-
PDF
- Split View
-
Views
-
Cite
Cite
Natalie Banniettis, Roopali Sharma, Ivan Hand, Charles A. Peloquin, Stephan Kohlhoff, Margaret R. Hammerschlag, Steady-state pharmacokinetics of oral linezolid suspension in a premature infant with osteomyelitis, Journal of Antimicrobial Chemotherapy, Volume 71, Issue 6, June 2016, Page 1738, https://doi.org/10.1093/jac/dkv507
Close - Share Icon Share
Sir,
Linezolid has activity against a broad range of Gram-positive bacteria, including MRSA. As infections with these organisms have become more common in infants, linezolid may have an important role, particularly in preterm newborns. Most of the published data on pharmacokinetics, efficacy and safety of linezolid are from adult studies.1,2 It is established that the pharmacokinetics of linezolid, especially clearance, varies with age. Children younger than 12 years of age have a smaller AUC, faster clearance and shorter elimination half-life than adults. Paediatric data, including from neonates, are limited and were mainly evaluated using the intravenous (iv) formulation.2–6 These studies reported considerable interindividual variability in plasma concentrations within the study populations. To date, there are no steady-state pharmacokinetic studies assessing oral linezolid suspension in infants. We report the steady-state pharmacokinetic parameters of oral linezolid suspension in a premature infant with osteomyelitis. The project was approved by the institutional review board, informed consent was obtained and the research was conducted in accordance with the Declaration of Helsinki.
A 4-month-old male infant born at 25 weeks gestation (birth weight 750 g) was being managed in the neonatal ICU for ongoing medical issues since birth; iv access proved challenging throughout the hospital course. On the 44th day of life, the infant developed a skin and soft-tissue infection of the left forearm at an old iv site, along with spontaneously draining pustules at the umbilicus. Cultures obtained from the forearm abscess grew MRSA with a vancomycin MIC of 2 mg/L. The isolate also was resistant to clindamycin. The infant was treated with vancomycin iv for 10 days, although a therapeutic trough was not achieved (3.6 mg/L). On the 89th day of life, the infant developed left ankle swelling at another old iv access site. Imaging revealed significant osteomyelitis of the distal tibia and fibula. Cultures from the bone grew a latex-negative Staphylococcus. However, further speciation and susceptibilities were not conducted as the laboratory discarded the sample. The infant was started on linezolid, iv at 10 mg/kg (29 mg) q8h. Treatment was monitored with serial serum inflammatory markers. Parenteral therapy was disrupted on several occasions as iv access was compromised. After 14 days of parenteral therapy, the infant was switched to oral linezolid suspension, 10 mg/kg q8h, for an additional 4 weeks. Blood samples obtained during the last week of oral linezolid therapy were analysed for quantification of linezolid using HPLC. Blood samples (0.5 mL) were obtained at time 0 before dosing and at 1, 1.5, 2, 4, 6 and 8 h after the oral dose of linezolid. An additional sample, 10 h after the prior dose and 2 h after the afternoon dose, also was obtained. Serum samples were shipped to the Infectious Disease Pharmacokinetics Laboratory in Gainesville (FL, USA) for serum concentration analysis. Linezolid concentrations were determined using a validated HPLC assay described previously.7
The plasma standard curve for linezolid ranged from 0.50 to 30 mg/L, with linearity extending below 0.50. The within-sample precision (percentage coefficient of variation) of validation in a single standard concentration was 0.69% and the overall validation precision across all standards was 1.04%–4.39%. Non-compartmental analysis of the data was performed with Phoenix software (v.6.4, Pharsight) to obtain the steady-state pharmacokinetic parameters. The 0 and 8 h sample concentrations (minimum plasma concentration, Cmin) were both 0.32 mg/L, confirming that steady-state had been achieved (Figure 1). The steady-state peak plasma concentration (Cmax) was 5.51 mg/L and the time to peak plasma concentration (Tmax) was 2 h. The second 2 h sample (10 h after the prior oral dose) was 3.99 mg/L, somewhat lower than the prior Cmax of 5.51 mg/L. This suggests that the rate of oral absorption varied from dose to dose. Typical linezolid peak concentrations are 12–26 mg/L ∼2 h after oral doses and trough concentrations are typically 3–9 mg/L.1–3 In this infant, despite q8h dosing, peak and trough concentrations were lower than typically seen. The volume of distribution divided by bioavailability (Vz/F), clearance divided by bioavailability (CL/F), elimination rate constant (kel, estimated with three concentrations), half-life (t1/2) and AUC0–8 were estimated at 3.48 L, 1.75 L/h, 0.50 h−1, 1.38 h and 16.56 mg · h/L, respectively.
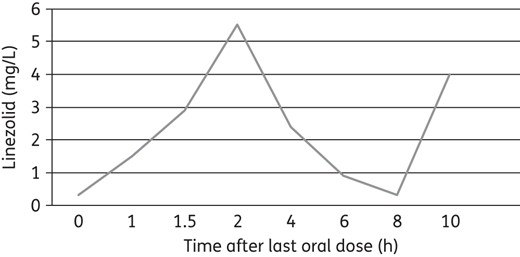
The pharmacokinetic profile of oral linezolid in our infant differed from what has been published in the literature for full-term infants. Full-term infants who received single doses of iv linezolid had much higher peak plasma concentrations, a larger AUC and a slower clearance.8 Our infant was born >3 months premature and was treated at a post-gestational age of 1 month (not equivalent to a full-term infant of the same age); therefore, direct correlation is challenging due to the differences in characteristics of the infants in the published studies.5,6,8,9 The Cmax and AUC were lower in our infant and apparent elimination t1/2 faster than what has been reported in full-term infants. Reasons for this may include incomplete absorption (F < 1), faster clearance or a smaller actual volume of distribution. We were only able to estimate Vz/F, leaving our assessment of this parameter less than optimal. Pharmacokinetic data reported in studies in infants and neonates have utilized the iv formulation of linezolid and studied the disposition of the drug after a single dose, whereas our infant received oral linezolid suspension and pharmacokinetic analysis was done at steady-state. This also may explain some of the differences in pharmacokinetic parameters. Kosaka et al.10 reported plasma levels in four children (aged <30 months) receiving linezolid for MRSA mediastinitis. A serum concentration of <2 mg/L linezolid was needed to inhibit growth for 90% of organisms (MIC90). Two patients were treated iv with a 90 min infusion at 10 mg/kg q8h initially and were switched to an oral regimen at the same dose. Two other patients were given linezolid orally at a dose of 10 or 15 mg/kg q8h. The linezolid trough concentrations were ≥3.5 mg/L in patients treated with iv linezolid. Lower trough concentrations were seen with oral administration of linezolid. In one 3-month-old child, who was the youngest, undetectable serum trough concentration (<0.1 mg/L) was documented when iv linezolid was changed to oral therapy at a dose of 10 mg/kg q8h; hence, the dose of linezolid was increased from 10 to 15 mg/kg.
Preterm infants are at increased risk of nosocomial bacterial infection with multiresistant Gram-positive pathogens. Linezolid seems to be a safe and potent alternative treatment for these infants. The benefits of linezolid compared with vancomycin include its availability as an oral formulation. Owing to the variability of linezolid kinetics in infants, especially in premature infants as in our case, we would recommend therapeutic serum concentrations be monitored at steady-state and dosing adjusted accordingly. Additional studies are needed to evaluate the steady-state pharmacokinetic disposition of oral linezolid in children.
Funding
This study was carried out as part of our routine work.
Transparency declarations
None to declare.



