68 (92) | 0.178 | P = 0.190 | P = 0.123 |
| 1+ | 12 (15) | 6 (8) | 2.00 (0.71–5.64) | 2.31 (0.80–6.68) | |
| Number of missed doses of maraviroc or Kaletra® over 14 days if completed 14 days of allocated PEP regimen, N = 184 | |||||
| 0 | 80 (85) | 73 (81) | 0.865 | P = 0.409 | P = 0.384 |
| 1 | 12 (13) | 11 (12) | 0.72 (0.33–1.56) | 0.71 (0.33–1.54) | |
| 2+ | 2 (2) | 6 (7) | |||
| Number of missed doses of maraviroc or Kaletra® over 28 days, if completed 28 days of allocated PEP regimen, N = 154 | |||||
| 0 | 62 (78) | 58 (78) | 0.853 | P = 0.829 | P = 0.867 |
| 1 | 8 (10) | 9 (12) | 1.09 (0.51–2.32) | 1.07 (0.50–2.28) | |
| 2+ | 10 (12) | 7 (10) | |||
| Number of doses of antidiarrhoeal medication taken over 28 days, N = 196 | |||||
| 0 | 74 (75) | 32 (33) | P < 0.001 | P < 0.001 | P < 0.001 |
| 1–5 | 18 (18) | 28 (29) | |||
| 6+ | 7 (7) | 37 (38) | 0.16 (0.09–0.28) | 0.15 (0.08–0.28) | |
| Number of doses of antiemetic taken over 28 days, N = 196 | |||||
| 0 | 58 (59) | 49 (51) | 0.059 | P = 0.157 | P = 0.158 |
| 1–5 | 22 (22) | 20 (21) | 0.68 (0.39–1.16) | 0.68 (0.39–1.16) | |
| 6+ | 19 (19) | 28 (29) | |||
| Number of days absent from work or college over 28 days (not including days absent for clinic visits), N = 173 | |||||
| 0 | 77 (87) | 73 (87) | 0.843 | P = 0.905 | P = 0.863 |
| 1–5 | 5 (6) | 6 (7) | 1.05 (0.44–2.53) | 1.08 (0.45–2.61) | |
| 6+ | 7 (8) | 5 (6) | |||
| HIV seroconversion 4 months after exposure, N = 119 | |||||
| No | 66 (100) | 53 (100) | 1.000 | – | – |
| Yes | 0 (0) | 0 (0) | |||
| Additional visits, N = 196 | |||||
| No | 88 (90) | 91 (93) | 0.446 | P = 0.449 | P = 0.338 |
| Yes | 10 (10) | 7 (7) | 1.48 (0.54–4.05) | 1.67 (0.58–4.90) | |
| Outcome measure (N = denominator across arms) . | Maraviroc arm n (%), N = 107 . | Kaletra® arm n (%), N = 106 . | P valuea . | P value, OR (95% CI) . | |
|---|---|---|---|---|---|
| unadjustedb . | adjustedb,c . | ||||
| Primary: completion of 28 days of allocated PEP regimen without grade 3 or 4 CAEs or laboratory AEs related to PEP, N = 196 | |||||
| No | 28 (29) | 34 (35) | 0.357 | P = 0.357 | P = 0.254 |
| Yes | 70 (71) | 64 (65) | 1.33 (0.73–2.43) | 1.44 (0.77–2.70) | |
| Primary after imputation: completion of 28 days of allocated PEP regimen without grade 3 or 4 CAEs or laboratory AEs related to PEP, N = 213 | |||||
| No | 27% | 33% | 0.350 | P = 0.330 | P = 0.262 |
| Yes | 73% | 67% | 1.35 (0.74–2.46) | 1.43 (0.77–2.65) | |
| Completion of 28 days of allocated PEP regimen, N = 193 | |||||
| No | 17 (18) | 22 (23) | 0.351 | P = 0.352 | P = 0.309 |
| Yes | 80 (82) | 74 (77) | 1.40 (0.69–2.84) | 1.46 (0.70–3.04) | |
| Laboratory abnormalities, highest grade reported, N = 196 | |||||
| 0 | 37 (37) | 23 (24) | 0.108 | P = 0.056 | P = 0.079 |
| 1 | 29 (29) | 30 (31) | 0.61 (0.37–1.01) | 0.63 (0.38–1.05) | |
| 2 | 20 (20) | 31 (33) | |||
| 3 | 13 (13) | 13 (12) | |||
| Grade 3+ laboratory abnormality related to PEP, N = 196 | |||||
| No | 87 (88) | 85 (88) | 0.957 | P = 0.957 | P = 0.911 |
| Yes | 12 (12) | 12 (12) | 0.98 (0.42–2.30) | 0.95 (0.39–2.29) | |
| CAEs, highest grade reported, N = 196 | |||||
| 0 | 30 (30) | 9 (9) | 0.001 | P < 0.001 | P < 0.001 |
| 1 | 59 (60) | 69 (71) | 0.32 (0.17–0.59) | 0.32 (0.17–0.59) | |
| 2 | 10 (10) | 19 (20) | |||
| Number of missed doses of Truvada® over 14 days, if completed 14 days of allocated PEP regimen, N = 184 | |||||
| 0 | 89 (95) | 83 (92) | 0.493 | P = 0.502 | P = 0.514 |
| 1+ | 5 (5) | 7 (8) | 0.67 (0.20–2.18) | 0.67 (0.20–2.22) | |
| Number of missed doses of Truvada® over 28 days, if completed 28 days of allocated PEP regimen, N = 154 | |||||
| 0 | 68 (85) | 68 (92) | 0.178 | P = 0.190 | P = 0.123 |
| 1+ | 12 (15) | 6 (8) | 2.00 (0.71–5.64) | 2.31 (0.80–6.68) | |
| Number of missed doses of maraviroc or Kaletra® over 14 days if completed 14 days of allocated PEP regimen, N = 184 | |||||
| 0 | 80 (85) | 73 (81) | 0.865 | P = 0.409 | P = 0.384 |
| 1 | 12 (13) | 11 (12) | 0.72 (0.33–1.56) | 0.71 (0.33–1.54) | |
| 2+ | 2 (2) | 6 (7) | |||
| Number of missed doses of maraviroc or Kaletra® over 28 days, if completed 28 days of allocated PEP regimen, N = 154 | |||||
| 0 | 62 (78) | 58 (78) | 0.853 | P = 0.829 | P = 0.867 |
| 1 | 8 (10) | 9 (12) | 1.09 (0.51–2.32) | 1.07 (0.50–2.28) | |
| 2+ | 10 (12) | 7 (10) | |||
| Number of doses of antidiarrhoeal medication taken over 28 days, N = 196 | |||||
| 0 | 74 (75) | 32 (33) | P < 0.001 | P < 0.001 | P < 0.001 |
| 1–5 | 18 (18) | 28 (29) | |||
| 6+ | 7 (7) | 37 (38) | 0.16 (0.09–0.28) | 0.15 (0.08–0.28) | |
| Number of doses of antiemetic taken over 28 days, N = 196 | |||||
| 0 | 58 (59) | 49 (51) | 0.059 | P = 0.157 | P = 0.158 |
| 1–5 | 22 (22) | 20 (21) | 0.68 (0.39–1.16) | 0.68 (0.39–1.16) | |
| 6+ | 19 (19) | 28 (29) | |||
| Number of days absent from work or college over 28 days (not including days absent for clinic visits), N = 173 | |||||
| 0 | 77 (87) | 73 (87) | 0.843 | P = 0.905 | P = 0.863 |
| 1–5 | 5 (6) | 6 (7) | 1.05 (0.44–2.53) | 1.08 (0.45–2.61) | |
| 6+ | 7 (8) | 5 (6) | |||
| HIV seroconversion 4 months after exposure, N = 119 | |||||
| No | 66 (100) | 53 (100) | 1.000 | – | – |
| Yes | 0 (0) | 0 (0) | |||
| Additional visits, N = 196 | |||||
| No | 88 (90) | 91 (93) | 0.446 | P = 0.449 | P = 0.338 |
| Yes | 10 (10) | 7 (7) | 1.48 (0.54–4.05) | 1.67 (0.58–4.90) | |
χ2, Fisher's exact and Mann–Whitney tests were used as appropriate.
ORs based on logistic regression for binary outcome or ordinal logistic regression assuming proportional odds for ordinal outcome.
Adjusted for age (continuous) and site.
| CAE type . | Maraviroc arm n (%), N = 99 . | Kaletra® arm n (%), N = 97 . | OR (95% CI)a, P value . | ||||
|---|---|---|---|---|---|---|---|
| Grade: . | 0 . | 1 . | 2 . | 0 . | 1 . | 2 . | |
| CNS | 85 (86) | 12 (12) | 2 (2) | 79 (81) | 17 (18) | 1 (1) | 0.68 (0.31–1.48), P = 0.330 |
| Headache | 89 (90) | 8 (8) | 2 (2) | 83 (86) | 13 (13) | 1 (1) | |
| Sleeping disorder | 93 (94) | 6 (6) | 0 (0) | 91 (94) | 6 (6) | 0 (0) | |
| Other CNS | 97 (98) | 2 (2) | 0 (0) | 96 (99) | 1 (1) | 0 (0) | |
| GI | 46 (47) | 46 (47) | 6 (6) | 14 (14) | 68 (70) | 15 (16) | 0.32 (0.18–0.56), P < 0.0001 |
| Nausea/vomiting | 69 (70) | 27 (27) | 3 (3) | 59 (61) | 31 (32) | 7 (7) | |
| Diarrhoea | 80 (81) | 18 (18) | 1 (1) | 25 (26) | 61 (63) | 11 (11) | |
| Constipation | 95 (96) | 3 (3) | 1 (1) | 95 (98) | 2 (2) | 0 (0) | |
| Other GI | 83 (84) | 15 (15) | 1 (1) | 60 (62) | 30 (31) | 7 (7) | |
| Skin | 96 (97) | 3 (3) | 0 (0) | 88 (91) | 8 (8) | 1 (1) | 0.36 (0.09–1.38) P = 0.135 |
| Rash | 97 (98) | 2 (2) | 0 (0) | 89 (92) | 7 (7) | 1 (1) | |
| Other skin | 98 (99) | 1 (1) | 0 (0) | 96 (99) | 1 (1) | 0 (0) | |
| Tiredness, fatigue, etc. | 63 (64) | 30 (30) | 6 (6) | 59 (61) | 35 (36) | 3 (3) | 1.05 (0.58–1.87) P = 0.882 |
| Other | 81 (82) | 14 (14) | 4 (4) | 70 (72) | 24 (25) | 3 (3) | 0.59 (0.30–1.16) P = 0.128 |
| CAE type . | Maraviroc arm n (%), N = 99 . | Kaletra® arm n (%), N = 97 . | OR (95% CI)a, P value . | ||||
|---|---|---|---|---|---|---|---|
| Grade: . | 0 . | 1 . | 2 . | 0 . | 1 . | 2 . | |
| CNS | 85 (86) | 12 (12) | 2 (2) | 79 (81) | 17 (18) | 1 (1) | 0.68 (0.31–1.48), P = 0.330 |
| Headache | 89 (90) | 8 (8) | 2 (2) | 83 (86) | 13 (13) | 1 (1) | |
| Sleeping disorder | 93 (94) | 6 (6) | 0 (0) | 91 (94) | 6 (6) | 0 (0) | |
| Other CNS | 97 (98) | 2 (2) | 0 (0) | 96 (99) | 1 (1) | 0 (0) | |
| GI | 46 (47) | 46 (47) | 6 (6) | 14 (14) | 68 (70) | 15 (16) | 0.32 (0.18–0.56), P < 0.0001 |
| Nausea/vomiting | 69 (70) | 27 (27) | 3 (3) | 59 (61) | 31 (32) | 7 (7) | |
| Diarrhoea | 80 (81) | 18 (18) | 1 (1) | 25 (26) | 61 (63) | 11 (11) | |
| Constipation | 95 (96) | 3 (3) | 1 (1) | 95 (98) | 2 (2) | 0 (0) | |
| Other GI | 83 (84) | 15 (15) | 1 (1) | 60 (62) | 30 (31) | 7 (7) | |
| Skin | 96 (97) | 3 (3) | 0 (0) | 88 (91) | 8 (8) | 1 (1) | 0.36 (0.09–1.38) P = 0.135 |
| Rash | 97 (98) | 2 (2) | 0 (0) | 89 (92) | 7 (7) | 1 (1) | |
| Other skin | 98 (99) | 1 (1) | 0 (0) | 96 (99) | 1 (1) | 0 (0) | |
| Tiredness, fatigue, etc. | 63 (64) | 30 (30) | 6 (6) | 59 (61) | 35 (36) | 3 (3) | 1.05 (0.58–1.87) P = 0.882 |
| Other | 81 (82) | 14 (14) | 4 (4) | 70 (72) | 24 (25) | 3 (3) | 0.59 (0.30–1.16) P = 0.128 |
OR for maraviroc arm relative to Kaletra®. Ordered logistic regression assuming proportional odds for GI (grades 0, 1, 2). Logistic regression for CNS, skin, tiredness, other (binary outcome grade 1+).
| CAE type . | Maraviroc arm n (%), N = 99 . | Kaletra® arm n (%), N = 97 . | OR (95% CI)a, P value . | ||||
|---|---|---|---|---|---|---|---|
| Grade: . | 0 . | 1 . | 2 . | 0 . | 1 . | 2 . | |
| CNS | 85 (86) | 12 (12) | 2 (2) | 79 (81) | 17 (18) | 1 (1) | 0.68 (0.31–1.48), P = 0.330 |
| Headache | 89 (90) | 8 (8) | 2 (2) | 83 (86) | 13 (13) | 1 (1) | |
| Sleeping disorder | 93 (94) | 6 (6) | 0 (0) | 91 (94) | 6 (6) | 0 (0) | |
| Other CNS | 97 (98) | 2 (2) | 0 (0) | 96 (99) | 1 (1) | 0 (0) | |
| GI | 46 (47) | 46 (47) | 6 (6) | 14 (14) | 68 (70) | 15 (16) | 0.32 (0.18–0.56), P < 0.0001 |
| Nausea/vomiting | 69 (70) | 27 (27) | 3 (3) | 59 (61) | 31 (32) | 7 (7) | |
| Diarrhoea | 80 (81) | 18 (18) | 1 (1) | 25 (26) | 61 (63) | 11 (11) | |
| Constipation | 95 (96) | 3 (3) | 1 (1) | 95 (98) | 2 (2) | 0 (0) | |
| Other GI | 83 (84) | 15 (15) | 1 (1) | 60 (62) | 30 (31) | 7 (7) | |
| Skin | 96 (97) | 3 (3) | 0 (0) | 88 (91) | 8 (8) | 1 (1) | 0.36 (0.09–1.38) P = 0.135 |
| Rash | 97 (98) | 2 (2) | 0 (0) | 89 (92) | 7 (7) | 1 (1) | |
| Other skin | 98 (99) | 1 (1) | 0 (0) | 96 (99) | 1 (1) | 0 (0) | |
| Tiredness, fatigue, etc. | 63 (64) | 30 (30) | 6 (6) | 59 (61) | 35 (36) | 3 (3) | 1.05 (0.58–1.87) P = 0.882 |
| Other | 81 (82) | 14 (14) | 4 (4) | 70 (72) | 24 (25) | 3 (3) | 0.59 (0.30–1.16) P = 0.128 |
| CAE type . | Maraviroc arm n (%), N = 99 . | Kaletra® arm n (%), N = 97 . | OR (95% CI)a, P value . | ||||
|---|---|---|---|---|---|---|---|
| Grade: . | 0 . | 1 . | 2 . | 0 . | 1 . | 2 . | |
| CNS | 85 (86) | 12 (12) | 2 (2) | 79 (81) | 17 (18) | 1 (1) | 0.68 (0.31–1.48), P = 0.330 |
| Headache | 89 (90) | 8 (8) | 2 (2) | 83 (86) | 13 (13) | 1 (1) | |
| Sleeping disorder | 93 (94) | 6 (6) | 0 (0) | 91 (94) | 6 (6) | 0 (0) | |
| Other CNS | 97 (98) | 2 (2) | 0 (0) | 96 (99) | 1 (1) | 0 (0) | |
| GI | 46 (47) | 46 (47) | 6 (6) | 14 (14) | 68 (70) | 15 (16) | 0.32 (0.18–0.56), P < 0.0001 |
| Nausea/vomiting | 69 (70) | 27 (27) | 3 (3) | 59 (61) | 31 (32) | 7 (7) | |
| Diarrhoea | 80 (81) | 18 (18) | 1 (1) | 25 (26) | 61 (63) | 11 (11) | |
| Constipation | 95 (96) | 3 (3) | 1 (1) | 95 (98) | 2 (2) | 0 (0) | |
| Other GI | 83 (84) | 15 (15) | 1 (1) | 60 (62) | 30 (31) | 7 (7) | |
| Skin | 96 (97) | 3 (3) | 0 (0) | 88 (91) | 8 (8) | 1 (1) | 0.36 (0.09–1.38) P = 0.135 |
| Rash | 97 (98) | 2 (2) | 0 (0) | 89 (92) | 7 (7) | 1 (1) | |
| Other skin | 98 (99) | 1 (1) | 0 (0) | 96 (99) | 1 (1) | 0 (0) | |
| Tiredness, fatigue, etc. | 63 (64) | 30 (30) | 6 (6) | 59 (61) | 35 (36) | 3 (3) | 1.05 (0.58–1.87) P = 0.882 |
| Other | 81 (82) | 14 (14) | 4 (4) | 70 (72) | 24 (25) | 3 (3) | 0.59 (0.30–1.16) P = 0.128 |
OR for maraviroc arm relative to Kaletra®. Ordered logistic regression assuming proportional odds for GI (grades 0, 1, 2). Logistic regression for CNS, skin, tiredness, other (binary outcome grade 1+).
A total of 127 and 158 grade 1, 2 or 3 laboratory AEs were observed in patients randomized to the maraviroc and the Kaletra® arm, respectively, by day 28. Most grade 3 laboratory AEs were related to renal function measurements. Thirteen participants had grade 3 hypophosphataemia, but with no difference between randomization arms. There were 57 participants with hypercholesterolaemia with a significant difference between randomization arms (17 and 40 in patients randomized to maraviroc and Kaletra®, respectively; P < 0.0001) but the only grade 3 hypercholesterolaemia reported by day 28 was in a participant in the maraviroc arm; this patient had persistent hypercholesterolaemia during the entire study period, including the month 4 visit, which suggests that this was a pre-existing condition not related to study medication (no baseline cholesterol measurement was available). Laboratory abnormalities reflecting metabolic disturbances (lipids and glucose) were less frequently observed in participants on maraviroc (OR = 0.27; 95% CI = 0.14–0.50; P < 0.0001), and predominantly due to grade 1–2 hypertriglyceridaemia. The distribution of laboratory AEs is summarized in Table 4. By 4 months after starting PEP, 93% of grade 2, 3 and 4 laboratory AEs were documented as having resolved.
| LAE type . | Maraviroc arm n (%), N = 99 . | Kaletra® arm n (%), N = 97 . | OR (95% CI)a, P value . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade: . | 0 . | 1 . | 2 . | 3 . | 0 . | 1 . | 2 . | 3 . | |
| Renal | 45 (45) | 23 (23) | 19 (19) | 12 (12) | 46 (47) | 21 (22) | 19 (20) | 11 (11) | 1.06 (0.63–1.78), P = 0.820 |
| Sodium | 94 (96) | 4 (4) | 1 (1) | 0 (0) | 95 (98) | 2 (2) | 0 (0) | 0 (0) | |
| Urea | 92 (93) | 0 (0) | 0 (0) | 7 (7) | 95 (98) | 0 (0) | 0 (0) | 2 (2) | |
| Creatinine | 98 (99) | 1 (1) | 0 (0) | 0 (0) | 97 (100) | 0 (0) | 0 (0) | 0 (0) | |
| Phosphateb | 66 (67) | 13 (13) | 16 (1) | 4 (4) | 56 (58) | 14 (14) | 18 (19) | 9 (9) | |
| UPCR | 82 (83) | 14 (14) | 2 (2) | 1 (1) | 89 (86) | 9 (9) | 2 (2) | 0 (0) | |
| Liver | 83 (84) | 13 (13) | 2 (2) | 1 (1) | 82 (85) | 12 (12) | 3 (3) | 0 (0) | 1.05 (0.49–2.27), P = 0.894 |
| Bilirubin | 89 (90) | 8 (8) | 1 (1) | 1 (1) | 88 (91) | 7 (7) | 2 (2) | 0 (0) | |
| ALT | 93 (94) | 5 (5) | 1 (1) | 0 (0) | 90 (93) | 6 (6) | 1 (1) | 0 (0) | |
| Metabolic | 76 (77) | 20 (20) | 1 (1) | 2 (2) | 47 (49) | 28 (29) | 21 (22) | 1 (1) | 0.27 (0.14–0.50), P < 0.0001 |
| Glucose | 95 (96) | 4 (4) | 0 (0) | 0 (0) | 89 (92) | 7 (7) | 1 (1) | 0 (0) | |
| Total cholesterol | 82 (83) | 14 (14) | 2 (2) | 1 (1) | 57 (59) | 20 (21) | 20 (21) | 0 (0) | |
| LDL | 90 (91) | 8 (8) | 0 (0) | 1 (1) | 78 (80) | 12 (12) | 6 (6) | 1 (1) | |
| Triglyceride | 94 (95) | 5 (5) | 0 (0) | 0 (0) | 73 (75) | 22 (23) | 2 (2) | 0 (0) | |
| Bone | 65 (66) | 14 (14) | 16 (16) | 4 (4) | 55 (57) | 15 (15) | 18 (19) | 9 (9) | 0.66 (0.38–1.15), P = 0.145 |
| Phosphateb | 66 (67) | 13 (13) | 16 (16) | 4 (4) | 56 (58) | 14 (14) | 18 (19) | 9 (9) | |
| Calcium | 98 (99) | 1 (1) | 0 (0) | 0 (0) | 95 (99) | 2 (2) | 0 (0) | 0 (0) | |
| LAE type . | Maraviroc arm n (%), N = 99 . | Kaletra® arm n (%), N = 97 . | OR (95% CI)a, P value . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade: . | 0 . | 1 . | 2 . | 3 . | 0 . | 1 . | 2 . | 3 . | |
| Renal | 45 (45) | 23 (23) | 19 (19) | 12 (12) | 46 (47) | 21 (22) | 19 (20) | 11 (11) | 1.06 (0.63–1.78), P = 0.820 |
| Sodium | 94 (96) | 4 (4) | 1 (1) | 0 (0) | 95 (98) | 2 (2) | 0 (0) | 0 (0) | |
| Urea | 92 (93) | 0 (0) | 0 (0) | 7 (7) | 95 (98) | 0 (0) | 0 (0) | 2 (2) | |
| Creatinine | 98 (99) | 1 (1) | 0 (0) | 0 (0) | 97 (100) | 0 (0) | 0 (0) | 0 (0) | |
| Phosphateb | 66 (67) | 13 (13) | 16 (1) | 4 (4) | 56 (58) | 14 (14) | 18 (19) | 9 (9) | |
| UPCR | 82 (83) | 14 (14) | 2 (2) | 1 (1) | 89 (86) | 9 (9) | 2 (2) | 0 (0) | |
| Liver | 83 (84) | 13 (13) | 2 (2) | 1 (1) | 82 (85) | 12 (12) | 3 (3) | 0 (0) | 1.05 (0.49–2.27), P = 0.894 |
| Bilirubin | 89 (90) | 8 (8) | 1 (1) | 1 (1) | 88 (91) | 7 (7) | 2 (2) | 0 (0) | |
| ALT | 93 (94) | 5 (5) | 1 (1) | 0 (0) | 90 (93) | 6 (6) | 1 (1) | 0 (0) | |
| Metabolic | 76 (77) | 20 (20) | 1 (1) | 2 (2) | 47 (49) | 28 (29) | 21 (22) | 1 (1) | 0.27 (0.14–0.50), P < 0.0001 |
| Glucose | 95 (96) | 4 (4) | 0 (0) | 0 (0) | 89 (92) | 7 (7) | 1 (1) | 0 (0) | |
| Total cholesterol | 82 (83) | 14 (14) | 2 (2) | 1 (1) | 57 (59) | 20 (21) | 20 (21) | 0 (0) | |
| LDL | 90 (91) | 8 (8) | 0 (0) | 1 (1) | 78 (80) | 12 (12) | 6 (6) | 1 (1) | |
| Triglyceride | 94 (95) | 5 (5) | 0 (0) | 0 (0) | 73 (75) | 22 (23) | 2 (2) | 0 (0) | |
| Bone | 65 (66) | 14 (14) | 16 (16) | 4 (4) | 55 (57) | 15 (15) | 18 (19) | 9 (9) | 0.66 (0.38–1.15), P = 0.145 |
| Phosphateb | 66 (67) | 13 (13) | 16 (16) | 4 (4) | 56 (58) | 14 (14) | 18 (19) | 9 (9) | |
| Calcium | 98 (99) | 1 (1) | 0 (0) | 0 (0) | 95 (99) | 2 (2) | 0 (0) | 0 (0) | |
LAEs, laboratory adverse events; UPCR, urine protein to creatinine ratio.
OR for maraviroc arm relative to Kaletra®. Ordered logistic regression assuming proportional odds for renal (grades 0, 1, 2, 3), metabolic (grades 0, 1, 2+), bone (grades 0, 1, 2+). Logistic regression for liver (binary outcome grade 1+).
Phosphate results contribute to both renal and bone groups.
| LAE type . | Maraviroc arm n (%), N = 99 . | Kaletra® arm n (%), N = 97 . | OR (95% CI)a, P value . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade: . | 0 . | 1 . | 2 . | 3 . | 0 . | 1 . | 2 . | 3 . | |
| Renal | 45 (45) | 23 (23) | 19 (19) | 12 (12) | 46 (47) | 21 (22) | 19 (20) | 11 (11) | 1.06 (0.63–1.78), P = 0.820 |
| Sodium | 94 (96) | 4 (4) | 1 (1) | 0 (0) | 95 (98) | 2 (2) | 0 (0) | 0 (0) | |
| Urea | 92 (93) | 0 (0) | 0 (0) | 7 (7) | 95 (98) | 0 (0) | 0 (0) | 2 (2) | |
| Creatinine | 98 (99) | 1 (1) | 0 (0) | 0 (0) | 97 (100) | 0 (0) | 0 (0) | 0 (0) | |
| Phosphateb | 66 (67) | 13 (13) | 16 (1) | 4 (4) | 56 (58) | 14 (14) | 18 (19) | 9 (9) | |
| UPCR | 82 (83) | 14 (14) | 2 (2) | 1 (1) | 89 (86) | 9 (9) | 2 (2) | 0 (0) | |
| Liver | 83 (84) | 13 (13) | 2 (2) | 1 (1) | 82 (85) | 12 (12) | 3 (3) | 0 (0) | 1.05 (0.49–2.27), P = 0.894 |
| Bilirubin | 89 (90) | 8 (8) | 1 (1) | 1 (1) | 88 (91) | 7 (7) | 2 (2) | 0 (0) | |
| ALT | 93 (94) | 5 (5) | 1 (1) | 0 (0) | 90 (93) | 6 (6) | 1 (1) | 0 (0) | |
| Metabolic | 76 (77) | 20 (20) | 1 (1) | 2 (2) | 47 (49) | 28 (29) | 21 (22) | 1 (1) | 0.27 (0.14–0.50), P < 0.0001 |
| Glucose | 95 (96) | 4 (4) | 0 (0) | 0 (0) | 89 (92) | 7 (7) | 1 (1) | 0 (0) | |
| Total cholesterol | 82 (83) | 14 (14) | 2 (2) | 1 (1) | 57 (59) | 20 (21) | 20 (21) | 0 (0) | |
| LDL | 90 (91) | 8 (8) | 0 (0) | 1 (1) | 78 (80) | 12 (12) | 6 (6) | 1 (1) | |
| Triglyceride | 94 (95) | 5 (5) | 0 (0) | 0 (0) | 73 (75) | 22 (23) | 2 (2) | 0 (0) | |
| Bone | 65 (66) | 14 (14) | 16 (16) | 4 (4) | 55 (57) | 15 (15) | 18 (19) | 9 (9) | 0.66 (0.38–1.15), P = 0.145 |
| Phosphateb | 66 (67) | 13 (13) | 16 (16) | 4 (4) | 56 (58) | 14 (14) | 18 (19) | 9 (9) | |
| Calcium | 98 (99) | 1 (1) | 0 (0) | 0 (0) | 95 (99) | 2 (2) | 0 (0) | 0 (0) | |
| LAE type . | Maraviroc arm n (%), N = 99 . | Kaletra® arm n (%), N = 97 . | OR (95% CI)a, P value . | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Grade: . | 0 . | 1 . | 2 . | 3 . | 0 . | 1 . | 2 . | 3 . | |
| Renal | 45 (45) | 23 (23) | 19 (19) | 12 (12) | 46 (47) | 21 (22) | 19 (20) | 11 (11) | 1.06 (0.63–1.78), P = 0.820 |
| Sodium | 94 (96) | 4 (4) | 1 (1) | 0 (0) | 95 (98) | 2 (2) | 0 (0) | 0 (0) | |
| Urea | 92 (93) | 0 (0) | 0 (0) | 7 (7) | 95 (98) | 0 (0) | 0 (0) | 2 (2) | |
| Creatinine | 98 (99) | 1 (1) | 0 (0) | 0 (0) | 97 (100) | 0 (0) | 0 (0) | 0 (0) | |
| Phosphateb | 66 (67) | 13 (13) | 16 (1) | 4 (4) | 56 (58) | 14 (14) | 18 (19) | 9 (9) | |
| UPCR | 82 (83) | 14 (14) | 2 (2) | 1 (1) | 89 (86) | 9 (9) | 2 (2) | 0 (0) | |
| Liver | 83 (84) | 13 (13) | 2 (2) | 1 (1) | 82 (85) | 12 (12) | 3 (3) | 0 (0) | 1.05 (0.49–2.27), P = 0.894 |
| Bilirubin | 89 (90) | 8 (8) | 1 (1) | 1 (1) | 88 (91) | 7 (7) | 2 (2) | 0 (0) | |
| ALT | 93 (94) | 5 (5) | 1 (1) | 0 (0) | 90 (93) | 6 (6) | 1 (1) | 0 (0) | |
| Metabolic | 76 (77) | 20 (20) | 1 (1) | 2 (2) | 47 (49) | 28 (29) | 21 (22) | 1 (1) | 0.27 (0.14–0.50), P < 0.0001 |
| Glucose | 95 (96) | 4 (4) | 0 (0) | 0 (0) | 89 (92) | 7 (7) | 1 (1) | 0 (0) | |
| Total cholesterol | 82 (83) | 14 (14) | 2 (2) | 1 (1) | 57 (59) | 20 (21) | 20 (21) | 0 (0) | |
| LDL | 90 (91) | 8 (8) | 0 (0) | 1 (1) | 78 (80) | 12 (12) | 6 (6) | 1 (1) | |
| Triglyceride | 94 (95) | 5 (5) | 0 (0) | 0 (0) | 73 (75) | 22 (23) | 2 (2) | 0 (0) | |
| Bone | 65 (66) | 14 (14) | 16 (16) | 4 (4) | 55 (57) | 15 (15) | 18 (19) | 9 (9) | 0.66 (0.38–1.15), P = 0.145 |
| Phosphateb | 66 (67) | 13 (13) | 16 (16) | 4 (4) | 56 (58) | 14 (14) | 18 (19) | 9 (9) | |
| Calcium | 98 (99) | 1 (1) | 0 (0) | 0 (0) | 95 (99) | 2 (2) | 0 (0) | 0 (0) | |
LAEs, laboratory adverse events; UPCR, urine protein to creatinine ratio.
OR for maraviroc arm relative to Kaletra®. Ordered logistic regression assuming proportional odds for renal (grades 0, 1, 2, 3), metabolic (grades 0, 1, 2+), bone (grades 0, 1, 2+). Logistic regression for liver (binary outcome grade 1+).
Phosphate results contribute to both renal and bone groups.
Attendance at study visits was high in both groups, with 80% of participants in the maraviroc arm and 81% in the Kaletra® arm attending the day 28 study visit. Adherence to the allocated PEP regimen at day 28 was similar in both arms with 27% of participants reporting missing at least one dose of Truvada®, whereas 22% of participants missed at least one dose of maraviroc or Kaletra®. There was no difference between arms in the number of days absent from work, or in the number of additional clinic visits during the course of PEP (Table 2).
The number of participants attending their month 4 study visits was 67% and 53% in the maraviroc and Kaletra® arms, respectively. By the end of the study follow-up there were no HIV seroconversions reported.
At the baseline visit, 12% of participants were diagnosed with a sexually transmitted infection (STI). At the day 28 visit, when all participants were screened for STIs again, 13% were diagnosed with an STI (Table 5). During the study period, participants randomized to each group showed similar sexual behaviour patterns. By the day 14 visit 55% and 62% in the maraviroc and Kaletra® arms, respectively (P = 0.344), reported no sexual activity since PEP initiation (Table 6). By combining the study arms, we see that 15% of participants reported ≥3 sexual partners since baseline by day 28 and 37% reported ≥3 sexual partners since baseline by 4 months. The proportion of participants reporting unprotected sex since baseline was 8% at day 28 and 26% at 4 months.
| Variables . | Maraviroc arm n (%) . | Kaletra® arm n (%) . |
|---|---|---|
| Had STI screen at 14 days, n = 191 | ||
| No | 28 (29) | 29 (30) |
| Yes | 69 (71) | 66 (70) |
| Any STIs at 14 days visit if screened, n = 134 | ||
| No | 57 (84) | 57 (86) |
| Yes | 11 (16) | 9 (14) |
| Syphilis at 14 days if tested, n = 52 | ||
| No | 26 (100) | 26 (100) |
| Yes | 0 (0) | 0 (0) |
| Had STI screen at 28 days, n = 172 | ||
| No | 57 (67) | 54 (64) |
| Yes | 28 (33) | 31 (36) |
| Any STIs at 28 days visit if screened, n = 59 | ||
| No | 23 (82) | 28 (90) |
| Yes | 5 (18) | 3 (10) |
| Syphilis at 28 days if tested, n = 49 | ||
| No | 23 (100) | 26 (100) |
| Yes | 0 (0) | 0 (0) |
| Had STI screen at 4 months, n = 120 | ||
| No | 28 (42) | 32 (59) |
| Yes | 38 (58) | 22 (41) |
| Any STIs at 4 months visit if screened, n = 60 | ||
| No | 38 (100) | 22 (100) |
| Yes | 0 (0) | 0 (0) |
| Syphilis at 4 months if tested, n = 25 | ||
| No | 12 (100) | 10 (8) |
| Yes | 0 (0) | 3 (23) |
| Variables . | Maraviroc arm n (%) . | Kaletra® arm n (%) . |
|---|---|---|
| Had STI screen at 14 days, n = 191 | ||
| No | 28 (29) | 29 (30) |
| Yes | 69 (71) | 66 (70) |
| Any STIs at 14 days visit if screened, n = 134 | ||
| No | 57 (84) | 57 (86) |
| Yes | 11 (16) | 9 (14) |
| Syphilis at 14 days if tested, n = 52 | ||
| No | 26 (100) | 26 (100) |
| Yes | 0 (0) | 0 (0) |
| Had STI screen at 28 days, n = 172 | ||
| No | 57 (67) | 54 (64) |
| Yes | 28 (33) | 31 (36) |
| Any STIs at 28 days visit if screened, n = 59 | ||
| No | 23 (82) | 28 (90) |
| Yes | 5 (18) | 3 (10) |
| Syphilis at 28 days if tested, n = 49 | ||
| No | 23 (100) | 26 (100) |
| Yes | 0 (0) | 0 (0) |
| Had STI screen at 4 months, n = 120 | ||
| No | 28 (42) | 32 (59) |
| Yes | 38 (58) | 22 (41) |
| Any STIs at 4 months visit if screened, n = 60 | ||
| No | 38 (100) | 22 (100) |
| Yes | 0 (0) | 0 (0) |
| Syphilis at 4 months if tested, n = 25 | ||
| No | 12 (100) | 10 (8) |
| Yes | 0 (0) | 3 (23) |
STI screen may include at least one of the following tests: Chlamydia trachomatis (up to three sites), gonorrhoea (up to three sites), lymphogranuloma venereum (up to three sites), herpes, human papillomavirus, non-specific urethritis, syphilis, hepatitis B and C.
| Variables . | Maraviroc arm n (%) . | Kaletra® arm n (%) . |
|---|---|---|
| Had STI screen at 14 days, n = 191 | ||
| No | 28 (29) | 29 (30) |
| Yes | 69 (71) | 66 (70) |
| Any STIs at 14 days visit if screened, n = 134 | ||
| No | 57 (84) | 57 (86) |
| Yes | 11 (16) | 9 (14) |
| Syphilis at 14 days if tested, n = 52 | ||
| No | 26 (100) | 26 (100) |
| Yes | 0 (0) | 0 (0) |
| Had STI screen at 28 days, n = 172 | ||
| No | 57 (67) | 54 (64) |
| Yes | 28 (33) | 31 (36) |
| Any STIs at 28 days visit if screened, n = 59 | ||
| No | 23 (82) | 28 (90) |
| Yes | 5 (18) | 3 (10) |
| Syphilis at 28 days if tested, n = 49 | ||
| No | 23 (100) | 26 (100) |
| Yes | 0 (0) | 0 (0) |
| Had STI screen at 4 months, n = 120 | ||
| No | 28 (42) | 32 (59) |
| Yes | 38 (58) | 22 (41) |
| Any STIs at 4 months visit if screened, n = 60 | ||
| No | 38 (100) | 22 (100) |
| Yes | 0 (0) | 0 (0) |
| Syphilis at 4 months if tested, n = 25 | ||
| No | 12 (100) | 10 (8) |
| Yes | 0 (0) | 3 (23) |
| Variables . | Maraviroc arm n (%) . | Kaletra® arm n (%) . |
|---|---|---|
| Had STI screen at 14 days, n = 191 | ||
| No | 28 (29) | 29 (30) |
| Yes | 69 (71) | 66 (70) |
| Any STIs at 14 days visit if screened, n = 134 | ||
| No | 57 (84) | 57 (86) |
| Yes | 11 (16) | 9 (14) |
| Syphilis at 14 days if tested, n = 52 | ||
| No | 26 (100) | 26 (100) |
| Yes | 0 (0) | 0 (0) |
| Had STI screen at 28 days, n = 172 | ||
| No | 57 (67) | 54 (64) |
| Yes | 28 (33) | 31 (36) |
| Any STIs at 28 days visit if screened, n = 59 | ||
| No | 23 (82) | 28 (90) |
| Yes | 5 (18) | 3 (10) |
| Syphilis at 28 days if tested, n = 49 | ||
| No | 23 (100) | 26 (100) |
| Yes | 0 (0) | 0 (0) |
| Had STI screen at 4 months, n = 120 | ||
| No | 28 (42) | 32 (59) |
| Yes | 38 (58) | 22 (41) |
| Any STIs at 4 months visit if screened, n = 60 | ||
| No | 38 (100) | 22 (100) |
| Yes | 0 (0) | 0 (0) |
| Syphilis at 4 months if tested, n = 25 | ||
| No | 12 (100) | 10 (8) |
| Yes | 0 (0) | 3 (23) |
STI screen may include at least one of the following tests: Chlamydia trachomatis (up to three sites), gonorrhoea (up to three sites), lymphogranuloma venereum (up to three sites), herpes, human papillomavirus, non-specific urethritis, syphilis, hepatitis B and C.
| Variables . | Maraviroc arm, n (%) . | Kaletra® arm, n (%) . |
|---|---|---|
| Number of sex partners since baseline reported at 14 days visit, n = 188 | ||
| No sex | 53 (55) | 57 (62) |
| 1 | 3 (31) | 25 (27) |
| 2 | 7 (7) | 5 (5) |
| 3–9 | 5 (5) | 5 (5) |
| 10+ | 1 (1) | 0 (0) |
| Number of sex partners since baseline reported at 28 days visit, n = 172 | ||
| No sex | 35 (41) | 44 (51) |
| 1 | 32 (37) | 24 (28) |
| 2 | 6 (7) | 6 (7) |
| 3–9 | 12 (14) | 11 (13) |
| 10+ | 1 (1) | 1 (1) |
| Number of sex partners since baseline reported at 4 months visit, n = 125 | ||
| No sex | 13 (19) | 12 (23) |
| 1 | 21 (30) | 14 (25) |
| 2 | 9 (13) | 10 (18) |
| 3–9 | 15 (21) | 12 (22) |
| 10+ | 12 (17) | 7 (13) |
| Unprotected sex since baseline reported at 14 days, n = 191 | ||
| No | 88 (92) | 88 (93) |
| Yes | 8 (8) | 4 (4) |
| Don’t know | 0 (0) | 3 (3) |
| Unprotected sex since baseline reported at 28 days, n = 172 | ||
| No | 78 (91) | 80 (93) |
| Yes | 8 (9) | 6 (7) |
| Don’t know | 0 (0) | 0 (0) |
| Unprotected sex since baseline reported at 4 months, n = 126 | ||
| No | 49 (70) | 43 (77) |
| Yes | 21 (30) | 12 (21) |
| Don’t know | 0 (0) | 1 (2) |
| Variables . | Maraviroc arm, n (%) . | Kaletra® arm, n (%) . |
|---|---|---|
| Number of sex partners since baseline reported at 14 days visit, n = 188 | ||
| No sex | 53 (55) | 57 (62) |
| 1 | 3 (31) | 25 (27) |
| 2 | 7 (7) | 5 (5) |
| 3–9 | 5 (5) | 5 (5) |
| 10+ | 1 (1) | 0 (0) |
| Number of sex partners since baseline reported at 28 days visit, n = 172 | ||
| No sex | 35 (41) | 44 (51) |
| 1 | 32 (37) | 24 (28) |
| 2 | 6 (7) | 6 (7) |
| 3–9 | 12 (14) | 11 (13) |
| 10+ | 1 (1) | 1 (1) |
| Number of sex partners since baseline reported at 4 months visit, n = 125 | ||
| No sex | 13 (19) | 12 (23) |
| 1 | 21 (30) | 14 (25) |
| 2 | 9 (13) | 10 (18) |
| 3–9 | 15 (21) | 12 (22) |
| 10+ | 12 (17) | 7 (13) |
| Unprotected sex since baseline reported at 14 days, n = 191 | ||
| No | 88 (92) | 88 (93) |
| Yes | 8 (8) | 4 (4) |
| Don’t know | 0 (0) | 3 (3) |
| Unprotected sex since baseline reported at 28 days, n = 172 | ||
| No | 78 (91) | 80 (93) |
| Yes | 8 (9) | 6 (7) |
| Don’t know | 0 (0) | 0 (0) |
| Unprotected sex since baseline reported at 4 months, n = 126 | ||
| No | 49 (70) | 43 (77) |
| Yes | 21 (30) | 12 (21) |
| Don’t know | 0 (0) | 1 (2) |
| Variables . | Maraviroc arm, n (%) . | Kaletra® arm, n (%) . |
|---|---|---|
| Number of sex partners since baseline reported at 14 days visit, n = 188 | ||
| No sex | 53 (55) | 57 (62) |
| 1 | 3 (31) | 25 (27) |
| 2 | 7 (7) | 5 (5) |
| 3–9 | 5 (5) | 5 (5) |
| 10+ | 1 (1) | 0 (0) |
| Number of sex partners since baseline reported at 28 days visit, n = 172 | ||
| No sex | 35 (41) | 44 (51) |
| 1 | 32 (37) | 24 (28) |
| 2 | 6 (7) | 6 (7) |
| 3–9 | 12 (14) | 11 (13) |
| 10+ | 1 (1) | 1 (1) |
| Number of sex partners since baseline reported at 4 months visit, n = 125 | ||
| No sex | 13 (19) | 12 (23) |
| 1 | 21 (30) | 14 (25) |
| 2 | 9 (13) | 10 (18) |
| 3–9 | 15 (21) | 12 (22) |
| 10+ | 12 (17) | 7 (13) |
| Unprotected sex since baseline reported at 14 days, n = 191 | ||
| No | 88 (92) | 88 (93) |
| Yes | 8 (8) | 4 (4) |
| Don’t know | 0 (0) | 3 (3) |
| Unprotected sex since baseline reported at 28 days, n = 172 | ||
| No | 78 (91) | 80 (93) |
| Yes | 8 (9) | 6 (7) |
| Don’t know | 0 (0) | 0 (0) |
| Unprotected sex since baseline reported at 4 months, n = 126 | ||
| No | 49 (70) | 43 (77) |
| Yes | 21 (30) | 12 (21) |
| Don’t know | 0 (0) | 1 (2) |
| Variables . | Maraviroc arm, n (%) . | Kaletra® arm, n (%) . |
|---|---|---|
| Number of sex partners since baseline reported at 14 days visit, n = 188 | ||
| No sex | 53 (55) | 57 (62) |
| 1 | 3 (31) | 25 (27) |
| 2 | 7 (7) | 5 (5) |
| 3–9 | 5 (5) | 5 (5) |
| 10+ | 1 (1) | 0 (0) |
| Number of sex partners since baseline reported at 28 days visit, n = 172 | ||
| No sex | 35 (41) | 44 (51) |
| 1 | 32 (37) | 24 (28) |
| 2 | 6 (7) | 6 (7) |
| 3–9 | 12 (14) | 11 (13) |
| 10+ | 1 (1) | 1 (1) |
| Number of sex partners since baseline reported at 4 months visit, n = 125 | ||
| No sex | 13 (19) | 12 (23) |
| 1 | 21 (30) | 14 (25) |
| 2 | 9 (13) | 10 (18) |
| 3–9 | 15 (21) | 12 (22) |
| 10+ | 12 (17) | 7 (13) |
| Unprotected sex since baseline reported at 14 days, n = 191 | ||
| No | 88 (92) | 88 (93) |
| Yes | 8 (8) | 4 (4) |
| Don’t know | 0 (0) | 3 (3) |
| Unprotected sex since baseline reported at 28 days, n = 172 | ||
| No | 78 (91) | 80 (93) |
| Yes | 8 (9) | 6 (7) |
| Don’t know | 0 (0) | 0 (0) |
| Unprotected sex since baseline reported at 4 months, n = 126 | ||
| No | 49 (70) | 43 (77) |
| Yes | 21 (30) | 12 (21) |
| Don’t know | 0 (0) | 1 (2) |
Discussion
The completion rate of PEP was high in both arms of this trial, and did not differ between arms. Furthermore, there were no differences observed in the rate of PEP completion in the absence of grade 3 or 4 clinical and laboratory adverse abnormalities comparing maraviroc- versus Kaletra®-based combinations. This was the combined primary endpoint of the trial.
The favourable maraviroc tolerability and safety profile, demonstrated in previous studies in both treatment and prevention, make it an attractive option to be considered as part of a PEP regimen.15–18,22 In this study, the benefit of using maraviroc was limited to fewer mild to moderate gastrointestinal side effects in the maraviroc arm (OR = 0.32; 95% CI = 0.18–0.56; P < 0.0001). The excess of gastrointestinal symptoms in the Kaletra® arm led to increased use of antidiarrhoeal and antiemetic medication. Low completion rates of PEP have been reported in many settings; therefore, PEP regimens with a more favourable tolerability profile may help in this regard.8,22–25 Recent data suggest that another ritonavir-boosted PI, darunavir, may be better tolerated.26 However, all ritonavir-boosted PI-based combinations have a higher risk for drug–drug interactions than alternatives such as raltegravir or rilpivirine.27,28
We did not observe any serious CAEs. Laboratory abnormalities were mild to moderate grade 1–3, mostly reflecting hypertriglyceridaemia and hypophosphataemia. Laboratory abnormalities associated with PEP exposure were transient and returned to normal after cessation of PEP.
It was decided not to include lipids in the primary endpoint because hyperlipidaemia is a recognized effect of Kaletra®, which would be transient and with no clinically significant sequelae in the context of short-term treatment.
The results of the study indicate that when using Truvada® and maraviroc the routine prescription of antiemetic or antidiarrhoeal drugs is not necessary. Where Kaletra® is used, routine provision of antiemetic and antidiarrhoeal drugs is standard practice in the UK and this study suggests that this should continue to be considered.
Although proximal renal tubular dysfunction and Fanconi’s syndrome are well reported in HIV-positive individuals on tenofovir disoproxil-based ART,29 these have not been reported in the setting of PEP and were not seen in this study.
We recruited a high-risk population, of whom many (54%) had received PEP previously, often more than once (45%). This may in part explain the high rate of treatment completion and adherence we observed. We also observed high rates of STIs, both at baseline and during the follow-up period, but no HIV seroconversions, consistent with previous reports.30 High-risk individuals may be suitable candidates for PrEP rather than repeated courses of PEP.31
The strengths of this trial are the low withdrawal and lost-to-follow-up rates and the design being representative of routine clinical care. Potential limitations include the restriction of the study population to those at risk from sexual exposure, and almost all MSM. There were no occupational exposure cases. The fact that many of the participants had received PEP previously, and were seeking PEP again means that those who had had more severe AEs of current PEP regimens may have been less likely to be seeking PEP again, and so less likely to be included in the study. Previous users of these medications may also have a different perception of the AEs. Although at the close of recruitment 213 participants out of an initial planned sample size of 280 had been enrolled, due to a change in the standard of care regimen, we had a better than expected follow-up rate. We had 196 participants who provided data for the primary outcome, which is only just short of the planned 210 and did not therefore materially compromise the power of the study.
In conclusion, maraviroc-based PEP has demonstrated advantages in comparison with a Kaletra®-based PEP regimen in terms of tolerability, even if that did not translate into a significant increase in completion rates in this trial.
Acknowledgements
We thank all patients and staff from all the centres participating in the MiPEP trial. This study was presented at the 15th European AIDS Conference, Barcelona, Spain 2015 (Abstract PE18/11).
Members of the MiPEP Trial Team
The Mortimer Market Centre, Central and North West London NHS Foundation Trust, London: Sarah Pett, Dianne Morris, Sarah McNamara, Gina Carrick, Nahum DeEsteban, Pierre Pellegrino, Lewis Haddow, Ian Williams, Laura Waters, June Minton, Rita Gupta. The Claude Nicol Unit, Brighton and Sussex University Hospitals NHS Trust, Brighton: Fiona Cresswell, Elaney Youssef, Nicky Perry, Celia Richardson, Wendy Hadley, Julia Pollard, Claire Richardson, Elisa Souto. Manchester Centre for Sexual Health, Manchester Royal Infirmary, Manchester: Lisa Southon, Stephanie Yau, Matthew Phillips, Carolyn Davies. The John Hunter Clinic, Chelsea and Westminster NHS Foundation Trust, London: Chris Higgs, Alex Meijer, Aminata Sy, Kathryn McCormick. Ambrose King Centre, Royal London Hospital, London: Angelina Twumasi.
Trial Steering Committee: Nick Paton (chair), Fiona Burns, Chris Sanford, Martin Fisher, Andrew Copas (trial statistician), Richard Gilson (chief investigator).
Data Monitoring Committee: Charles Lacey (chair), David Dunn, Alan Winston.
Funding
This study was funded by an investigator-initiated research grant from Pfizer®.
Transparency declarations
P. B. was MiPEP Trial Chief Investigator until January 2014. Between January 2014 and December 2015, P. B. was employed full time by Gilead Sciences UK, in the HIV franchise; from January 2016 onwards, he was employed full time by ViiV Healthcare. All other authors: none to declare.
Author contributions
P. B., R. G., A. A. P., A. Copas and A. M. designed the study. A. M., A. A. P., P. B. and R. G. co-ordinated and oversaw the study. N. B. and A. Copas did the statistical analysis. All authors interpreted data. A. M., P. B., A. A. P., N. B., A. Copas and R. G. drafted the report. All the authors provided input into the report and approved the final version of the report.
Disclaimer
The views and opinions expressed herein are those of the authors.
References
Kaletra Summary of Product Characteristics. Last Updated on eMC 29-Dec-
Author notes
Professor Martin Fisher died in April 2015—he made a significant contribution to this study and our speciality as a whole—he is greatly missed.
Members are listed in Acknowledgements section.



