-
PDF
- Split View
-
Views
-
Cite
Cite
Samir Agrawal, Rosemary Barnes, Roger J. Brüggemann, Riina Rautemaa-Richardson, Adilia Warris, The role of the multidisciplinary team in antifungal stewardship, Journal of Antimicrobial Chemotherapy, Volume 71, Issue suppl_2, November 2016, Pages ii37–ii42, https://doi.org/10.1093/jac/dkw395
Close - Share Icon Share
Abstract
There are a variety of challenges faced in the management of invasive fungal diseases (IFD), including high case-fatality rates, high cost of antifungal drugs and development of antifungal resistance. The diagnostic challenges and poor outcomes associated with IFD have resulted in excessive empirical use of antifungals in various hospital settings, exposing many patients without IFD to potential drug toxicities as well as causing spiralling antifungal drug costs. Further complexity arises as different patient groups show marked variation in their risk for IFD, fungal epidemiology, sensitivity and specificity of diagnostic tests and the pharmacokinetics and pharmacodynamics of antifungal drugs. To address these issues and to ensure optimal management of IFD, specialist knowledge and experience from a range of backgrounds is required, which extends beyond the remit of most antibiotic stewardship programmes. The first step in the development of any antifungal stewardship (AFS) programme is to build a multidisciplinary team encompassing the necessary expertise in the management of IFD to develop and implement the AFS programme. The specific roles of the key individuals within the AFS team and the importance of collaboration are discussed in this article.
Introduction
The primary aim of antifungal stewardship (AFS) programmes is to optimize antifungal drug use by integrating specialist experience and knowledge to tackle the issues preventing appropriate use of antifungal drugs. The formation of a multidisciplinary team with the necessary expertise is key to the development of any AFS programme. The core members of the AFS team should consist of individuals who possess sufficient knowledge of, and experience in, the clinical management of relevant patient populations, fungal epidemiology and susceptibility patterns, the laboratory diagnosis of invasive fungal disease (IFD), pharmacokinetics (PK) of antifungal drugs, dosing and drug–drug interactions. In this article the authors offer their views on the remit of the multidisciplinary team, the specific roles of key individuals within the team and the importance of collaboration.
Clinical pharmacist
Antifungal drugs are used for heterogeneous groups of patients. Consequently, knowledge of the PK behaviour of antifungal drugs is crucial in order to select the most appropriate drug at the correct dose. Those patients at risk of IFD belong to special populations, including neonates, paediatric patients, those in the ICU, pregnant women, obese patients and frail elderly patients who show substantial pathology-mediated PK variations.1 Quite often, specific information on the differences between these patient populations is lacking and extrapolation of knowledge from other subjects is required. The clinical application of knowledge of the PK behaviour of a given drug must be undertaken with caution and by someone with extensive knowledge of the field, as mistakes may lead to suboptimal or even toxic therapy. In addition to deciding on the appropriate selection of a drug, the clinician is also often confronted with pathophysiological states, such as renal dysfunction, the need for extracorporeal elimination techniques, as well as drug–drug interactions that require immediate attention.
Changes in renal function have a significant impact on drugs that are cleared renally, such as fluconazole and flucytosine (5-fluorocytosine), but there are also a variety of other factors that can contribute to significant intra- and inter-patient variability in response to antifungal agents, especially drug–drug interactions. Managing potential drug interactions can pose a particular challenge to both clinicians and pharmacists, especially when several interacting agents are being administered.2,3
Theoretical data are not always sufficient to predict PK interactions, as unexpected drug interactions with antifungal drugs may occur, which adds to the complexity. It is also worth remembering that a lack of data to support an interaction does not necessarily mean the absence of the interaction. Ideally, the healthcare professional should have access to a comprehensive, up-to-date overview of drug interactions with antifungal drugs. The clinical pharmacist should have a full understanding of the underlying mechanisms and scientific evidence for different antifungal agents, as well as extensive knowledge of drug PKs to be able to offer tailor-made advice on how to manage drug–drug interactions and select the most suitable antifungal for a given clinical condition and IFD.
It is essential that therapeutic drug monitoring (TDM) is performed for antifungal drugs since they have a narrow therapeutic range and large inter-individual variation in PK and pharmacodynamics, and can cause severe adverse effects.4,5 In the clinical setting, there is evidence to support the observation that plasma concentrations above a certain concentration may be predictive of efficacy for voriconazole, posaconazole, itraconazole and flucytosine,5,6 though this has yet to be confirmed in prospective studies. Nevertheless, the importance of TDM of these antifungal drugs is widely accepted and TDM is recommended in guidelines for the treatment of IFD [presented at the European Conference on Infections in Leukaemia (ECIL), 2015; http://kobe.fr/ecil]. The clinical pharmacist clearly has a key role in this complex process by interpreting the results, advising on dose adaptation when required and coordinating the whole cycle of events.5
Microbiologist
Much of the inappropriate use of antifungal agents arises from the inability to diagnose IFD reliably. In addition to a combination of host factors, and clinical signs and symptoms to define the likelihood of the presence of IFD, several mycological tests can be used to increase the certainty of IFD being diagnosed.7,8 Understanding the accessibility, performance and interpretation of the available mycological tests is the specific expertise of the microbiologist (or medical mycologist).
Modern techniques for diagnosing fungal infection include biomarker testing to provide evidence of a fungal infection, and molecular tools such as PCR to detect the fungus itself. Biomarker tests available for the diagnosis of IFD include detection of Aspergillus galactomannan (GM) by ELISA, glycoprotein antigen detected by the immunochromatographic lateral flow device, cryptococcal antigen, Candida antigens (mannan, germ tube antigen), pan-fungal markers [1-3-β-d-glucan (BDG)] and either a fungal species-specific or pan-fungal PCR. In addition, modern developments in mass spectrometry, proteomics and breath tests are leading to the introduction of newer diagnostic techniques, although few are in mainstream use yet. A decision needs to be made between employing these tests in a screening strategy to rule out IFD (allowing a move away from empirical therapy) or as diagnostic tests to rule in disease (targeted therapy).9 Both strategies can be used within the same patient population depending on the underlying risk of IFD, prevalence and pre-test probability of disease, and use of antifungal prophylaxis.
For the diagnosis of aspergillosis, the GM test and standardized PCR methodologies show high sensitivity with a high negative predictive value, and can be used to screen at-risk patients to exclude invasive disease.10,11
However, due to limited specificity and the relative rarity of IFD, the positive predictive value of individual biomarkers is rarely sufficient to diagnose disease.11–13 Recent studies have shown the utility of combinations of biomarkers and their ability to detect infection early, before radiological evidence of disease is present, and to improve patient outcomes.14–16 All show a reduction in empirical antifungal usage and improved targeting of drugs to patients who need them. Biomarkers can also be used to monitor response to therapy and can inform decisions on when to stop treatment when the test is negative.
Microbiological culture is necessary to establish proven IFD and can provide susceptibility profiles for optimizing therapy. The microbiologist can guide appropriate sampling (e.g. large-volume blood cultures for candidaemia) and can interpret the significance of results (e.g. identifying Candida species in lower respiratory samples as contaminants). PCR can be designed to identify to a species level providing diagnostic information, but also informs on epidemiological trends. Several PCR-based methods for the detection of fungal pathogens, including mutations conferring resistance to specific antifungals (e.g. echinocandin resistance in Candida species and azole resistance in Aspergillus fumigatus), are also currently available.
The role of the microbiologist is to direct therapy more accurately by identifying patients who are unlikely to have IFD (no antifungal therapy needed), to ensure early identification of IFD before clinical symptoms develop and to diagnose those patients with IFD to enable therapy targeted to the causative fungal pathogen.
Paediatric infectious diseases specialist
There are differences in the underlying conditions between adults and children that predispose the latter to IFD. However, paediatric patients are also unique in terms of the epidemiology of IFD, the usefulness of non-culture-based microbiological tests and the pharmacology and dosing of antifungal agents. High-risk paediatric populations include very low birthweight infants admitted to neonatal ICUs, children with primary immunodeficiencies, infants and children with malignancies and those receiving haematopoietic stem cell transplants. IFD of very low birth weight neonates is predominantly due to invasive candidiasis, which is disseminated to the CNS in up to 23%.17Candida albicans and Candida parapsilosis are the most common species encountered. This differs from adults and needs to be taken into account when treatment is started before cultures become positive.18 The epidemiology of invasive aspergillosis in the paediatric population is remarkable for the higher incidence of cutaneous aspergillosis19 as well as the observation that Aspergillus nidulans is the second-most common Aspergillus species causing IFD in children with chronic granulomatous disease.20 The performance of several diagnostic procedures used to diagnose IFD is also different from that found for adults. Typical abnormalities on a chest CT of adults with pulmonary invasive aspergillosis are less common in children.21,22 The BDG test is not validated in children and the cut-off has yet to be determined.23,24
There is also a lack of Phase III clinical trials involving paediatric patients to assess the efficacy of antifungal agents. Consequently, recommendations on the treatment of IFD in neonates and children (specific antifungal agent and dosing) must often rely on evidence from efficacy trials in adults, which needs to be combined with paediatric PK and safety data, and, ideally, supportive efficacy data from published case reports and series.
Another specific issue for the paediatric population is the consideration of regulatory approval for the use of a certain antifungal agent in a particular age group by the EMA.25 The paediatric infectious disease (ID) specialist with the necessary expertise and knowledge is best placed to address all these challenges to ensure optimal antifungal drug prescription to children and neonates.26 The recently published paediatric guidelines for the management of IFD also support this by offering separate and expert guidance in recognition of the uniqueness of this patient population.27,28
Adult infectious diseases specialist
ID specialists are trained to manage complex patients, have experience of assessing the clinical signs and symptoms in different types of patients and understand the challenges associated with this. They also have expertise in assessing and managing various risk factors for IFD and in understanding the feasibility of performing assessments and obtaining samples for diagnostics. Most antifungal agents are prescribed prophylactically or empirically for patients at risk for IFD. These patients are typically looked after by experienced specialists such as intensivists, haematologists and transplant surgeons. Influencing and leading these experts is a challenge that requires credibility and good interpersonal skills. The ID specialists' hands-on experience can be very helpful when recommendations or guidelines are challenged by the clinical teams, or when new diagnostic tests or drugs are being introduced. For example, antifungal agents are overused empirically in many ICUs as the mortality due to candidaemia is high and the adverse events associated with echinocandin use are minimal. ID specialists can guide the clinicians looking after critically ill patients on the selection and interpretation of diagnostic test results in a spectrum of clinical scenarios (e.g. the BDG antigen test with a high negative predictive value in low incidence settings),29,30 and use their clinical expertise to encourage withholding or stopping antifungals when they are not needed.
Additionally, their awareness of global trends in antifungal resistance development and the driving forces behind these is valuable when developing local guidelines. ID specialists should have a key role in ‘stewarding’ the introduction of new diagnostic approaches and antifungal agents by discussing each suspected or likely case of IFD with the clinical team, being present at the bedside and sharing the responsibility of decision making.
Haematologist
The treatment of patients with acute leukaemia and those undergoing allogeneic transplantation typically falls to haematologists and their departments. As a consequence, particularly in large transplant centres, haematologists acquire significant expertise in the management of IFD in this high-risk and complex patient group. The acute leukaemia groups–both acute myeloid leukaemia (AML) and acute lymphoblastic leukaemia (ALL)–and the allogeneic transplant patient groups represent some of the highest-risk populations for IFD.
A typical clinical scenario is one of a patient with chemotherapy-induced neutropenia, who then develops fever that persists despite broad-spectrum antibiotics, in the absence of localizing signs for infection and microbiological evidence. After 72–96 h from the initial fever, a chest CT is performed, fungal diagnostic tests are requested and antifungal therapy is commenced empirically.31 While pulmonary signs or symptoms compatible with IFD can include cough, dyspnoea, haemoptysis and pleuritic chest pain, these findings, along with fever, may be due to pathologies other than IFD. The haematologist has sufficient knowledge and experience to review not just the diagnostic test results, but also the clinical condition, duration of neutropenia and whether additional immunosuppressive agents have been used, and can estimate the a priori likelihood of the IFD based on the underlying malignancy and the use of antifungal prophylaxis. This then allows a decision to be made on whether the empirical regimen should be continued, modified or stopped altogether.
If imaging shows localized abnormalities, the haematologist will also be able to judge whether the patient's clinical condition is sufficient to allow invasive diagnostic procedures to be undertaken to identify the cause of the pulmonary abnormalities. Detection and identification of a fungus will then allow antifungal therapy to be targeted, which is important, particularly when the susceptibility of the fungus is known.
The multidisciplinary team
The first step in the development and implementation of AFS is to build a multidisciplinary team encompassing the necessary expertise in the management of IFD. The individual team members will be proficient in their clinical speciality, hold a specific interest and have expertise in complementary aspects of the management of IFD. They should also be seen as local authorities and opinion leaders (Figure 1).
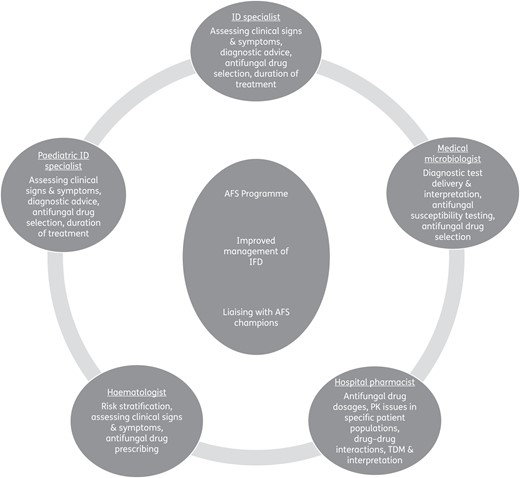
It is important that the key clinical specialities are fully represented in the AFS team as unsolicited guidance is more palatable when it is provided by peers and clinical team members rather than by external individuals such as auditors or regulators. The credibility of the team depends on the knowledge and experience of its members and the roles they have within their organization. In addition, to be effective, members of the AFS team need to possess good communication and networking skills, be able to collaborate and show a willingness to share responsibilities. The exact composition of the multidisciplinary team will depend on local circumstances but as a minimum should consist of a hospital pharmacist, a microbiologist and a clinician, each of whom should be actively engaged in the management of IFD.
The role of ID specialists in AFS programmes varies between countries and healthcare systems. Nonetheless, ID specialists are often integral members of AFS teams as ID is a broad speciality of internal medicine with links to all other specialities. The spectrum of patients and specialities will determine the need to involve, on a case-by-case basis, other specialists, such as paediatric ID specialists, haematologists, ICU consultants, respiratory physicians and surgeons, in the multidisciplinary AFS team.
The multidisciplinary team should define the roles and responsibilities of each member. Establishing a programme lead and a governance structure is key to developing a functional and coherent team. Team members should be assigned tasks related to their specific expertise and networks. Their responsibilities would normally include education, developing institutional guidelines, performing audits and their physical clinical presence to provide support. Liaison with senior clinicians representing various clinical teams is vital for the AFS principles to be fully integrated into standard clinical care. Identifying and involving opinion leaders (often called ‘AFS champions’) is strategically very important, as is official approval and the support of, and empowerment by, the hospital managers; this is a prerequisite to the success of any AFS programme.
The democratic leadership model, an inclusive strategy and consensus in decision making can prove extremely useful to AFS programmes. This approach is commonly used in clinical multidisciplinary team meetings so clinicians are used to taking advice from their peers in this setting. Obviously, this is more likely to work in settings that are familiar with democratic leadership models so different approaches may prove more useful in other settings. For example, in smaller centres with few high-risk patients and low antifungal consumption, adopting a standard antimicrobial stewardship approach may be sufficient. There may also be settings where restrictions, monitoring and penalties work best. However, true stewardship cannot thrive on fear or restrictions; it must focus on a genuine commitment to ‘doing the right thing’ for the benefit of patients. The role of the multidisciplinary team is to support the decision-making process by bringing together the available knowledge and expertise for the management of IFD. AFS is clearly needed given that the high level of antifungal drug prescribing and consumption in many centres is not commensurate with the number of patients suffering from IFD, and also because of the increasing prevalence of antifungal resistance.
Conclusions
Antifungal stewardship requires the integration of patient risk factors and interpretation of conventional tests, biomarkers, molecular diagnostics, and imaging, followed by optimal choice of antifungal therapy. The complexity of the patient population at risk of developing IFD necessitates a multidisciplinary team to develop and implement AFS programmes within hospitals. The members of the multidisciplinary team complement each other with respect to specific expertise in the management of IFD.
Transparency declarations
S. A. has received sponsorship for meetings, unrestricted educational grants, research grants and consultancy work from Astellas, Basilea, Gilead, MSD and Pfizer. R. Barnes has served on advisory boards, received sponsorship and travel expenses to attend meetings, and received honoraria for lectures/symposia from Merck, Sharp and Dohme, Astellas, Gilead Sciences and Pfizer. In addition, she has received educational grants, scientific fellowship awards and independent researcher grants from Gilead Sciences and Pfizer. She is a steering board member of the European Aspergillus PCR Initiative of the International Society for Human and Animal Mycology. R. Brüggemann has served as a consultant to, and has received unrestricted and research grants from, Astellas Pharma Inc., Gilead Sciences, Merck Sharpe and Dohme Corp., and Pfizer Inc. All payments were invoiced by the Radboud University Medical Center, The Netherlands. R. R.-R. has given lectures/had consultancy contracts for Gilead, Astellas, MSD and Pfizer. A. W. has received consultancy fees from Basilea and Gilead; educational grants from Pfizer and Gilead; and is supported by the Wellcome Trust Strategic Award for Medical Mycology and Fungal Immunology 097377.
This article forms part of a Supplement sponsored and funded by Gilead Sciences Europe Ltd; editorial assistance was provided by Synergy Medical. The content of this Supplement is based on the sessions presented at the CARE VIII meeting, held in Madrid in November 2015.



