-
PDF
- Split View
-
Views
-
Cite
Cite
Victoire de Lastours, Elena Maugy, Vincent Mathy, Françoise Chau, Benjamin Rossi, François Guérin, Vincent Cattoir, Bruno Fantin, for the CIPHARES Study Group, Ecological impact of ciprofloxacin on commensal enterococci in healthy volunteers, Journal of Antimicrobial Chemotherapy, Volume 72, Issue 6, June 2017, Pages 1574–1580, https://doi.org/10.1093/jac/dkx043
Close - Share Icon Share
Background: The ecological impact of ciprofloxacin on commensal enterococci is unknown.
Methods: Forty-eight healthy volunteers received ciprofloxacin from day (D) 0 to D14; stools were collected on D7, D14 and D42. Fluoroquinolone-susceptible and -resistant enterococci (FQ-SE and FQ-RE) were detected and quantified by culture, and identified by MALDI-TOF MS. The relative abundance of FQ-RE over FQ-SE was determined. The genetic basis of fluoroquinolone resistance was deciphered by partial sequencing of gyrA and parC genes. Clonal relatedness was determined by random amplification of polymorphic DNA PCR. Clinical trial no.: NCT00190151.
Results: Enterococci were carried by 47/48 (98%) subjects. Total counts were reduced during ciprofloxacin therapy (4.0 and 3.9 log cfu/g on D7 and D14 versus 5.9 log cfu/g before and 6.9 log cfu/g after treatment; P < 0.05). Twenty-one out of 48 (44%) carried FQ-RE; among them, 21/21 carried Enterococcus faecium, 19 carried Enterococcus faecalis and 11 carried other species. Five out of 48 (10%) harboured FQ-RE (ciprofloxacin MIC >4 mg/L) before treatment (all E. faecium), 6 on D7 (3 E. faecium and 3 E. faecalis), 8 on D14 (4 E. faecium and 4 E. faecalis) and 10 (21%) on D42 (9 E. faecium and 1 E. faecalis). The relative abundance of FQ-RE increased from 44% on D0 to 73% and 75% on D7 and D14, respectively. No acquisition of fluoroquinolone resistance among endogenous D0 strains was evidenced. All (14/14) distinct Fluoroquinolone-resistant E. faecalis clones were gyrA/parC double mutants with high-level resistance (ciprofloxacin MIC >64 mg/L). In contrast, 34/35 E. faecium exhibited low-level resistance (ciprofloxacin MIC 4–32 mg/L) with no gyrA/parC mutation, but overexpressed the chromosomal Efmqnr gene. As compared with Fluoroquinolone-susceptible strains, Fluoroquinolone-resistant E. faecium were more frequently ampicillin resistant and Fluoroquinolone-resistant E. faecalis were more highly resistant to gentamicin.
Conclusions: Although intrinsically poorly susceptible to fluoroquinolones, gut populations of enterococci are highly impacted both quantitatively and qualitatively by ciprofloxacin.
Introduction
Enterococci, mainly Enterococcus faecalis and Enterococcus faecium, have become major opportunistic pathogens in human medicine as they are responsible for numerous infections, including urinary tract and intra-abdominal infections, bacteraemia and endocarditis. They are increasingly difficult to treat due to the rapid emergence of MDR and hospital-adapted clones, especially in E. faecium.1,2 Enterococci are primarily gut colonizers in most humans and also in many animals. Since they are both commensals and opportunistic pathogens, the gastrointestinal tract plays a crucial role in the pathogenesis of enterococcal infections, but also in the emergence of antimicrobial resistance and the dissemination of MDR enterococci, especially VRE. Enterococci are intrinsically resistant to many antibiotics, such as cephalosporins, aminoglycosides (low level) and sulphonamides, and are prone to easily acquire resistance determinants, especially van operons in VRE. This confers a high selective advantage in particular in the hospital setting, where increasing numbers of patients are immunocompromised, receive many antibiotics, are hospitalized in intensive care units and carry foreign bodies such as intravenous and urinary catheters. These factors increase the risk of infections by MDR organisms such as enterococci, explaining their rise as major nosocomial pathogens.1
Fluoroquinolones, especially ciprofloxacin, have a major ecological impact on the human gut microbiota as they reach very high local concentrations.3,4 Both experimental and epidemiological data now exist concerning the impact of fluoroquinolones on commensal Enterobacteriaceae, leading to the rapid disappearance of susceptible strains from the gut microbiota and its recolonization with resistant clones.5–9 Fluoroquinolones, of which extensive use around the world has led to the rapid emergence of bacterial resistance, are poorly active against enterococci.10 Acquisition of high-level fluoroquinolone resistance in enterococci mainly results from chromosomal mutations in primary fluoroquinolone targets, i.e. DNA gyrase and topoisomerase IV. Other mechanisms of fluoroquinolone resistance, such as active efflux (e.g. EmeA pump) and target protection (Qnr-like determinants), have also been described in enterococci.11,12
Use of ciprofloxacin has been associated with increases in VRE density in faecal flora in animal models, but contradictory findings were reported in patients.13,14 Additionally, very little is known about the impact of fluoroquinolone therapy both on the dynamics of enterococcal gut populations and the emergence of fluoroquinolone resistance. We hypothesized that despite a reduced susceptibility to fluoroquinolones, enterococcal populations may accumulate different mechanisms of resistance over time, with the consequence of co-selecting clones resistant to other antibiotics. Therefore, in this work, we analysed the quantitative and qualitative impact of ciprofloxacin treatment received by 48 healthy volunteers for 14 days on commensal enterococcal populations in terms of total densities, species distribution and emergence of fluoroquinolone resistance in vivo.
Patients and methods
Subjects
Forty-eight healthy volunteers received different therapeutic regimens of oral ciprofloxacin for 14 days, ranging from 500 to 1500 mg/day.9 Subjects had not received any antimicrobial in the 3 months before the trial and received none during the whole follow-up. Faecal samples were collected on day (D) 0, D8, D14 and D42. At D42, no ciprofloxacin was detected in the faeces.9 Clinical trial no. NCT00190151.
Screening, identification and antimicrobial susceptibility testing for enterococci
Immediately after stool sampling, ∼10–20 mg of faeces was diluted into 1 mL of brain heart infusion (BHI) broth with 20% glycerol and stored at −80°C.9 This work was done with freshly thawed stool samples. After thawing, 100 μL was plated on bile-esculin-azide (BEA) agar plates (Bio-Rad, Marnes-la-Coquette, France), one with no antibiotic and one containing 4 mg/L levofloxacin to detect fluoroquinolone-resistant mutants, and cultured for up to 48 h at 37°C. The faecal densities of enterococci were determined by plating serial dilutions of the broth onto BEA agar. According to the quantity of stools in each sample (10–20 mg), we estimated the lower limit of detection of enterococci strains to be 103 cfu/g of faeces. Up to five colonies with distinct morphotypes growing on plates without levofloxacin and three growing on plates with levofloxacin were identified by MALDI-TOF MS (Microflex LT; Bruker Daltonics, Bremen, Germany) and tested for in vitro antimicrobial susceptibility by the disc diffusion method according to the CA-SFM guidelines (www.sfm-microbiologie.org/). MICs of ciprofloxacin (with or without reserpine (10 mg/L) to detect an active efflux) were determined by the broth microdilution method and results were interpreted using EUCAST breakpoints (www.eucast.org/).
Relative abundance of FQ-resistant enterococci (FQ-RE)
Relative densities of total enterococci and FQ-RE were determined by plating serial dilutions onto BEA agar, with or without 4 mg/L levofloxacin. FQ-RE relative abundance was calculated as the ratio of the FQ-RE counts divided by the total number of enterococci, expressed as a percentage.15 Clones were considered dominant if they represented ≥50% of the total enterococcal population.
Molecular characterization of FQ-RE
All enterococcal clinical isolates from patients carrying FQ-RE at least once were compared phenotypically (species identification, antimicrobial susceptibility profile) and were genotypically compared by random amplification of polymorphic DNA PCR (RAPD–PCR) if they belonged to the same species and had similar antibiotic resistance profiles.16 Strains with distinct RAPD–PCR patterns were considered to be distinct clones. Mutations in the quinolone-resistance determining region (QRDR) of both gyrA and parC genes were screened for by PCR sequencing for each unique clone, as described elsewhere.17 Additionally, quantitative RT–PCR (qRT–PCR) experiments were performed using the QuantiFast SYBR Green RT–PCR Kit according to the manufacturer’s instructions. Transcript levels of Efmqnr (E. faecium) and Efsqnr (E. faecalis) were determined in the presence or absence of ciprofloxacin at subinhibitory concentrations (i.e. 1/8 MIC) by the ΔΔ Ct method, using adk (E. faecium) or 23S rRNA (E. faecalis) as housekeeping control genes. The following pairs of primers were used: Efmqnr-F (5′-TGGGAACACGTTATTCGAGA-3′) and Efmqnr-R (5′-GCGCTTGATAAGACAGAGTGC-3′) for Efmqnr; Efsqnr-F (5′-TGTCAAATTGCAACGAAGGA-3′) and Efsqnr-R (5′-GGGATGCTGTTTTCGATCAT-3′) for Efsqnr; adk-F (5′-GCGATGCAAAACGAAACAG-3′) and adk-R (5′-CGCTCTTTCACGATTCCATT-3′) for adk; and 23S-F (5′-CCCCAAGAGTCCACATCG-3′) and 23S-R (5′-GCGTTTATCCCTTCCCTAG-3′) for 23S rRNA.18 Six different strains were tested for each species and each experiment was done in triplicate.
Statistics
Densities of enterococci and relative abundances of FQ-RE over total enterococci between collection dates were compared using Student’s t-test or the Wilcoxon rank-sum test for continuous variables and the χ2 test or Fisher’s exact test for categorical variables, when appropriate. A P value <0.05 was considered to be significant. Statistical analyses were performed using STATA 13 (StataCorp, College Station, TX, USA) software.
Ethics
All subjects had given informed consent and the study was approved by the institutional review Board (IRB #00008522).
Results
Enterococcal populations
Among the 48 volunteers, 47 (98%) carried enterococci in their gut at one collection date or more while 21 (44%) carried FQ-RE at least once. In this work, we concentrated on enterococcal isolates collected from the 21 subjects who at one point had FQ-RE in their stools.
Quantitative impact of ciprofloxacin on enterococcal populations
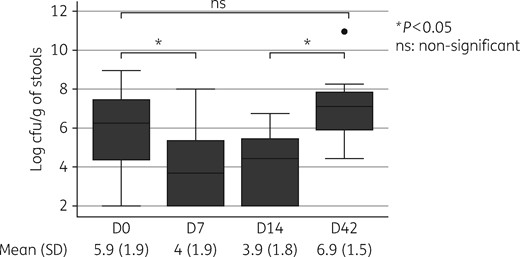
Densities of enterococcal species in the stools amongst the 21 healthy volunteers (in log cfu/g of stools by day of collection). Ciprofloxacin treatment was given from D0 (after sampling) to D14.
Impact of ciprofloxacin on enterococcal diversity
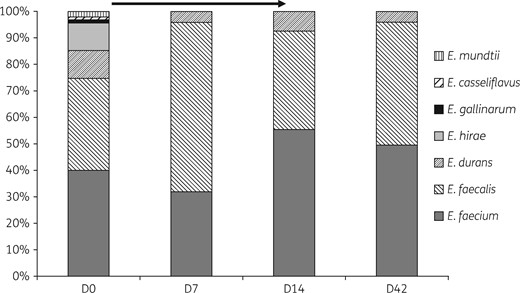
Proportion of the different Enterococcus spp. found in the faeces of 48 healthy volunteers by day of collection. Ciprofloxacin treatment was received from D0 (after sampling) to D14 (indicated as an arrow).
Resistant enterococcal strains

Transcript levels (fold changes in expression) of Efmqnr (E. faecium) and Efsqnr (E. faecalis) in the presence and absence of subinhibitory concentrations of ciprofloxacin (i.e. 1/8 MIC).
Dynamics of the emergence of FQ-RE
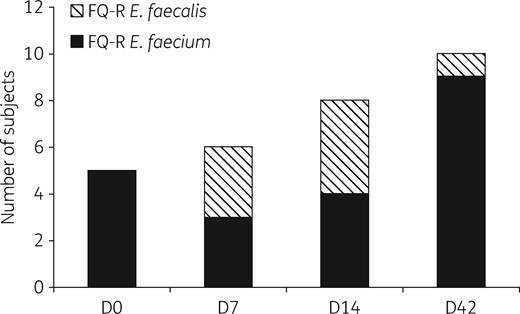
Dynamics of the emergence of FQ-RE among 21 subjects treated with ciprofloxacin from D0 to D14, with FQ-RE isolated at least on 1 day of sampling. Emergence of resistance was maximal during treatment for E. faecalis and after the end of treatment for E. faecium. On D42, 10 subjects carried FQ-RE versus 5 before treatment (P = 0.2). FQ-R, fluoroquinolone resistant.
On D0, five subjects carried fluoroquinolone-resistant E. faecium while none carried fluoroquinolone-resistant E. faecalis. Fluoroquinolone-resistant E. faecalis emerged during ciprofloxacin treatment in three subjects on D7 and four subjects on D14; however, only one subject carried fluoroquinolone-resistant E. faecalis after the end of treatment at D42. These seven subjects carried 14 distinct high-level fluoroquinolone-resistant E. faecalis, which all had an elevated ciprofloxacin MIC (>32 mg/L) and were gyrA/parC double mutants.
Interestingly, the dynamics of the emergence of fluoroquinolone resistance was completely different for E. faecium strains: on D0, four subjects carried low-level FQ-RE (ciprofloxacin MICs between 4 and 32 mg/L) with no chromosomal mutations, and one was a double mutant with a ciprofloxacin MIC of 128 mg/L. On D7 and D14, two and one of the four initial subjects had no FQ-RE detected in their stools, respectively. On D42, nine subjects carried fluoroquinolone-resistant E. faecium, among which eight subjects had low-level resistant mutants with no chromosomal mutations and one subject carried the same high-level resistant mutant as on D0.
Co-resistances
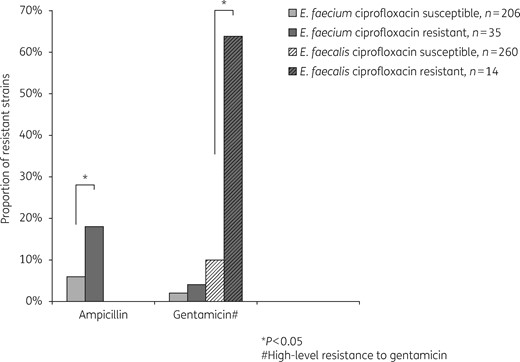
Proportion of co-resistances to ampicillin and gentamicin (high-level resistance) found in E. faecalis and E. faecium strains according to their fluoroquinolone resistance profiles.
Origin of FQ-RE
All resistant enterococci collected during or after ciprofloxacin therapy were compared with susceptible isolates belonging to the same species and collected from the same patient at D0. No acquisition of resistance was evidenced among endogenous enterococci present before ciprofloxacin treatment.
Discussion
Thanks to a unique collection of stool samples collected from healthy volunteers who were treated with ciprofloxacin for 14 days, we were able to study both the qualitative and the quantitative impact of ciprofloxacin on commensal populations of enterococci and analyse the dynamics of the emergence of fluoroquinolone resistance. Several major results emerge from these data. First, ciprofloxacin significantly decreased the densities of total enterococci during treatment, but these densities returned to pre-exposure levels at the end of treatment; however, the diversity of enterococcal species decreased and did not return to the pre-treatment levels. Second, during ciprofloxacin treatment, FQ-RE always belonged to a single species (either E. faecium or E. faecalis) and became the dominant enterococcal clone (relative abundance >50%). Third, emergence of fluoroquinolone resistance was frequent, with twice as many FQ-RE carriers after the end of ciprofloxacin treatment than before. Additionally, E. faecium and E. faecalis appeared to behave very differently mechanistically: all 14 E. faecalis isolates were highly resistant to fluoroquinolones in association with gyrA/parC double mutations, whereas almost all (34/35, 97%) E. faecium exhibited low-level fluoroquinolone resistance with no mutations in gyrA and parC QRDRs. Finally, we could not detect a quinolone-susceptible counterpart to the emerging FQ-RE strains in the initial microbiota before ciprofloxacin treatment, suggesting that resistant strains were exogenously acquired, as previously shown for E. coli and staphylococci.5,19
Data in the literature concerning the effect of fluoroquinolones on enterococci were contradictory until this work: in a study among cirrhotic patients, the relative abundance of enterococci was increased after treatment with ciprofloxacin,20 whereas other works conducted on 12 healthy volunteers found, like us, a decreased abundance of enterococci under treatment and a return to normal counts after the end of treatment.21,22 Unfortunately, these studies from the 1980s and 1990s did not include a description of resistance phenotypes. Here, we studied a large group of 48 healthy volunteers who had not received previous antibiotic treatments and the results are straightforward: the quantitative effect of ciprofloxacin that decreases the counts of enterococci in the gut is temporary, as they return to normal after the end of treatment; however, the qualitative impact is persistent, with a loss of species diversity, the consequences of which are unknown. Indeed, all minor species of enterococci apart from Enterococcus durans (including Enterococcus mundtii) were eliminated from the faeces by ciprofloxacin treatment. These minor species appear less adapted than E. faecalis and E. faecium to antibiotic pressure, which may explain why they are very rarely responsible for infections. This adds data specifically concerning enterococci on the major impact on the gut microbiota of antibiotics in general and that of ciprofloxacin in particular, which profoundly modifies also the diversity of the microbiota, including anaerobes, without returning to pre-antibiotic status even several months after treatment.23
Enterococci usually represent a small proportion of the gut microbiota,24 but an important step towards nosocomial infection is the increased density of gut colonization.1 In the case of VRE, it was shown in the clinical setting that intestinal domination by these microorganisms preceded bloodstream infection in patients undergoing allogeneic HSCT, suggesting that the increase in the relative abundance of FQ-RE, which become dominant under ciprofloxacin treatment, may put subjects at risk of infection by VRE.25
An interesting point here is that E. faecium and E. faecalis appear to behave in completely different ways in the face of ciprofloxacin treatment. Low-level resistance in E. faecium appears to be relatively common as it was present in 5/21 (24%) healthy subjects who had not received antibiotics in the last 3 months. During treatment, high concentrations of ciprofloxacin in the stools inhibited the growth of these low-level resistant mutants, but the impact of ciprofloxacin treatment was best seen after the end of treatment, when concentrations of ciprofloxacin had decreased. Altogether, the number of subjects carrying fluoroquinolone-resistant E. faecium nearly doubled from 5 to 9/21 (43%) after 14 days of ciprofloxacin treatment. We found that 34/35 fluoroquinolone-resistant E. faecium did not carry QRDR chromosomal mutations, but rather overexpressed the chromosome-encoded Efmqnr gene, likely responsible for the low-level resistance phenotype.11
Concerning E. faecalis, no subject carried fluoroquinolone-resistant E. faecalis before treatment, but high-level fluoroquinolone resistance emerged in seven subjects during treatment. Only one of them had persistent carriage of E. faecalis at D42, when no more ciprofloxacin was found in the stools. This suggests that these highly resistant E. faecalis strains were advantaged during ciprofloxacin treatment, but were probably not well adapted to the commensal lifestyle and were not able to survive once the selective advantage had disappeared. This is clearly not the case for fluoroquinolone-resistant E. faecium strains, which appear to survive even in healthy subjects before any antibiotic treatment and despite the absence of ciprofloxacin at D42. This suggests that overexpression of Efmqnr may carry lower biological costs in terms of fitness than QRDR mutations, responsible for high-level resistance, therefore favouring the persistence of the low-level resistant E. faecium strains, even after the clearance of ciprofloxacin.
This is of major clinical importance because E. faecium is by far the most difficult species to treat, as these organisms are often ampicillin resistant and are particularly affected by vancomycin resistance, especially in the USA.1 Additionally, the FQ-RE carried co-resistances to other antibiotics of clinical importance. FQ-RE were indeed more likely than their susceptible counterparts to be ampicillin resistant (for E. faecium) and to exhibit high-level resistance to gentamicin (for E. faecalis). Because VRE are very rare in France (<1% among blood isolates), we did not find evidence any of these isolates; however, in a setting where VRE are epidemic, co-selecting such strains may likely occur.
Although it may appear as a limitation, we purposefully only used culture techniques in this work. Indeed, we were interested in enterococci, which represent a minor taxa among the bacteria populating the gut microbiota. Deep sequencing techniques such as those used to perform metagenomic studies of the microbiota are unable to address the changes in very low-abundance taxa. Indeed, the exploration of the impact of antibiotic therapy on gut microbiota using recently described ‘culturomics’ techniques offers complementary information regarding minority populations, which remains of great value, in the era of deep sequencing.26–28
Altogether, this work is a unique description of the ecological impact of ciprofloxacin on the gut microbiota and specifically on enterococcal populations in both quantitative and qualitative terms and on the risk of emergence of resistant strains. Interestingly, although fluoroquinolones are poorly active against enterococcal species from a therapeutic point of view, the impact of a fluoroquinolone treatment on gut commensal enterococci should not be underestimated from an ecological point of view. Indeed, our results highlight once again the major impact of fluoroquinolones on human microbiota both in qualitative and quantitative terms,23 but also on the risk of emergence of bacterial resistance,4,6,19 even against bacterial species that are not primary therapeutic targets.
Acknowledgements
This work was presented as a poster at the Twenty-sixth European Congress of Clinical Microbiology and Infectious Diseases, Amsterdam, The Netherlands, 2016 (Abstract EV0320).
Funding
The CIPHARES study was funded by a grant from the Direction de la Recherche Clinique, Assistance Publique-Hôpitaux de Paris (Contrat d’Investigation et de Recherche Clinique) in 2007.9
This work was funded by the IAME Research Group, UMR-1137, Inserm and University Paris Diderot.
Transparency declarations
None to declare.



