-
PDF
- Split View
-
Views
-
Cite
Cite
P. H. Van, P. T. Binh, N. H. L. Minh, I. Morrissey, D. Torumkuney, Results from the Survey of Antibiotic Resistance (SOAR) 2009–11 in Vietnam, Journal of Antimicrobial Chemotherapy, Volume 71, Issue suppl_1, May 2016, Pages i93–i102, https://doi.org/10.1093/jac/dkw069
Close - Share Icon Share
Abstract
To investigate the susceptibility of respiratory tract infection pathogens collected between 2009 and 2011 from the SOAR study in Vietnam.
MICs were determined using Etest® and susceptibility was assessed using CLSI, EUCAST and pharmacokinetic/pharmacodynamic (PK/PD) breakpoints.
Two hundred and eighty-nine Streptococcus pneumoniae and 195 Haemophilus influenzae were collected from 11 centres. Overall, 4.8% of S. pneumoniae were penicillin susceptible (CLSI oral and EUCAST breakpoints). Using CLSI intravenous breakpoints, 86.9% were penicillin susceptible. Susceptibility to high-dose amoxicillin/clavulanic acid (or amoxicillin) using PK/PD breakpoints, cefuroxime (using CLSI or PK/PD breakpoints), cefaclor (CLSI breakpoint) and azithromycin (CLSI breakpoint) was 96.9%, 18.7%, 8% and 4.2%, respectively. Ofloxacin susceptibility was 93.4% by CLSI but 0% by EUCAST. All S. pneumoniae were fully vancomycin susceptible. S. pneumoniae from children were significantly less susceptible to most antimicrobials than those from the elderly. For ofloxacin, however, the reverse was true.
Among H. influenzae isolates, 40.5% produced β-lactamase and 13.8% were β-lactamase negative but ampicillin resistant (BLNAR) by CLSI. H. influenzae were highly susceptible (97.4%) in vitro to amoxicillin/clavulanic acid and also to ceftriaxone by CLSI and PK/PD breakpoints but not EUCAST breakpoints. However, BLNAR isolates should be considered clinically resistant, with susceptibility reduced to 84.1%. With EUCAST breakpoints, amoxicillin/clavulanic acid susceptibility was lower, at 63.1%. Azithromycin susceptibility was 79.5% (CLSI).
Resistance to antibacterials in Vietnam was high, with amoxicillin/clavulanic acid being the most active agent. Ceftriaxone was highly active against H. influenzae while ofloxacin appeared highly active against S. pneumoniae using CLSI but not by EUCAST breakpoints. Ongoing surveillance through SOAR will further assist in understanding susceptibility trends over time.
Introduction
Despite a substantial reduction since 1990, community-acquired pneumonia (CAP) and other lower respiratory tract infections (LRTIs) still rank as the second most frequent cause of all-age premature deaths at the global level.1,2Streptococcus pneumoniae and Haemophilus influenzae are the most common bacteria associated with CAP. Generally, S. pneumoniae is the major pathogen of the two, but a recent study in Vietnam found the prevalence of H. influenzae in sputum to be higher than that of S. pneumoniae (28% versus 23%).3
Antimicrobial resistance in such clinical indications is common and resistance surveillance plays an important role in the reporting and management of such disease. One such study is the ongoing Survey of Antibiotic Resistance (SOAR), which is an antimicrobial resistance surveillance study of key respiratory pathogens conducted in the Middle East, Africa, Latin America, Asia-Pacific and the Commonwealth of Independent States countries and has been running since 2002. Here, we report recent data from SOAR for major community-acquired respiratory tract infection pathogens collected from hospital sites in Vietnam.
Materials and methods
Collaborating centres
The following centres (n = 11) took part in the study: An Binh Hospital, Ho Chi Minh City; Bach Mai Hospital, Hanoi; Can Tho Central Hospital, Can Tho; Da Nang Hospital, Da Nang; Hue Central Hospital, Hue; Children's No. 1 Hospital, Ho Chi Minh City; Children's No. 2 Hospital, Ho Chi Minh City; Gia Dinh People's Hospital, Ho Chi Minh City; Nguyen Tri Phuong Hospital, Ho Chi Minh City; National Hospital of Pediatrics, Hanoi; and Ear, Nose and Throat Hospital, Ho Chi Minh City.
Clinical isolates
Isolates of S. pneumoniae and H. influenzae were obtained from clinical materials taken from adults and paediatric patients (outpatients who attended the university hospital) with community-acquired respiratory infections. Organisms were identified using conventional methods (optochin susceptibility/bile solubility for S. pneumoniae and X/V factor requirement for H. influenzae). Duplicate isolates from the same patient were not accepted.
The presence of β-lactamase was tested for all isolates of H. influenzae by a chromogenic cephalosporin (nitrocefin) disc method.
Susceptibility testing
MICs were determined using the Etest® susceptibility method according to the manufacturer's instructions (bioMérieux, Marcy l'Etoile, France). Disc diffusion susceptibility testing was carried out according to CLSI methodology.4 Susceptibility of H. influenzae to amoxicillin/clavulanic acid (2/1), ampicillin, azithromycin, cefaclor, ceftriaxone, cefuroxime and clarithromycin was evaluated by Etest®. Trimethoprim/sulfamethoxazole (1/19), tetracycline and chloramphenicol susceptibility were evaluated by disc diffusion. Susceptibility of S. pneumoniae to penicillin, amoxicillin/clavulanic acid (2/1), azithromycin, cefaclor, cefuroxime ofloxacin and vancomycin was evaluated by Etest®. Erythromycin, clindamycin, trimethoprim/sulfamethoxazole (1/19), tetracycline and chloramphenicol susceptibilities were evaluated by disc diffusion. CLSI breakpoints were applied,5 except for macrolides and clindamycin, where bioMérieux Etest® breakpoints for incubation in CO2 were used. In addition, susceptibilities, as defined by EUCAST and pharmacokinetic/pharmacodynamic (PK/PD) breakpoints, were reported where applicable6,7 to assess whether adoption of these breakpoints would affect susceptibility. EUCAST and PK/PD breakpoints were not applied for macrolides or clindamycin because, unlike CLSI, these are not adjusted for incubation in CO2 by bioMérieux. The MIC breakpoints used are shown in Table 1 and the disc diffusion zone diameter breakpoints are shown in Table 2.
| . | Breakpoints [S/I/R (mg/L)] . | . | |||
|---|---|---|---|---|---|
| . | S. pneumoniae . | H. influenzae . | . | ||
| Antimicrobial . | CLSI . | EUCAST . | CLSI . | EUCAST . | MIC (mg/L) (S only) PK/PD . |
| Amoxicillin/clavulanic acida | ≤2/4/≥8 | NA | ≤4/–/≥8 | ≤2/–/≥4 | ≤2 (≤4) |
| Ampicillin | NT | NT | ≤1/2/≥4 | ≤1/–/≥2 | NA |
| Azithromycinb | ≤4/8/≥16 | NA | ≤8/–/– | NA | NA |
| Cefaclor | ≤1/2/≥4 | ≤0.03/0.06–0.5/≥1 | ≤8/16/≥32 | ≤0.5/–/≥1 | ≤0.5 |
| Cefuroximec | ≤1/2/≥4 | ≤0.25/0.5/≥1 | ≤4/8/≥16 | ≤0.12/0.25–1/≥2 | ≤1 |
| Clarithromycinb | NT | NT | ≤16/32/≥64 | NA | NA |
| Ofloxacin | ≤2/4/≥8 | ≤0.12/0.25–4/≥8 | NT | NT | NA |
| Penicillin (iv non-meningitis) | ≤2/4/≥8 | see noted | NT | NT | NA |
| Penicillin (oral) | ≤0.06/0.12–1/≥2 | ≤0.06/0.12–2/≥4 | NA | NA | NA |
| Vancomycin | ≤1/–/– | NA | NT | NT | NA |
| . | Breakpoints [S/I/R (mg/L)] . | . | |||
|---|---|---|---|---|---|
| . | S. pneumoniae . | H. influenzae . | . | ||
| Antimicrobial . | CLSI . | EUCAST . | CLSI . | EUCAST . | MIC (mg/L) (S only) PK/PD . |
| Amoxicillin/clavulanic acida | ≤2/4/≥8 | NA | ≤4/–/≥8 | ≤2/–/≥4 | ≤2 (≤4) |
| Ampicillin | NT | NT | ≤1/2/≥4 | ≤1/–/≥2 | NA |
| Azithromycinb | ≤4/8/≥16 | NA | ≤8/–/– | NA | NA |
| Cefaclor | ≤1/2/≥4 | ≤0.03/0.06–0.5/≥1 | ≤8/16/≥32 | ≤0.5/–/≥1 | ≤0.5 |
| Cefuroximec | ≤1/2/≥4 | ≤0.25/0.5/≥1 | ≤4/8/≥16 | ≤0.12/0.25–1/≥2 | ≤1 |
| Clarithromycinb | NT | NT | ≤16/32/≥64 | NA | NA |
| Ofloxacin | ≤2/4/≥8 | ≤0.12/0.25–4/≥8 | NT | NT | NA |
| Penicillin (iv non-meningitis) | ≤2/4/≥8 | see noted | NT | NT | NA |
| Penicillin (oral) | ≤0.06/0.12–1/≥2 | ≤0.06/0.12–2/≥4 | NA | NA | NA |
| Vancomycin | ≤1/–/– | NA | NT | NT | NA |
S, susceptible; I, intermediate; R, resistant; NT, not tested; NA, not applicable.
aAmoxicillin/clavulanic acid was tested at a 2:1 amoxicillin to clavulanic acid ratio; breakpoints are expressed as the amoxicillin component. PK/PD breakpoints based on high dose (4 g of amoxicillin with 250 mg of clavulanate per day for adults) are shown in parentheses, which is the same as CLSI for H. influenzae and one dilution higher for S. pneumoniae.
bbioMérieux Etest® breakpoints for incubation in CO2 used for macrolides.
cBreakpoints used are for cefuroxime axetil.
dEUCAST do not give iv breakpoints but dose-specific susceptible breakpoints are noted for pneumonia: 1.2 g × 4 (MIC ≤0.5 mg/L = susceptible), 1.2 g × 6 or 2.4 g × 4 (MIC ≤1 mg/L = susceptible) and 2.4 g × 6 (MIC ≤2 mg/L = susceptible).
| . | Breakpoints [S/I/R (mg/L)] . | . | |||
|---|---|---|---|---|---|
| . | S. pneumoniae . | H. influenzae . | . | ||
| Antimicrobial . | CLSI . | EUCAST . | CLSI . | EUCAST . | MIC (mg/L) (S only) PK/PD . |
| Amoxicillin/clavulanic acida | ≤2/4/≥8 | NA | ≤4/–/≥8 | ≤2/–/≥4 | ≤2 (≤4) |
| Ampicillin | NT | NT | ≤1/2/≥4 | ≤1/–/≥2 | NA |
| Azithromycinb | ≤4/8/≥16 | NA | ≤8/–/– | NA | NA |
| Cefaclor | ≤1/2/≥4 | ≤0.03/0.06–0.5/≥1 | ≤8/16/≥32 | ≤0.5/–/≥1 | ≤0.5 |
| Cefuroximec | ≤1/2/≥4 | ≤0.25/0.5/≥1 | ≤4/8/≥16 | ≤0.12/0.25–1/≥2 | ≤1 |
| Clarithromycinb | NT | NT | ≤16/32/≥64 | NA | NA |
| Ofloxacin | ≤2/4/≥8 | ≤0.12/0.25–4/≥8 | NT | NT | NA |
| Penicillin (iv non-meningitis) | ≤2/4/≥8 | see noted | NT | NT | NA |
| Penicillin (oral) | ≤0.06/0.12–1/≥2 | ≤0.06/0.12–2/≥4 | NA | NA | NA |
| Vancomycin | ≤1/–/– | NA | NT | NT | NA |
| . | Breakpoints [S/I/R (mg/L)] . | . | |||
|---|---|---|---|---|---|
| . | S. pneumoniae . | H. influenzae . | . | ||
| Antimicrobial . | CLSI . | EUCAST . | CLSI . | EUCAST . | MIC (mg/L) (S only) PK/PD . |
| Amoxicillin/clavulanic acida | ≤2/4/≥8 | NA | ≤4/–/≥8 | ≤2/–/≥4 | ≤2 (≤4) |
| Ampicillin | NT | NT | ≤1/2/≥4 | ≤1/–/≥2 | NA |
| Azithromycinb | ≤4/8/≥16 | NA | ≤8/–/– | NA | NA |
| Cefaclor | ≤1/2/≥4 | ≤0.03/0.06–0.5/≥1 | ≤8/16/≥32 | ≤0.5/–/≥1 | ≤0.5 |
| Cefuroximec | ≤1/2/≥4 | ≤0.25/0.5/≥1 | ≤4/8/≥16 | ≤0.12/0.25–1/≥2 | ≤1 |
| Clarithromycinb | NT | NT | ≤16/32/≥64 | NA | NA |
| Ofloxacin | ≤2/4/≥8 | ≤0.12/0.25–4/≥8 | NT | NT | NA |
| Penicillin (iv non-meningitis) | ≤2/4/≥8 | see noted | NT | NT | NA |
| Penicillin (oral) | ≤0.06/0.12–1/≥2 | ≤0.06/0.12–2/≥4 | NA | NA | NA |
| Vancomycin | ≤1/–/– | NA | NT | NT | NA |
S, susceptible; I, intermediate; R, resistant; NT, not tested; NA, not applicable.
aAmoxicillin/clavulanic acid was tested at a 2:1 amoxicillin to clavulanic acid ratio; breakpoints are expressed as the amoxicillin component. PK/PD breakpoints based on high dose (4 g of amoxicillin with 250 mg of clavulanate per day for adults) are shown in parentheses, which is the same as CLSI for H. influenzae and one dilution higher for S. pneumoniae.
bbioMérieux Etest® breakpoints for incubation in CO2 used for macrolides.
cBreakpoints used are for cefuroxime axetil.
dEUCAST do not give iv breakpoints but dose-specific susceptible breakpoints are noted for pneumonia: 1.2 g × 4 (MIC ≤0.5 mg/L = susceptible), 1.2 g × 6 or 2.4 g × 4 (MIC ≤1 mg/L = susceptible) and 2.4 g × 6 (MIC ≤2 mg/L = susceptible).
CLSI zone diameter breakpoints (mm) used for S. pneumoniae and H. influenzae isolates
| . | Disc diffusion breakpoints [R/I/S (mm)] . | |
|---|---|---|
| Antimicrobial . | S. pneumoniae . | H. influenzae . |
| Chloramphenicol | ≤20/–/≥21 | ≤25/26–28/≥29 |
| Clindamycin | ≤15/16–18/≥19 | NT |
| Erythromycin | ≤15/16–20/≥21 | NT |
| Tetracycline | ≤24/25–27/≥28 | ≤25/26–28/≥29 |
| Trimethoprim/sulfamethoxazole | ≤15/16–18/≥19 | ≤10/11–15/≥16 |
| . | Disc diffusion breakpoints [R/I/S (mm)] . | |
|---|---|---|
| Antimicrobial . | S. pneumoniae . | H. influenzae . |
| Chloramphenicol | ≤20/–/≥21 | ≤25/26–28/≥29 |
| Clindamycin | ≤15/16–18/≥19 | NT |
| Erythromycin | ≤15/16–20/≥21 | NT |
| Tetracycline | ≤24/25–27/≥28 | ≤25/26–28/≥29 |
| Trimethoprim/sulfamethoxazole | ≤15/16–18/≥19 | ≤10/11–15/≥16 |
S, susceptible; I, intermediate; R, resistant; NT, not tested.
CLSI zone diameter breakpoints (mm) used for S. pneumoniae and H. influenzae isolates
| . | Disc diffusion breakpoints [R/I/S (mm)] . | |
|---|---|---|
| Antimicrobial . | S. pneumoniae . | H. influenzae . |
| Chloramphenicol | ≤20/–/≥21 | ≤25/26–28/≥29 |
| Clindamycin | ≤15/16–18/≥19 | NT |
| Erythromycin | ≤15/16–20/≥21 | NT |
| Tetracycline | ≤24/25–27/≥28 | ≤25/26–28/≥29 |
| Trimethoprim/sulfamethoxazole | ≤15/16–18/≥19 | ≤10/11–15/≥16 |
| . | Disc diffusion breakpoints [R/I/S (mm)] . | |
|---|---|---|
| Antimicrobial . | S. pneumoniae . | H. influenzae . |
| Chloramphenicol | ≤20/–/≥21 | ≤25/26–28/≥29 |
| Clindamycin | ≤15/16–18/≥19 | NT |
| Erythromycin | ≤15/16–20/≥21 | NT |
| Tetracycline | ≤24/25–27/≥28 | ≤25/26–28/≥29 |
| Trimethoprim/sulfamethoxazole | ≤15/16–18/≥19 | ≤10/11–15/≥16 |
S, susceptible; I, intermediate; R, resistant; NT, not tested.
Quality control and data analysis
Quality control strains recommended by CLSI were used on each day of testing. Any Etest® MIC results that were between doubling dilutions were rounded up to the next doubling dilution MIC for data analysis. S. pneumoniae ATCC 49619, H. influenzae ATCC 49247, H. influenzae ATCC 49766, Escherichia coli ATCC 25922 and E. coli ATCC 32518 were included on each day of testing. Results of susceptibility testing were accepted if the results of the control strains were within published limits. Differences in susceptibility between age groups were assessed for statistical significance with Fisher's exact test using XLSTAT version 2011.1.05. A P value <0.05 was considered statistically significant.
Results
S. pneumoniae
A total of 289 S. pneumoniae were collected from 11 different centres in Vietnam from 2009–11. The source of the S. pneumoniae isolates included sputum (n = 114; 39.4%) and bronchoalveolar lavage (n = 82; 28.4%). Smaller numbers of isolates were from middle ear effusion (n = 38; 13.1%), sinuses (n = 34; 11.8%) and blood (n = 21; 7.3%). Most isolates (n = 182, 63.0%) came from paediatric patients (aged ≤12 years), 50 (17.3%) were from elderly patients (aged ≥65 years) and 34 (11.8%) were from adults (aged 13–64 years). The remaining 23 isolates were from patients of unknown age.
Summary MIC and susceptibility data for all 289 S. pneumoniae are shown in Table 3. MIC distribution data are given in Table 4 and Figure 1. Susceptibility data for antimicrobials tested using disc diffusion are shown in Table 5.
MIC and percentage susceptibility data for S. pneumoniae isolates (n = 289)
| . | Susceptibility . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | MIC (mg/L) . | CLSI . | PK/PD . | EUCAST . | |||||||
| Antimicrobial . | 50% . | 90% . | min . | max . | %S . | %I . | %R . | %S . | %S . | %I . | %R . |
| Amoxicillin/clavulanic acida,b | 1 | 4 | 0.03 | 8 | 76.1 | 20.8 | 3.1 | 76.1 (96.9) | NA | NA | NA |
| Azithromycinc | 256 | 256 | 0.5 | 256 | 4.2 | 2.8 | 93.1 | NA | NA | NA | NA |
| Cefaclor | 32 | 256 | 0.5 | 256 | 8.0 | 3.8 | 88.2 | 4.8 | 0 | 4.8 | 95.2 |
| Cefuroximed | 2 | 8 | 0.03 | 64 | 18.7 | 32.5 | 48.8 | 18.7 | 10.7 | 1.4 | 87.9 |
| Ofloxacin | 2 | 2 | 0.5 | 32 | 93.4 | 1.0 | 5.5 | NA | 0.0 | 94.5 | 5.5 |
| Penicillin (iv) | 1 | 4 | 0.03 | 32 | 86.9 | 11.1 | 2.1 | NA | NA | NA | NA |
| Penicillin (oral) | 1 | 4 | 0.03 | 32 | 4.8 | 47.4 | 47.8 | NA | 4.8 | 82.0 | 13.1 |
| Penicillin dose 1.2 g × 4 | 1 | 4 | 0.03 | 32 | NA | NA | NA | NA | 25.6 | NA | NA |
| Penicillin dose 2.4 g × 4 or 1.2 g × 6 | 1 | 4 | 0.03 | 32 | NA | NA | NA | NA | 52.2 | NA | NA |
| Penicillin dose 2.4 g × 6 | 1 | 4 | 0.03 | 32 | NA | NA | NA | NA | 86.9 | NA | NA |
| Vancomycin | 0.25 | 0.5 | 0.06 | 1 | 100 | — | — | NA | NA | NA | NA |
| . | Susceptibility . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | MIC (mg/L) . | CLSI . | PK/PD . | EUCAST . | |||||||
| Antimicrobial . | 50% . | 90% . | min . | max . | %S . | %I . | %R . | %S . | %S . | %I . | %R . |
| Amoxicillin/clavulanic acida,b | 1 | 4 | 0.03 | 8 | 76.1 | 20.8 | 3.1 | 76.1 (96.9) | NA | NA | NA |
| Azithromycinc | 256 | 256 | 0.5 | 256 | 4.2 | 2.8 | 93.1 | NA | NA | NA | NA |
| Cefaclor | 32 | 256 | 0.5 | 256 | 8.0 | 3.8 | 88.2 | 4.8 | 0 | 4.8 | 95.2 |
| Cefuroximed | 2 | 8 | 0.03 | 64 | 18.7 | 32.5 | 48.8 | 18.7 | 10.7 | 1.4 | 87.9 |
| Ofloxacin | 2 | 2 | 0.5 | 32 | 93.4 | 1.0 | 5.5 | NA | 0.0 | 94.5 | 5.5 |
| Penicillin (iv) | 1 | 4 | 0.03 | 32 | 86.9 | 11.1 | 2.1 | NA | NA | NA | NA |
| Penicillin (oral) | 1 | 4 | 0.03 | 32 | 4.8 | 47.4 | 47.8 | NA | 4.8 | 82.0 | 13.1 |
| Penicillin dose 1.2 g × 4 | 1 | 4 | 0.03 | 32 | NA | NA | NA | NA | 25.6 | NA | NA |
| Penicillin dose 2.4 g × 4 or 1.2 g × 6 | 1 | 4 | 0.03 | 32 | NA | NA | NA | NA | 52.2 | NA | NA |
| Penicillin dose 2.4 g × 6 | 1 | 4 | 0.03 | 32 | NA | NA | NA | NA | 86.9 | NA | NA |
| Vancomycin | 0.25 | 0.5 | 0.06 | 1 | 100 | — | — | NA | NA | NA | NA |
S, susceptible; I, intermediate; R, resistant; NT, not tested; NA, not applicable.
aAmoxicillin/clavulanic acid was tested at a 2:1 amoxicillin to clavulanic acid ratio; breakpoints are expressed as the amoxicillin component. PK/PD breakpoints based on high dose (4 g of amoxicillin with 250 mg of clavulanate per day for adults) shown in parentheses, which is the same as CLSI for H. influenzae and one dilution higher for S. pneumoniae.
bFor S. pneumoniae, susceptibility to amoxicillin alone can be inferred from amoxicillin/clavulanic acid data.
cbioMérieux Etest® breakpoints for incubation in CO2 used for macrolides.
dBreakpoints used are for cefuroxime axetil.
MIC and percentage susceptibility data for S. pneumoniae isolates (n = 289)
| . | Susceptibility . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | MIC (mg/L) . | CLSI . | PK/PD . | EUCAST . | |||||||
| Antimicrobial . | 50% . | 90% . | min . | max . | %S . | %I . | %R . | %S . | %S . | %I . | %R . |
| Amoxicillin/clavulanic acida,b | 1 | 4 | 0.03 | 8 | 76.1 | 20.8 | 3.1 | 76.1 (96.9) | NA | NA | NA |
| Azithromycinc | 256 | 256 | 0.5 | 256 | 4.2 | 2.8 | 93.1 | NA | NA | NA | NA |
| Cefaclor | 32 | 256 | 0.5 | 256 | 8.0 | 3.8 | 88.2 | 4.8 | 0 | 4.8 | 95.2 |
| Cefuroximed | 2 | 8 | 0.03 | 64 | 18.7 | 32.5 | 48.8 | 18.7 | 10.7 | 1.4 | 87.9 |
| Ofloxacin | 2 | 2 | 0.5 | 32 | 93.4 | 1.0 | 5.5 | NA | 0.0 | 94.5 | 5.5 |
| Penicillin (iv) | 1 | 4 | 0.03 | 32 | 86.9 | 11.1 | 2.1 | NA | NA | NA | NA |
| Penicillin (oral) | 1 | 4 | 0.03 | 32 | 4.8 | 47.4 | 47.8 | NA | 4.8 | 82.0 | 13.1 |
| Penicillin dose 1.2 g × 4 | 1 | 4 | 0.03 | 32 | NA | NA | NA | NA | 25.6 | NA | NA |
| Penicillin dose 2.4 g × 4 or 1.2 g × 6 | 1 | 4 | 0.03 | 32 | NA | NA | NA | NA | 52.2 | NA | NA |
| Penicillin dose 2.4 g × 6 | 1 | 4 | 0.03 | 32 | NA | NA | NA | NA | 86.9 | NA | NA |
| Vancomycin | 0.25 | 0.5 | 0.06 | 1 | 100 | — | — | NA | NA | NA | NA |
| . | Susceptibility . | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| . | MIC (mg/L) . | CLSI . | PK/PD . | EUCAST . | |||||||
| Antimicrobial . | 50% . | 90% . | min . | max . | %S . | %I . | %R . | %S . | %S . | %I . | %R . |
| Amoxicillin/clavulanic acida,b | 1 | 4 | 0.03 | 8 | 76.1 | 20.8 | 3.1 | 76.1 (96.9) | NA | NA | NA |
| Azithromycinc | 256 | 256 | 0.5 | 256 | 4.2 | 2.8 | 93.1 | NA | NA | NA | NA |
| Cefaclor | 32 | 256 | 0.5 | 256 | 8.0 | 3.8 | 88.2 | 4.8 | 0 | 4.8 | 95.2 |
| Cefuroximed | 2 | 8 | 0.03 | 64 | 18.7 | 32.5 | 48.8 | 18.7 | 10.7 | 1.4 | 87.9 |
| Ofloxacin | 2 | 2 | 0.5 | 32 | 93.4 | 1.0 | 5.5 | NA | 0.0 | 94.5 | 5.5 |
| Penicillin (iv) | 1 | 4 | 0.03 | 32 | 86.9 | 11.1 | 2.1 | NA | NA | NA | NA |
| Penicillin (oral) | 1 | 4 | 0.03 | 32 | 4.8 | 47.4 | 47.8 | NA | 4.8 | 82.0 | 13.1 |
| Penicillin dose 1.2 g × 4 | 1 | 4 | 0.03 | 32 | NA | NA | NA | NA | 25.6 | NA | NA |
| Penicillin dose 2.4 g × 4 or 1.2 g × 6 | 1 | 4 | 0.03 | 32 | NA | NA | NA | NA | 52.2 | NA | NA |
| Penicillin dose 2.4 g × 6 | 1 | 4 | 0.03 | 32 | NA | NA | NA | NA | 86.9 | NA | NA |
| Vancomycin | 0.25 | 0.5 | 0.06 | 1 | 100 | — | — | NA | NA | NA | NA |
S, susceptible; I, intermediate; R, resistant; NT, not tested; NA, not applicable.
aAmoxicillin/clavulanic acid was tested at a 2:1 amoxicillin to clavulanic acid ratio; breakpoints are expressed as the amoxicillin component. PK/PD breakpoints based on high dose (4 g of amoxicillin with 250 mg of clavulanate per day for adults) shown in parentheses, which is the same as CLSI for H. influenzae and one dilution higher for S. pneumoniae.
bFor S. pneumoniae, susceptibility to amoxicillin alone can be inferred from amoxicillin/clavulanic acid data.
cbioMérieux Etest® breakpoints for incubation in CO2 used for macrolides.
dBreakpoints used are for cefuroxime axetil.
| . | Number of isolates at MIC (mg/L) . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial . | 0.03 . | 0.06 . | 0.12 . | 0.25 . | 0.5 . | 1 . | 2 . | 4 . | 8 . | 16 . | 32 . | 64 . | 128 . | 256 . |
| Amoxicillin/clavulanic acid | 9 | 5 | 8 | 13 | 48 | 95 | 42 | 60 | 9 | |||||
| Azithromycin | 3 | 5 | 4 | 8 | 6 | 9 | 5 | 249 | ||||||
| Cefaclor | 1 | 13 | 9 | 11 | 9 | 28 | 31 | 74 | 48 | 29 | 36 | |||
| Cefuroxime | 10 | 5 | 3 | 13 | 4 | 19 | 94 | 104 | 24 | 6 | 5 | 2 | ||
| Ofloxacin | 1 | 4 | 78 | 187 | 3 | 3 | 1 | 12 | ||||||
| Penicillin | 11 | 3 | 8 | 11 | 41 | 77 | 100 | 32 | 5 | 1 | ||||
| Vancomycin | 1 | 18 | 153 | 116 | 1 | |||||||||
| . | Number of isolates at MIC (mg/L) . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial . | 0.03 . | 0.06 . | 0.12 . | 0.25 . | 0.5 . | 1 . | 2 . | 4 . | 8 . | 16 . | 32 . | 64 . | 128 . | 256 . |
| Amoxicillin/clavulanic acid | 9 | 5 | 8 | 13 | 48 | 95 | 42 | 60 | 9 | |||||
| Azithromycin | 3 | 5 | 4 | 8 | 6 | 9 | 5 | 249 | ||||||
| Cefaclor | 1 | 13 | 9 | 11 | 9 | 28 | 31 | 74 | 48 | 29 | 36 | |||
| Cefuroxime | 10 | 5 | 3 | 13 | 4 | 19 | 94 | 104 | 24 | 6 | 5 | 2 | ||
| Ofloxacin | 1 | 4 | 78 | 187 | 3 | 3 | 1 | 12 | ||||||
| Penicillin | 11 | 3 | 8 | 11 | 41 | 77 | 100 | 32 | 5 | 1 | ||||
| Vancomycin | 1 | 18 | 153 | 116 | 1 | |||||||||
| . | Number of isolates at MIC (mg/L) . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial . | 0.03 . | 0.06 . | 0.12 . | 0.25 . | 0.5 . | 1 . | 2 . | 4 . | 8 . | 16 . | 32 . | 64 . | 128 . | 256 . |
| Amoxicillin/clavulanic acid | 9 | 5 | 8 | 13 | 48 | 95 | 42 | 60 | 9 | |||||
| Azithromycin | 3 | 5 | 4 | 8 | 6 | 9 | 5 | 249 | ||||||
| Cefaclor | 1 | 13 | 9 | 11 | 9 | 28 | 31 | 74 | 48 | 29 | 36 | |||
| Cefuroxime | 10 | 5 | 3 | 13 | 4 | 19 | 94 | 104 | 24 | 6 | 5 | 2 | ||
| Ofloxacin | 1 | 4 | 78 | 187 | 3 | 3 | 1 | 12 | ||||||
| Penicillin | 11 | 3 | 8 | 11 | 41 | 77 | 100 | 32 | 5 | 1 | ||||
| Vancomycin | 1 | 18 | 153 | 116 | 1 | |||||||||
| . | Number of isolates at MIC (mg/L) . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimicrobial . | 0.03 . | 0.06 . | 0.12 . | 0.25 . | 0.5 . | 1 . | 2 . | 4 . | 8 . | 16 . | 32 . | 64 . | 128 . | 256 . |
| Amoxicillin/clavulanic acid | 9 | 5 | 8 | 13 | 48 | 95 | 42 | 60 | 9 | |||||
| Azithromycin | 3 | 5 | 4 | 8 | 6 | 9 | 5 | 249 | ||||||
| Cefaclor | 1 | 13 | 9 | 11 | 9 | 28 | 31 | 74 | 48 | 29 | 36 | |||
| Cefuroxime | 10 | 5 | 3 | 13 | 4 | 19 | 94 | 104 | 24 | 6 | 5 | 2 | ||
| Ofloxacin | 1 | 4 | 78 | 187 | 3 | 3 | 1 | 12 | ||||||
| Penicillin | 11 | 3 | 8 | 11 | 41 | 77 | 100 | 32 | 5 | 1 | ||||
| Vancomycin | 1 | 18 | 153 | 116 | 1 | |||||||||
Susceptibility data for antimicrobials tested against S. pneumoniae isolates (n = 289) using CLSI disc diffusion
| . | Susceptible . | Intermediate . | Resistant . | |||
|---|---|---|---|---|---|---|
| Antimicrobial . | n . | (%) . | n . | (%) . | n . | (%) . |
| Chloramphenicol | 93 | (32.2) | 0 | (0) | 196 | (67.8) |
| Clindamycin | 40 | (13.8) | 3 | (1.0) | 246 | (85.1) |
| Erythromycin | 9 | (3.1) | 3 | (1.0) | 277 | (95.8) |
| Tetracycline | 26 | (9.0) | 19 | (6.6) | 244 | (84.4) |
| Trimethoprim/sulfamethoxazole | 13 | (4.5) | 3 | (1.0) | 273 | (94.5) |
| . | Susceptible . | Intermediate . | Resistant . | |||
|---|---|---|---|---|---|---|
| Antimicrobial . | n . | (%) . | n . | (%) . | n . | (%) . |
| Chloramphenicol | 93 | (32.2) | 0 | (0) | 196 | (67.8) |
| Clindamycin | 40 | (13.8) | 3 | (1.0) | 246 | (85.1) |
| Erythromycin | 9 | (3.1) | 3 | (1.0) | 277 | (95.8) |
| Tetracycline | 26 | (9.0) | 19 | (6.6) | 244 | (84.4) |
| Trimethoprim/sulfamethoxazole | 13 | (4.5) | 3 | (1.0) | 273 | (94.5) |
Susceptibility data for antimicrobials tested against S. pneumoniae isolates (n = 289) using CLSI disc diffusion
| . | Susceptible . | Intermediate . | Resistant . | |||
|---|---|---|---|---|---|---|
| Antimicrobial . | n . | (%) . | n . | (%) . | n . | (%) . |
| Chloramphenicol | 93 | (32.2) | 0 | (0) | 196 | (67.8) |
| Clindamycin | 40 | (13.8) | 3 | (1.0) | 246 | (85.1) |
| Erythromycin | 9 | (3.1) | 3 | (1.0) | 277 | (95.8) |
| Tetracycline | 26 | (9.0) | 19 | (6.6) | 244 | (84.4) |
| Trimethoprim/sulfamethoxazole | 13 | (4.5) | 3 | (1.0) | 273 | (94.5) |
| . | Susceptible . | Intermediate . | Resistant . | |||
|---|---|---|---|---|---|---|
| Antimicrobial . | n . | (%) . | n . | (%) . | n . | (%) . |
| Chloramphenicol | 93 | (32.2) | 0 | (0) | 196 | (67.8) |
| Clindamycin | 40 | (13.8) | 3 | (1.0) | 246 | (85.1) |
| Erythromycin | 9 | (3.1) | 3 | (1.0) | 277 | (95.8) |
| Tetracycline | 26 | (9.0) | 19 | (6.6) | 244 | (84.4) |
| Trimethoprim/sulfamethoxazole | 13 | (4.5) | 3 | (1.0) | 273 | (94.5) |

MIC distribution for (a) amoxicillin/clavulanic acid, azithromycin and penicillin and (b) cefaclor, cefuroxime and ofloxacin against all S. pneumoniae.
Overall, when applying CLSI oral or EUCAST breakpoints (these being identical), only 4.8% of the pneumococci (14/289) were penicillin-susceptible. However, due to differing intermediate and resistant breakpoints, 47.4% (137/289) were penicillin intermediate and 47.8% (138/289) penicillin resistant as defined by CLSI oral breakpoints, and 82.0% (237/289) were penicillin intermediate and 13.1% (38/289) penicillin resistant by EUCAST. EUCAST also provide susceptible-only breakpoints for higher doses of penicillin, and when applying these breakpoints the susceptibility was 25.6% (74/289) and 52.2% (151/289) with doses of 1.2 g × 4 and 2.4 g × 4 (or 1.2 g × 6), respectively. Applying the highest EUCAST dose-specific breakpoint (2.4 g × 6), susceptibility was 86.9% (251/289), which is the same as defined by the CLSI intravenous (iv) (non-meningitis) breakpoints. For the latter, 11.1% (32/289) were intermediate and 2.1% (6/289) were resistant (Table 3).
A total of 76.1% (220/289) isolates were susceptible to amoxicillin/clavulanic acid (and by inference amoxicillin alone) as defined by CLSI or low-dose PK/PD breakpoints; 96.9% (280/289) were susceptible when applying high-dose amoxicillin/clavulanic acid (and by inference amoxicillin alone) PK/PD breakpoints (Table 3). Apart from high susceptibility of isolates to ofloxacin [93.4% (270/289)], susceptibility to other antimicrobials, including macrolides and cephalosporins, was low, as shown in Tables 3 and 5. As defined by CLSI breakpoints, the least active antibiotics were azithromycin (4.2% susceptible), oral penicillin (4.8% susceptible) and cefaclor (8% susceptible). EUCAST breakpoints are not provided for amoxicillin/clavulanic acid, but susceptibility to other agents was generally lower than that defined by CLSI breakpoints. This included ofloxacin (93.4% using CLSI breakpoints, 0% using EUCAST breakpoints), where EUCAST breakpoints define almost all isolates as having intermediate susceptibility (Table 3).
CLSI guidelines indicate that isolates susceptible to penicillin G (MIC ≤0.06 mg/L) can be reported as susceptible to amoxicillin, amoxicillin/clavulanic acid, ceftriaxone, cefaclor and cefuroxime. This study confirmed this, in that all penicillin-susceptible S. pneumoniae were also susceptible to the β-lactams listed above. However, the reverse was not always found. Of the 275 penicillin-non-susceptible isolates, 206 were amoxicillin/clavulanic acid susceptible (74.9%) and 40 were cefuroxime susceptible (14.5%). However, only nine penicillin-susceptible S. pneumoniae were cefaclor susceptible (27.7%). A similar ‘expert rule’ is provided by EUCAST but for penicillins only, i.e. amoxicillin/clavulanic acid (amoxicillin) in this study. However, unlike CLSI, individual breakpoints are not provided by EUCAST for amoxicillin/clavulanate to make this comparison.
Prevalence of antibiotic susceptibility among penicillin-susceptible S. pneumoniae (PSSP), penicillin-intermediate S. pneumoniae (PISP) and penicillin-resistant S. pneumoniae (PRSP) isolates (based on CLSI oral breakpoints)
PSSP isolates were also fully susceptible to amoxicillin/clavulanic acid (amoxicillin) and other β-lactam agents (Figure 2). However only 9/14 isolates (64.3%) were susceptible to azithromycin, chloramphenicol or clindamycin. Of the PISP, ≥90% were susceptible to amoxicillin/clavulanic acid (amoxicillin) and ofloxacin, with <40% susceptible to the other antibiotics tested (Figure 2). For PRSP isolates activity of other agents was very poor, but amoxicillin/clavulanic acid (amoxicillin) was active against 52.9% (73/138) of penicillin-resistant pneumococci. Ofloxacin and vancomycin were active against 97.1% (134/138) and 100% (138/138) of these strains, respectively.
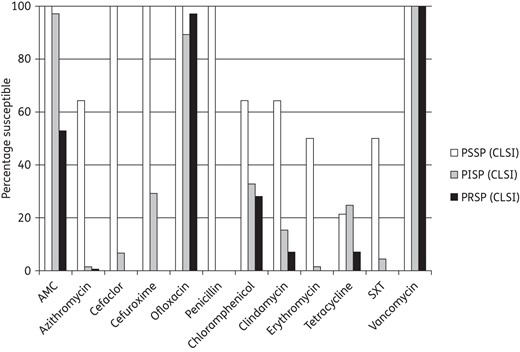
Percentage susceptible rates for antimicrobials according to CLSI breakpoints against penicillin-susceptible (PSSP), penicillin-intermediate (PISP) and penicillin-resistant S. pneumoniae (PRSP). AMC, amoxicillin/clavulanate; SXT, trimethoprim/sulfamethoxazole. Penicillin susceptibility category was based on oral penicillin CLSI breakpoints.
H. influenzae
A total of 195 H. influenzae were collected from 11 different centres in Vietnam from 2009–11. These isolates were mostly from sputum (n = 90; 46.2%) and bronchoalveolar lavage (n = 54; 27.7%). Less frequently, isolates were from blood (n= 17; 8.7%), CSF (n = 16; 8.2%) and sinuses (n= 15; 7.7%). The remaining three isolates were from middle ear pus/exudates. Most isolates of H. influenzae (n = 115, 59.0%) came from paediatric patients, 27 (13.8%) from elderly patients, 43 (22.1%) from adults and 10 (5.1%) from patients of unknown age.
Overall, 40.5% (79/195) were β-lactamase positive and 59.5% (116/195) were β-lactamase negative. Among the latter, 27 were β-lactamase negative but ampicillin resistant (BLNAR), comprising 13.8% of all H. influenzae isolates according to CLSI definitions (ampicillin MICs ≥4 mg/L). Using EUCAST definitions (ampicillin MIC ≥2 mg/L), 48 would be considered BLNAR (24.6% of total). For analysis the BLNAR were included within the β-lactamase-negative population.
Summary MIC susceptibility data and MIC distributions for all 195 H. influenzae are shown in Tables 6 and 7, respectively and Figure 3. Susceptibility data for antimicrobials tested using disc diffusion are shown in Table 8.
| Antimicrobial . | Isolate group . | . | . | . | . | . | Susceptibility . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) . | CLSI . | PK/PD . | EUCAST . | ||||||||||
| n . | 50% . | 90% . | min . | max . | %S . | %I . | %R . | %S . | %S . | %I . | %R . | ||
| Amoxicillin/ clavulanic acida,d | all | 195 | 1 | 4 | 0.25 | 8 | 97.4 (84.1)f | — | 2.6 (15.9)f | 79.5 (97.4) | 79.5 (63.1)f | — | 20.5 (36.9)f |
| BL neg | 116 | 1 | 4 | 0.25 | 8 | 99.1 | — | 0.9 | 84.5 (99.1) | 84.5 | — | 15.5 | |
| BL pos | 79 | 2 | 4 | 0.5 | 8 | 94.9 | — | 5.1 | 72.2 (94.9) | 72.2 | — | 27.8 | |
| Ampicillinb | all | 195 | 4 | 256 | 0.12 | 256 | 35.9 | 12.8 | 51.3 | NA | 35.9 | — | 64.1 |
| BL neg | 116 | 1 | 8 | 0.12 | 256 | 58.6 | 18.1 | 23.3 | NA | 58.6 | — | 41.4 | |
| BL pos | 79 | 256 | 256 | 0.25 | 256 | 2.5 | 5.1 | 92.4 | NA | 2.5 | — | 97.5 | |
| Azithromycinc | all | 195 | 4 | 256 | 0.25 | 256 | 79.5 | — | — | NA | NA | NA | NA |
| BL neg | 116 | 4 | 256 | 0.5 | 256 | 78.4 | — | — | NA | NA | NA | NA | |
| BL pos | 79 | 4 | 16 | 0.25 | 256 | 81 | — | — | NA | NA | NA | NA | |
| Cefaclord | all | 195 | 8 | 256 | 1 | 256 | 61.5 (54.9)f | 9.7 (8.2)f | 28.7 (36.9)f | 0.5 | NA | NA | NA |
| BL neg | 116 | 4 | 256 | 1 | 256 | 72.4 | 6.9 | 20.7 | 0.9 | NA | NA | NA | |
| BL pos | 79 | 16 | 256 | 2 | 256 | 45.6 | 13.9 | 40.5 | 0 | NA | NA | NA | |
| Ceftriaxone | all | 195 | 0.25 | 1 | 0.03 | 4 | 99.5 | — | — | 94.4 | 44.1 | — | 55.9 |
| BL neg | 116 | 0.12 | 1 | 0.03 | 4 | 99.1 | — | — | 96.6 | 51.7 | — | 48.3 | |
| BL pos | 79 | 0.5 | 1 | 0.03 | 2 | 100 | — | — | 91.1 | 32.9 | — | 67.1 | |
| Cefuroximed,e | all | 195 | 2 | 256 | 0.25 | 256 | 70.3 (65.1)f | 3.1 (1.5)f | 26.7 (33.3)f | 39 | 0.5 (0.5)f | 38.5 (35.4)f | 61 (64.1)f |
| BL neg | 116 | 2 | 256 | 0.25 | 256 | 76.7 | 4.3 | 19.0 | 45.7 | 0.9 | 44.8 | 54.3 | |
| BL pos | 79 | 2 | 128 | 0.25 | 256 | 60.8 | 1.3 | 38 | 29.1 | 0 | 29.1 | 70.9 | |
| Clarithromycin | all | 195 | 16 | 32 | 2 | 256 | 75.9 | 16.9 | 7.2 | NA | NA | NA | NA |
| BL neg | 116 | 16 | 32 | 4 | 256 | 75.9 | 17.2 | 6.9 | NA | NA | NA | NA | |
| BL pos | 79 | 16 | 32 | 2 | 256 | 75.9 | 16.5 | 7.6 | NA | NA | NA | NA | |
| Antimicrobial . | Isolate group . | . | . | . | . | . | Susceptibility . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) . | CLSI . | PK/PD . | EUCAST . | ||||||||||
| n . | 50% . | 90% . | min . | max . | %S . | %I . | %R . | %S . | %S . | %I . | %R . | ||
| Amoxicillin/ clavulanic acida,d | all | 195 | 1 | 4 | 0.25 | 8 | 97.4 (84.1)f | — | 2.6 (15.9)f | 79.5 (97.4) | 79.5 (63.1)f | — | 20.5 (36.9)f |
| BL neg | 116 | 1 | 4 | 0.25 | 8 | 99.1 | — | 0.9 | 84.5 (99.1) | 84.5 | — | 15.5 | |
| BL pos | 79 | 2 | 4 | 0.5 | 8 | 94.9 | — | 5.1 | 72.2 (94.9) | 72.2 | — | 27.8 | |
| Ampicillinb | all | 195 | 4 | 256 | 0.12 | 256 | 35.9 | 12.8 | 51.3 | NA | 35.9 | — | 64.1 |
| BL neg | 116 | 1 | 8 | 0.12 | 256 | 58.6 | 18.1 | 23.3 | NA | 58.6 | — | 41.4 | |
| BL pos | 79 | 256 | 256 | 0.25 | 256 | 2.5 | 5.1 | 92.4 | NA | 2.5 | — | 97.5 | |
| Azithromycinc | all | 195 | 4 | 256 | 0.25 | 256 | 79.5 | — | — | NA | NA | NA | NA |
| BL neg | 116 | 4 | 256 | 0.5 | 256 | 78.4 | — | — | NA | NA | NA | NA | |
| BL pos | 79 | 4 | 16 | 0.25 | 256 | 81 | — | — | NA | NA | NA | NA | |
| Cefaclord | all | 195 | 8 | 256 | 1 | 256 | 61.5 (54.9)f | 9.7 (8.2)f | 28.7 (36.9)f | 0.5 | NA | NA | NA |
| BL neg | 116 | 4 | 256 | 1 | 256 | 72.4 | 6.9 | 20.7 | 0.9 | NA | NA | NA | |
| BL pos | 79 | 16 | 256 | 2 | 256 | 45.6 | 13.9 | 40.5 | 0 | NA | NA | NA | |
| Ceftriaxone | all | 195 | 0.25 | 1 | 0.03 | 4 | 99.5 | — | — | 94.4 | 44.1 | — | 55.9 |
| BL neg | 116 | 0.12 | 1 | 0.03 | 4 | 99.1 | — | — | 96.6 | 51.7 | — | 48.3 | |
| BL pos | 79 | 0.5 | 1 | 0.03 | 2 | 100 | — | — | 91.1 | 32.9 | — | 67.1 | |
| Cefuroximed,e | all | 195 | 2 | 256 | 0.25 | 256 | 70.3 (65.1)f | 3.1 (1.5)f | 26.7 (33.3)f | 39 | 0.5 (0.5)f | 38.5 (35.4)f | 61 (64.1)f |
| BL neg | 116 | 2 | 256 | 0.25 | 256 | 76.7 | 4.3 | 19.0 | 45.7 | 0.9 | 44.8 | 54.3 | |
| BL pos | 79 | 2 | 128 | 0.25 | 256 | 60.8 | 1.3 | 38 | 29.1 | 0 | 29.1 | 70.9 | |
| Clarithromycin | all | 195 | 16 | 32 | 2 | 256 | 75.9 | 16.9 | 7.2 | NA | NA | NA | NA |
| BL neg | 116 | 16 | 32 | 4 | 256 | 75.9 | 17.2 | 6.9 | NA | NA | NA | NA | |
| BL pos | 79 | 16 | 32 | 2 | 256 | 75.9 | 16.5 | 7.6 | NA | NA | NA | NA | |
S, susceptible; I, intermediate; R, resistant; NT, not tested; NA, not applicable; BL, β-lactamase; min, minimum; max, maximum; pos, positive; neg, negative.
aAmoxicillin/clavulanic acid was tested at a 2:1 amoxicillin to clavulanic acid ratio; breakpoints are expressed as the amoxicillin component. PK/PD breakpoints based on high dose (4 g of amoxicillin with 250 mg of clavulanate per day for adults) are shown in parentheses, which is the same as CLSI for H. influenzae and one dilution higher for S. pneumoniae.
bIn clinical settings, all β-lactamase-positive H. influenzae should be considered resistant.
cbioMérieux Etest® breakpoints for incubation in CO2 used for macrolides.
dIn clinical settings, isolates of BLNAR are considered resistant to amoxicillin/clavulanic acid, cefaclor and cefuroxime (see main text).
eBreakpoints used are for cefuroxime axetil.
fClinical susceptibility to amoxicillin/clavulanic acid reduced (data in parenthesis) due to corrections according to BLNAR (see main text).
| Antimicrobial . | Isolate group . | . | . | . | . | . | Susceptibility . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) . | CLSI . | PK/PD . | EUCAST . | ||||||||||
| n . | 50% . | 90% . | min . | max . | %S . | %I . | %R . | %S . | %S . | %I . | %R . | ||
| Amoxicillin/ clavulanic acida,d | all | 195 | 1 | 4 | 0.25 | 8 | 97.4 (84.1)f | — | 2.6 (15.9)f | 79.5 (97.4) | 79.5 (63.1)f | — | 20.5 (36.9)f |
| BL neg | 116 | 1 | 4 | 0.25 | 8 | 99.1 | — | 0.9 | 84.5 (99.1) | 84.5 | — | 15.5 | |
| BL pos | 79 | 2 | 4 | 0.5 | 8 | 94.9 | — | 5.1 | 72.2 (94.9) | 72.2 | — | 27.8 | |
| Ampicillinb | all | 195 | 4 | 256 | 0.12 | 256 | 35.9 | 12.8 | 51.3 | NA | 35.9 | — | 64.1 |
| BL neg | 116 | 1 | 8 | 0.12 | 256 | 58.6 | 18.1 | 23.3 | NA | 58.6 | — | 41.4 | |
| BL pos | 79 | 256 | 256 | 0.25 | 256 | 2.5 | 5.1 | 92.4 | NA | 2.5 | — | 97.5 | |
| Azithromycinc | all | 195 | 4 | 256 | 0.25 | 256 | 79.5 | — | — | NA | NA | NA | NA |
| BL neg | 116 | 4 | 256 | 0.5 | 256 | 78.4 | — | — | NA | NA | NA | NA | |
| BL pos | 79 | 4 | 16 | 0.25 | 256 | 81 | — | — | NA | NA | NA | NA | |
| Cefaclord | all | 195 | 8 | 256 | 1 | 256 | 61.5 (54.9)f | 9.7 (8.2)f | 28.7 (36.9)f | 0.5 | NA | NA | NA |
| BL neg | 116 | 4 | 256 | 1 | 256 | 72.4 | 6.9 | 20.7 | 0.9 | NA | NA | NA | |
| BL pos | 79 | 16 | 256 | 2 | 256 | 45.6 | 13.9 | 40.5 | 0 | NA | NA | NA | |
| Ceftriaxone | all | 195 | 0.25 | 1 | 0.03 | 4 | 99.5 | — | — | 94.4 | 44.1 | — | 55.9 |
| BL neg | 116 | 0.12 | 1 | 0.03 | 4 | 99.1 | — | — | 96.6 | 51.7 | — | 48.3 | |
| BL pos | 79 | 0.5 | 1 | 0.03 | 2 | 100 | — | — | 91.1 | 32.9 | — | 67.1 | |
| Cefuroximed,e | all | 195 | 2 | 256 | 0.25 | 256 | 70.3 (65.1)f | 3.1 (1.5)f | 26.7 (33.3)f | 39 | 0.5 (0.5)f | 38.5 (35.4)f | 61 (64.1)f |
| BL neg | 116 | 2 | 256 | 0.25 | 256 | 76.7 | 4.3 | 19.0 | 45.7 | 0.9 | 44.8 | 54.3 | |
| BL pos | 79 | 2 | 128 | 0.25 | 256 | 60.8 | 1.3 | 38 | 29.1 | 0 | 29.1 | 70.9 | |
| Clarithromycin | all | 195 | 16 | 32 | 2 | 256 | 75.9 | 16.9 | 7.2 | NA | NA | NA | NA |
| BL neg | 116 | 16 | 32 | 4 | 256 | 75.9 | 17.2 | 6.9 | NA | NA | NA | NA | |
| BL pos | 79 | 16 | 32 | 2 | 256 | 75.9 | 16.5 | 7.6 | NA | NA | NA | NA | |
| Antimicrobial . | Isolate group . | . | . | . | . | . | Susceptibility . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) . | CLSI . | PK/PD . | EUCAST . | ||||||||||
| n . | 50% . | 90% . | min . | max . | %S . | %I . | %R . | %S . | %S . | %I . | %R . | ||
| Amoxicillin/ clavulanic acida,d | all | 195 | 1 | 4 | 0.25 | 8 | 97.4 (84.1)f | — | 2.6 (15.9)f | 79.5 (97.4) | 79.5 (63.1)f | — | 20.5 (36.9)f |
| BL neg | 116 | 1 | 4 | 0.25 | 8 | 99.1 | — | 0.9 | 84.5 (99.1) | 84.5 | — | 15.5 | |
| BL pos | 79 | 2 | 4 | 0.5 | 8 | 94.9 | — | 5.1 | 72.2 (94.9) | 72.2 | — | 27.8 | |
| Ampicillinb | all | 195 | 4 | 256 | 0.12 | 256 | 35.9 | 12.8 | 51.3 | NA | 35.9 | — | 64.1 |
| BL neg | 116 | 1 | 8 | 0.12 | 256 | 58.6 | 18.1 | 23.3 | NA | 58.6 | — | 41.4 | |
| BL pos | 79 | 256 | 256 | 0.25 | 256 | 2.5 | 5.1 | 92.4 | NA | 2.5 | — | 97.5 | |
| Azithromycinc | all | 195 | 4 | 256 | 0.25 | 256 | 79.5 | — | — | NA | NA | NA | NA |
| BL neg | 116 | 4 | 256 | 0.5 | 256 | 78.4 | — | — | NA | NA | NA | NA | |
| BL pos | 79 | 4 | 16 | 0.25 | 256 | 81 | — | — | NA | NA | NA | NA | |
| Cefaclord | all | 195 | 8 | 256 | 1 | 256 | 61.5 (54.9)f | 9.7 (8.2)f | 28.7 (36.9)f | 0.5 | NA | NA | NA |
| BL neg | 116 | 4 | 256 | 1 | 256 | 72.4 | 6.9 | 20.7 | 0.9 | NA | NA | NA | |
| BL pos | 79 | 16 | 256 | 2 | 256 | 45.6 | 13.9 | 40.5 | 0 | NA | NA | NA | |
| Ceftriaxone | all | 195 | 0.25 | 1 | 0.03 | 4 | 99.5 | — | — | 94.4 | 44.1 | — | 55.9 |
| BL neg | 116 | 0.12 | 1 | 0.03 | 4 | 99.1 | — | — | 96.6 | 51.7 | — | 48.3 | |
| BL pos | 79 | 0.5 | 1 | 0.03 | 2 | 100 | — | — | 91.1 | 32.9 | — | 67.1 | |
| Cefuroximed,e | all | 195 | 2 | 256 | 0.25 | 256 | 70.3 (65.1)f | 3.1 (1.5)f | 26.7 (33.3)f | 39 | 0.5 (0.5)f | 38.5 (35.4)f | 61 (64.1)f |
| BL neg | 116 | 2 | 256 | 0.25 | 256 | 76.7 | 4.3 | 19.0 | 45.7 | 0.9 | 44.8 | 54.3 | |
| BL pos | 79 | 2 | 128 | 0.25 | 256 | 60.8 | 1.3 | 38 | 29.1 | 0 | 29.1 | 70.9 | |
| Clarithromycin | all | 195 | 16 | 32 | 2 | 256 | 75.9 | 16.9 | 7.2 | NA | NA | NA | NA |
| BL neg | 116 | 16 | 32 | 4 | 256 | 75.9 | 17.2 | 6.9 | NA | NA | NA | NA | |
| BL pos | 79 | 16 | 32 | 2 | 256 | 75.9 | 16.5 | 7.6 | NA | NA | NA | NA | |
S, susceptible; I, intermediate; R, resistant; NT, not tested; NA, not applicable; BL, β-lactamase; min, minimum; max, maximum; pos, positive; neg, negative.
aAmoxicillin/clavulanic acid was tested at a 2:1 amoxicillin to clavulanic acid ratio; breakpoints are expressed as the amoxicillin component. PK/PD breakpoints based on high dose (4 g of amoxicillin with 250 mg of clavulanate per day for adults) are shown in parentheses, which is the same as CLSI for H. influenzae and one dilution higher for S. pneumoniae.
bIn clinical settings, all β-lactamase-positive H. influenzae should be considered resistant.
cbioMérieux Etest® breakpoints for incubation in CO2 used for macrolides.
dIn clinical settings, isolates of BLNAR are considered resistant to amoxicillin/clavulanic acid, cefaclor and cefuroxime (see main text).
eBreakpoints used are for cefuroxime axetil.
fClinical susceptibility to amoxicillin/clavulanic acid reduced (data in parenthesis) due to corrections according to BLNAR (see main text).
| Antimicrobial . | Number of isolates at MIC (mg/L) . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.03 . | 0.06 . | 0.12 . | 0.25 . | 0.5 . | 1 . | 2 . | 4 . | 8 . | 16 . | 32 . | 64 . | 128 . | 256 . | |
| Amoxicillin/clavulanic acid | 1 | 3 | 32 | 66 | 53 | 35 | 5 | |||||||
| Ampicillin | 1 | 4 | 24 | 18 | 23 | 25 | 21 | 4 | 12 | 12 | 5 | 4 | 42 | |
| Azithromycin | 3 | 1 | 13 | 53 | 65 | 20 | 16 | 2 | 22 | |||||
| Cefaclor | 1 | 4 | 52 | 39 | 24 | 19 | 7 | 6 | 43 | |||||
| Ceftriaxone | 72 | 9 | 5 | 16 | 50 | 32 | 10 | 1 | ||||||
| Cefuroxime | 1 | 6 | 21 | 48 | 39 | 22 | 6 | 7 | 11 | 11 | 3 | 20 | ||
| Clarithromycin | 2 | 13 | 63 | 70 | 33 | 6 | 1 | 7 | ||||||
| Antimicrobial . | Number of isolates at MIC (mg/L) . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.03 . | 0.06 . | 0.12 . | 0.25 . | 0.5 . | 1 . | 2 . | 4 . | 8 . | 16 . | 32 . | 64 . | 128 . | 256 . | |
| Amoxicillin/clavulanic acid | 1 | 3 | 32 | 66 | 53 | 35 | 5 | |||||||
| Ampicillin | 1 | 4 | 24 | 18 | 23 | 25 | 21 | 4 | 12 | 12 | 5 | 4 | 42 | |
| Azithromycin | 3 | 1 | 13 | 53 | 65 | 20 | 16 | 2 | 22 | |||||
| Cefaclor | 1 | 4 | 52 | 39 | 24 | 19 | 7 | 6 | 43 | |||||
| Ceftriaxone | 72 | 9 | 5 | 16 | 50 | 32 | 10 | 1 | ||||||
| Cefuroxime | 1 | 6 | 21 | 48 | 39 | 22 | 6 | 7 | 11 | 11 | 3 | 20 | ||
| Clarithromycin | 2 | 13 | 63 | 70 | 33 | 6 | 1 | 7 | ||||||
| Antimicrobial . | Number of isolates at MIC (mg/L) . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.03 . | 0.06 . | 0.12 . | 0.25 . | 0.5 . | 1 . | 2 . | 4 . | 8 . | 16 . | 32 . | 64 . | 128 . | 256 . | |
| Amoxicillin/clavulanic acid | 1 | 3 | 32 | 66 | 53 | 35 | 5 | |||||||
| Ampicillin | 1 | 4 | 24 | 18 | 23 | 25 | 21 | 4 | 12 | 12 | 5 | 4 | 42 | |
| Azithromycin | 3 | 1 | 13 | 53 | 65 | 20 | 16 | 2 | 22 | |||||
| Cefaclor | 1 | 4 | 52 | 39 | 24 | 19 | 7 | 6 | 43 | |||||
| Ceftriaxone | 72 | 9 | 5 | 16 | 50 | 32 | 10 | 1 | ||||||
| Cefuroxime | 1 | 6 | 21 | 48 | 39 | 22 | 6 | 7 | 11 | 11 | 3 | 20 | ||
| Clarithromycin | 2 | 13 | 63 | 70 | 33 | 6 | 1 | 7 | ||||||
| Antimicrobial . | Number of isolates at MIC (mg/L) . | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.03 . | 0.06 . | 0.12 . | 0.25 . | 0.5 . | 1 . | 2 . | 4 . | 8 . | 16 . | 32 . | 64 . | 128 . | 256 . | |
| Amoxicillin/clavulanic acid | 1 | 3 | 32 | 66 | 53 | 35 | 5 | |||||||
| Ampicillin | 1 | 4 | 24 | 18 | 23 | 25 | 21 | 4 | 12 | 12 | 5 | 4 | 42 | |
| Azithromycin | 3 | 1 | 13 | 53 | 65 | 20 | 16 | 2 | 22 | |||||
| Cefaclor | 1 | 4 | 52 | 39 | 24 | 19 | 7 | 6 | 43 | |||||
| Ceftriaxone | 72 | 9 | 5 | 16 | 50 | 32 | 10 | 1 | ||||||
| Cefuroxime | 1 | 6 | 21 | 48 | 39 | 22 | 6 | 7 | 11 | 11 | 3 | 20 | ||
| Clarithromycin | 2 | 13 | 63 | 70 | 33 | 6 | 1 | 7 | ||||||
Susceptibility data for antimicrobials tested against H. influenzae isolates (n = 195) using CLSI disc diffusion
| . | . | Susceptible . | Intermediate . | Resistant . | |||
|---|---|---|---|---|---|---|---|
| Antimicrobial . | . | n . | (%) . | n . | (%) . | n . | (%) . |
| Chloramphenicol | all | 8 | (4.1) | 35 | (17.9) | 152 | (77.9) |
| β-lactamase neg | 6 | (5.2) | 29 | (25.0) | 81 | (69.8) | |
| β-lactamase pos | 2 | (2.5) | 6 | (7.6) | 71 | (89.9) | |
| Tetracycline | all | 3 | (1.5) | 12 | (6.2) | 180 | (92.3) |
| β-lactamase neg | 2 | (1.7) | 11 | (9.5) | 103 | (88.8) | |
| β-lactamase pos | 1 | (1.3) | 1 | (1.3) | 77 | (97.5) | |
| Trimethoprim/sulfamethoxazole | all | 31 | (15.9) | 4 | (2.1) | 160 | (82.1) |
| β-lactamase neg | 27 | (23.3) | 2 | (1.7) | 87 | (75.0) | |
| β-lactamase pos | 4 | (5.1) | 2 | (2.5) | 73 | (92.4) | |
| . | . | Susceptible . | Intermediate . | Resistant . | |||
|---|---|---|---|---|---|---|---|
| Antimicrobial . | . | n . | (%) . | n . | (%) . | n . | (%) . |
| Chloramphenicol | all | 8 | (4.1) | 35 | (17.9) | 152 | (77.9) |
| β-lactamase neg | 6 | (5.2) | 29 | (25.0) | 81 | (69.8) | |
| β-lactamase pos | 2 | (2.5) | 6 | (7.6) | 71 | (89.9) | |
| Tetracycline | all | 3 | (1.5) | 12 | (6.2) | 180 | (92.3) |
| β-lactamase neg | 2 | (1.7) | 11 | (9.5) | 103 | (88.8) | |
| β-lactamase pos | 1 | (1.3) | 1 | (1.3) | 77 | (97.5) | |
| Trimethoprim/sulfamethoxazole | all | 31 | (15.9) | 4 | (2.1) | 160 | (82.1) |
| β-lactamase neg | 27 | (23.3) | 2 | (1.7) | 87 | (75.0) | |
| β-lactamase pos | 4 | (5.1) | 2 | (2.5) | 73 | (92.4) | |
pos, positive; neg, negative.
Susceptibility data for antimicrobials tested against H. influenzae isolates (n = 195) using CLSI disc diffusion
| . | . | Susceptible . | Intermediate . | Resistant . | |||
|---|---|---|---|---|---|---|---|
| Antimicrobial . | . | n . | (%) . | n . | (%) . | n . | (%) . |
| Chloramphenicol | all | 8 | (4.1) | 35 | (17.9) | 152 | (77.9) |
| β-lactamase neg | 6 | (5.2) | 29 | (25.0) | 81 | (69.8) | |
| β-lactamase pos | 2 | (2.5) | 6 | (7.6) | 71 | (89.9) | |
| Tetracycline | all | 3 | (1.5) | 12 | (6.2) | 180 | (92.3) |
| β-lactamase neg | 2 | (1.7) | 11 | (9.5) | 103 | (88.8) | |
| β-lactamase pos | 1 | (1.3) | 1 | (1.3) | 77 | (97.5) | |
| Trimethoprim/sulfamethoxazole | all | 31 | (15.9) | 4 | (2.1) | 160 | (82.1) |
| β-lactamase neg | 27 | (23.3) | 2 | (1.7) | 87 | (75.0) | |
| β-lactamase pos | 4 | (5.1) | 2 | (2.5) | 73 | (92.4) | |
| . | . | Susceptible . | Intermediate . | Resistant . | |||
|---|---|---|---|---|---|---|---|
| Antimicrobial . | . | n . | (%) . | n . | (%) . | n . | (%) . |
| Chloramphenicol | all | 8 | (4.1) | 35 | (17.9) | 152 | (77.9) |
| β-lactamase neg | 6 | (5.2) | 29 | (25.0) | 81 | (69.8) | |
| β-lactamase pos | 2 | (2.5) | 6 | (7.6) | 71 | (89.9) | |
| Tetracycline | all | 3 | (1.5) | 12 | (6.2) | 180 | (92.3) |
| β-lactamase neg | 2 | (1.7) | 11 | (9.5) | 103 | (88.8) | |
| β-lactamase pos | 1 | (1.3) | 1 | (1.3) | 77 | (97.5) | |
| Trimethoprim/sulfamethoxazole | all | 31 | (15.9) | 4 | (2.1) | 160 | (82.1) |
| β-lactamase neg | 27 | (23.3) | 2 | (1.7) | 87 | (75.0) | |
| β-lactamase pos | 4 | (5.1) | 2 | (2.5) | 73 | (92.4) | |
pos, positive; neg, negative.
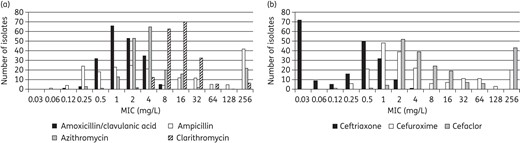
MIC distribution for (a) amoxicillin/clavulanic acid, azithromycin, clarithromycin and ampicillin and (b) cefaclor, cefuroxime and ceftriaxone against all H. influenzae.
A total of 35.9% (70/195) H. influenzae were susceptible to ampicillin as defined by CLSI or EUCAST breakpoints (Table 6). As would be expected, ampicillin was inactive against the β-lactamase-positive strains (Table 6). The older agents tested by disc diffusion were poorly active against H. influenzae (Table 8).
H. influenzae in vitro susceptibility to amoxicillin/clavulanic acid was high, at ∼95% or above, including β-lactamase-positive isolates, as defined by CLSI breakpoints and by high-dose PK/PD breakpoints (Table 6). Applying EUCAST and low-dose PK/PD breakpoints, susceptibility to amoxicillin/clavulanic acid was reduced to 79.5% (155/195) overall. As indicated with CLSI breakpoints, the BLNAR strains (n = 48 according to EUCAST) are also considered resistant to amoxicillin/clavulanic acid cefaclor and cefuroxime by EUCAST. This translates to a ‘clinical’ susceptibility to amoxicillin/clavulanic acid of 63.1% by EUCAST.
High susceptibility (≥90%) to ceftriaxone was observed, as defined by CLSI and PK/PD breakpoints (Table 6). However, with EUCAST breakpoints, susceptibility to ceftriaxone was reduced considerably to 44.1% (86/195) against all isolates and 32.9% (64/195) against the β-lactamase-positive isolates (Table 6).
Applying CLSI breakpoints, 61.5% (120/195) and 70.3% (137/195) of the H. influenzae isolates were susceptible to cefaclor and cefuroxime, respectively (Table 6). Susceptibility to cefuroxime was much lower, at 39.0% (76/195), and cefaclor susceptibility was virtually zero (Table 6) as defined by PK/PD breakpoints. (EUCAST does not provide breakpoints for cefaclor and H. influenzae).
A total of 75%–80% of H. influenzae (including β-lactamase-positive or -negative isolates) were susceptible to azithromycin and clarithromycin as defined by CLSI breakpoints (Table 6).
The overall trend was for H. influenzae susceptibility as defined by EUCAST breakpoints to be reduced compared with CLSI breakpoints for all antimicrobials (Figure 4).
Based on CLSI breakpoints, the most active antibiotics were ceftriaxone (99.5% susceptible) and amoxicillin/clavulanic acid (84.1% susceptible adjusted for BLNAR). Based on EUCAST breakpoints, the prevalence of susceptibility to amoxicillin/clavulanic acid was the highest (63.1% adjusted for BLNAR). Based on PK/PD breakpoints, the most active antibiotics were amoxicillin/clavulanic acid (high dose, 97.4%) and ceftriaxone (94.4%).
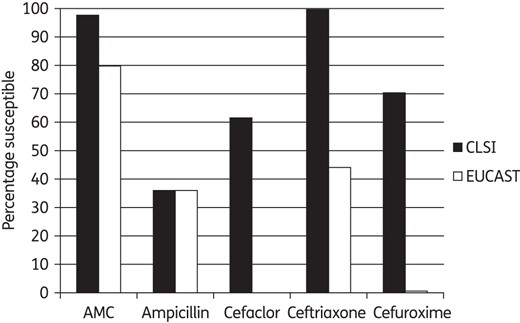
Percentage susceptible rates for antimicrobials according to CLSI and EUCAST breakpoints against H. influenzae. AMC, amoxicillin/clavulanate.
Sub-population analysis
An analysis was made comparing antimicrobial susceptibility among age groups. Susceptibility data by age groups as defined by CLSI breakpoints are given in Figures 5 and 6. There was no statistically significant difference in H. influenzae antimicrobial susceptibility by age group (Figure 5). Every antimicrobial except ofloxacin was statistically less active against S. pneumoniae isolates from paediatric patients than from the elderly (Figure 6). However, no statistically significant differences were observed between the adult group when compared with the paediatric or elderly groups, with the exception of azithromycin, where paediatric isolates were also significantly less susceptible than adult isolates. Ofloxacin was different from all other antimicrobials tested, with paediatric isolates more susceptible than those from both adults and the elderly (Figure 6).
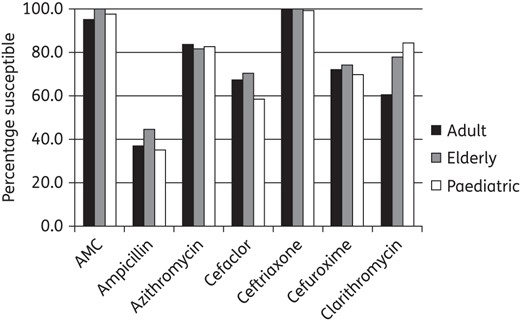
Percentage susceptible rates for antimicrobials against H. influenzae by age group according to CLSI breakpoints. No significant difference was found between the age groups. AMC, amoxicillin/clavulanate.
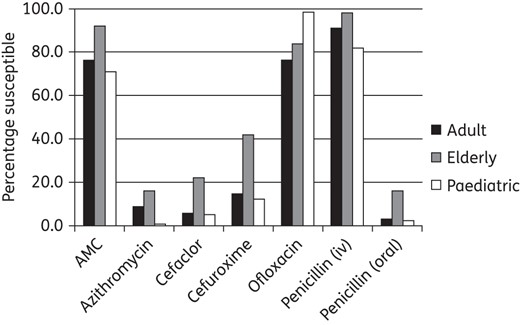
Percentage susceptibility rates for antimicrobials against S. pneumoniae by age group according to CLSI breakpoints. Paediatric susceptibility was significantly lower than in the elderly (but not adults) for amoxicillin/clavulanic acid (P = 0.001), cefaclor (P = 0.001), cefuroxime (P < 0.0001), penicillin iv (P = 0.003) and penicillin oral (P = 0.001). Paediatric susceptibility was significantly lower than in the elderly and adults for azithromycin (P < 0.0001 and 0.013, respectively). Paediatric susceptibility was significantly higher than in the elderly and adults for ofloxacin (P = 0.0003 and <0.0001, respectively).
Discussion
The increasing prevalence of antimicrobial resistance among the major pathogens responsible for community respiratory tract infections is a serious global problem that complicates the management of these infections. SOAR assesses antimicrobial resistance in key respiratory pathogens and is currently conducted throughout the Middle East, Africa, Latin America, Asia-Pacific and Commonwealth of Independent States countries, and has been running since 2002. The study was established to provide information on local resistance patterns among the two most common pulmonary pathogens, S. pneumoniae and H. influenzae. In S. pneumoniae, resistance to antibiotics is a growing problem and has become an important prognostic factor, since it is directly associated with persistent disease or disease mortality.8
Data from this current Vietnam SOAR study highlight numerous important features with respect to contemporary surveillance in Ho Chi Minh City and Hanoi. As defined by CLSI oral and standard EUCAST penicillin breakpoints, the vast majority (95.1%) of pneumococci were penicillin non-susceptible. However, applying the higher-dose EUCAST or the CLSI iv penicillin breakpoints, up to 86.9% may be considered susceptible to penicillin. In a similar way, 76.1% of pneumococci were susceptible as defined by CLSI and ‘normal-dose’ amoxicillin/clavulanic acid (and by inference amoxicillin) breakpoints, but higher-dose PK/PD breakpoints increased susceptibility to 96.9%. Therefore, the choice of appropriate breakpoints and dosing of penicillin and amoxicillin/clavulanic acid (or amoxicillin) are important factors to consider when assessing appropriate antimicrobial use. Other β-lactams (cefaclor and cefuroxime) were less active (≤18.7% susceptible) than amoxicillin/clavulanic acid (amoxicillin) or low-dose penicillin regardless of breakpoint methodology. Other classes of antimicrobial, including macrolides, were also poorly active. The exception was ofloxacin, where ∼94% of pneumococci were susceptible as defined by CLSI breakpoints. Therefore, >90% susceptibility of S. pneumoniae to an antimicrobial was only observed with amoxicillin/clavulanic acid (high dose)/amoxicillin and ofloxacin.
The data from this study confirm that isolates of S. pneumoniae susceptible to penicillin G are also susceptible to other penicillins as inferred by CLSI and EUCAST guidelines and cephalosporins as inferred by CLSI guidelines. Interestingly, the data from this study found the reverse was not always correct using CLSI breakpoints; i.e. most penicillin non-susceptible S. pneumoniae were susceptible to amoxicillin/clavulanic acid (amoxicillin). Therefore, either the amoxicillin/clavulanic acid breakpoints are not correct or the CLSI cross-resistance statement within the β-lactam class is not correct. This warrants further investigation.
Among the isolates of H. influenzae, 54.3% were ampicillin resistant, comprising 40.5% that were β-lactamase positive and 13.8% BLNAR. However, as defined by CLSI or high-dose PK/PD breakpoints, ≥95% of isolates (84.1% accounting for BLNAR) were susceptible to amoxicillin/clavulanic acid (amoxicillin) in vitro. The application of EUCAST and low-dose PK/PD breakpoints gave lower rates of overall susceptibility (∼80%) in vitro (63.1% accounting for BLNAR), with ∼70% of β-lactamase-positive isolates classified as susceptible. A similar, but more dramatic, breakpoint difference was observed with ceftriaxone, where 99% susceptibility was calculated applying CLSI breakpoints but only 44.1% for EUCAST breakpoints. Cefuroxime was also affected in this way. For cefaclor there are no EUCAST breakpoints, but PK/PD breakpoints indicate that this cephalosporin is inactive.
To the best of our knowledge this is the first publication to compare susceptibility of respiratory tract pathogens defined by CLSI, EUCAST and PK/PD breakpoints. For penicillin susceptibility in S. pneumoniae, the MIC distributions in this study indicate that high-dose therapy is absolutely essential if penicillin is to be used in Vietnam. For most other antimicrobials, susceptibility was low against this pathogen irrespective of the breakpoint methodology. A comparison of S. pneumoniae susceptibility with that of ofloxacin is interesting. Applying the higher CLSI breakpoint, the majority of isolates are categorized as susceptible whereas EUCAST would define these as intermediate. This is a good example of where breakpoint harmonization is required, particularly where ofloxacin susceptibility might be used as a marker for overall fluoroquinolone susceptibility. EUCAST does not provide breakpoints for amoxicillin/clavulanic acid but indicates that susceptibility to this β-lactam/β-lactamase inhibitor may be inferred from penicillin (low dose) susceptibility. This may not be the best approach when we know that dosing of amoxicillin/clavulanic acid has an effect on PK/PD parameters and that many penicillin-non-susceptible S. pneumoniae are susceptible to amoxicillin/clavulanic acid (amoxicillin). A further impact of differences between CLSI and EUCAST breakpoints was observed with H. influenzae, where, apart from amoxicillin/clavulanic acid, the antibiotic susceptibility of isolates was much lower when applying EUCAST criteria. This again requires harmonization. These breakpoint differences are also important to understand as there is anecdotal evidence to suggest that some non-European countries are moving towards EUCAST breakpoints.
The current study included a relatively large proportion of isolates from paediatric patients (63% of S. pneumoniae and 59% of H. influenzae). Analysis of data according to patient age group clearly indicates that there is no statistical difference in antimicrobial susceptibility for H. influenzae. However, for S. pneumoniae, isolates from paediatric patients are statistically less susceptible to most agents compared with those from the elderly, but not those from adults. For azithromycin, isolates from paediatric patients are less susceptible than those from both adults and the elderly. The exception was ofloxacin, where isolates from paediatric patients were more susceptible to this antimicrobial than those from both adults and the elderly, probably due to the fact that fluoroquinolones are not used in the paediatric population. This is an important observation in guiding empirical therapy and when comparing data from other surveillance studies where patient age distribution may vary.
Earlier studies have also indicated high antibiotic resistance in Vietnam. From 2002 to 2005, >70% of pneumococcal isolates were reported as resistant to macrolides or trimethoprim/sulfamethoxazole and 38% resistant to penicillin.9 Similarly, the same study group found a 49% prevalence of β-lactamase in H. influenzae.10 In another study from Vietnam over the same time period, 90% of S. pneumoniae, 68% of H. influenzae and 74% of M. catarrhalis acquired resistance to at least one antibiotic. Moreover, 88% of S. pneumoniae and 32% of H. influenzae were resistant to tetracycline, 32% and 44% resistant to trimethoprim/sulphonamide and 25% and 24% resistant to chloramphenicol. Also, 23% of S. pneumoniae were erythromycin resistant and 18% of H. influenzae isolates were resistant to ampicillin.11 In agreement with these single-site studies, the ANSORP study data from a few years earlier (2000–01) indicate that resistance to penicillin (71.4% as defined by the CLSI oral breakpoint) and erythromycin (92.1%) in pneumococci from Vietnam was the highest in Asia.12 In a more recent Vietnamese study,13 95% of S. pneumoniae were resistant to at least one antibiotic (401/421). Furthermore, resistance to trimethoprim/sulfamethoxazole, tetracycline, penicillin, erythromycin and ciprofloxacin was 78%, 75%, 75%, 70% and 28%, respectively. However, low resistance was noted for amoxicillin (4%), benzylpenicillin (4%) and cefotaxime (2%). The level of intermediate resistance to amoxicillin was 32%. Multidrug resistance was observed in 60% of isolates. The most common pattern was co-resistance to trimethoprim/sulfamethoxazole, tetracycline and erythromycin.13 Interestingly, the most recently published ANSORP data (isolates collected in 2008–09) showed a reduction in penicillin resistance (CLSI oral breakpoint) to 17.6% in Vietnam,14 which is lower than that reported by Hoa et al.13 and in this current study.
Data from the literature and from the current ongoing SOAR study strongly highlight the importance of antimicrobial resistance surveillance in Vietnam, particularly as resistance to common antimicrobial agents is relatively high in this country. The SOAR study continues to evolve and future new data from Vietnam will further assist in understanding the longitudinal implications related to antimicrobial resistance in this country.
Funding
This study was funded by GlaxoSmithKline.
Transparency declaration
This article forms part of a Supplement sponsored by GlaxoSmithKline. N.H.L. Minh and D. Torumkuney are employees of GlaxoSmithKline. D. Torumkuney also holds shares in GlaxoSmithKline. I. Morrissey is an employee of IHMA, a medical communication and consultancy company, who participated in the exploration, interpretation of the results and preparation of this manuscript on behalf of GSK. IHMA also provided medical writing support in the form of writing assistance, collating author's comments, grammatical editing and referencing that was paid for by GSK. All other authors declare that they have no conflict of interest.
Editorial assistance was provided by Tracey Morris, Livewire Editorial Communications.
Acknowledgements
For their susceptibility data used in this analysis, we thank D. M. Phuong, L. Q. Thinh, T. T. T. Trinh, N. T. H. Nga, N. N. Lien, D. T. T. Huong, N. T. N. Anh, N. S. M. Tuyet, L. T. Cuc, D. T. T. Hang from Bach Mai Hospital in Hanoi, Vietnam; Children's Hospital No. 1 in HCMC, Vietnam; An Binh Hospital in HCMC, Vietnam; Can Tho Central Hospital in Cantho, Vietnam; Hue Central Hospital in Hue, Vietnam; Da Nang General Hospital in Danang, Vietnam; Children's Hospital No. 2 in HCMC, Vietnam; Nhan Dan Gia Dinh Hospital in HCMC, Vietnam; ENT Hospital in HCMC, Vietnam; and Children's Central Hospital in Hanoi, Vietnam.
References
- amoxicillin
- ampicillin
- vancomycin
- ceftriaxone
- azithromycin
- antibiotic resistance, bacterial
- penicillin
- cefuroxime
- haemophilus influenzae
- amoxicillin-potassium clavulanate combination
- cefaclor
- child
- ofloxacin
- respiratory tract infections
- streptococcus pneumoniae
- vietnam
- pharmacodynamics
- pathogenic organism
- antimicrobials
- anti-bacterial agents
- older adult
- surveillance, medical
- malnutrition-inflammation-cachexia syndrome



