-
PDF
- Split View
-
Views
-
Cite
Cite
G. M. Chong, M. T. van der Beek, P. A. von dem Borne, J. Boelens, E. Steel, G. A. Kampinga, L. F. R. Span, K. Lagrou, J. A. Maertens, G. J. H. Dingemans, G. R. Gaajetaan, D. W. E. van Tegelen, J. J. Cornelissen, A. G. Vonk, B. J. A. Rijnders, PCR-based detection of Aspergillus fumigatus Cyp51A mutations on bronchoalveolar lavage: a multicentre validation of the AsperGenius assay® in 201 patients with haematological disease suspected for invasive aspergillosis, Journal of Antimicrobial Chemotherapy, Volume 71, Issue 12, 1 December 2016, Pages 3528–3535, https://doi.org/10.1093/jac/dkw323
Close - Share Icon Share
In patients with invasive aspergillosis (IA), fungal cultures are mostly negative. Consequently, azole resistance often remains undetected. The AsperGenius® multiplex real-time PCR assay identifies clinically relevant Aspergillus species and four resistance-associated mutations (RAMs; TR34/L98H/T289A/Y121F) in the Cyp51A gene. This multicentre study evaluated the diagnostic performance of this assay on bronchoalveolar lavage (BAL) fluid and correlated the presence of RAMs with azole treatment failure and mortality.
Stored BAL samples from patients with haematological diseases with suspected IA were used. BAL samples that were galactomannan/culture positive were considered positive controls for the presence of Aspergillus. Azole treatment failure and 6 week mortality were compared in patients with and without RAMs that had received ≥5 days of voriconazole monotherapy.
Two hundred and one patients each contributed one BAL sample, of which 88 were positive controls and 113 were negative controls. The optimal cycle threshold cut-off value for the Aspergillus species PCR was <38. With this cut-off, the PCR was positive in 74/88 positive controls. The sensitivity, specificity, positive predictive value and negative predictive value were 84%, 80%, 76% and 87%, respectively. 32/74 BAL samples were culture negative. Azole treatment failure was observed in 6/8 patients with a RAM compared with 12/45 patients without RAMs (P = 0.01). Six week mortality was 2.7 times higher in patients with RAMs (50.0% versus 18.6%; P = 0.07).
The AsperGenius® assay had a good diagnostic performance on BAL and differentiated WT from Aspergillus fumigatus with RAMs, including in culture-negative BAL samples. Most importantly, detection of RAMs was associated with azole treatment failure.
Introduction
Invasive aspergillosis (IA) is the most frequent pulmonary mould infection among immunocompromised patients with haematological diseases and is usually caused by Aspergillus fumigatus.1,2 The triazole voriconazole is currently recommended for first-line therapy.3 However, (pan)azole resistance in A. fumigatus has been reported increasingly over the past decade with a prevalence ranging from 1.0% to as high as 20.0%.4–11 This is worrisome because a study showed that the mortality in culture-positive IA caused by an azole-resistant strain was 88%.11 Azole resistance is often caused by mutations in the Cyp51A gene that encodes the lanosterol 14α-demethylase, the target enzyme for azoles. Two mutation patterns in this gene account for a large part of the azole resistance mechanisms: TR34/L98H and TR46/T289A/Y121F.6,9–12
Aspergillus cultures of respiratory specimens are positive in at most 26% of the IA cases.13,14 Given the low sensitivity of the cultures, most cases are diagnosed indirectly by detection of galactomannan (GM).13 However, in the absence of a positive culture, azole resistance remains undetected. Thus, the lack of a fast and readily available azole susceptibility test compromises the initiation of adequate treatment in the case of azole resistance. The commercially available AsperGenius® multiplex real-time PCR assay consists of two PCRs: the species PCR identifies the clinically relevant Aspergillus species, and the resistance PCR detects the TR34, L98H, T289A and Y121F resistance-associated mutations (RAMs) that represent the prevalent mutation combinations TR34/L98H and TR46/T289A/Y121F in the Cyp51A gene. In a recent single-centre study, the diagnostic performance of the species PCR on bronchoalveolar lavage (BAL) samples of patients with haematological diseases showed a sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of 89%, 89%, 73% and 96%, respectively, when a cycle threshold (Ct) value of <36 was used.15 Moreover, the resistance PCR was able to detect RAMs in a culture-negative patient with IA.15
The purpose of this retrospective multicentre study was to confirm the diagnostic performance of the AsperGenius® assay in a large population of patients with haematological diseases and to evaluate if the molecular detection of the above-mentioned RAMs correlates with azole treatment failure and mortality.
Methods
Study design
This retrospective study was performed at three Dutch and two Belgian hospitals (Erasmus University Medical Center, Leiden University Medical Center, University Medical Center Groningen, Ghent University Hospital and University Hospitals Leuven). The AsperGenius® assay was performed on 1 mL stored leftover BAL samples on which GM (Platelia™ Bio-Rad Inc.) and culture had already been performed because of a clinical suspicion of IA. Only BAL samples from patients with haematological diseases were included. The collection of BAL samples ended on 31 May 2015. All available leftover BAL samples with a minimum volume of 1 mL before this date were obtained. BAL samples were stored at −20°C in four hospitals and at −80°C in one hospital. The following information was retrieved from medical files: age, sex, underlying disease, duration of hospitalization, documentation of IA and the antifungal treatment given. In addition, 6 and 12 week mortality was documented. Because this was a retrospective study, no data on the BAL procedure itself was registered.
The study consisted of two parts. First, the optimal Ct value and diagnostic performance of the species probe of the PCR were determined. Second, treatment failure and 6 week mortality were determined in all patients who had received azole monotherapy for at least 5 days and in whom the resistance PCR successfully discriminated WT from Cyp51A mutated A. fumigatus. Patients with A. fumigatus without RAM (=WT) were compared with those with A. fumigatus containing a RAM. Patients were excluded from the azole treatment failure analysis if: (i) a non-A. fumigatus or mixed infection was present (e.g. A. fumigatus and Aspergillus terreus); (ii) patients were treated with non-azole therapy or combination therapy; (iii) the antifungal therapy or duration was unknown; or (iv) patients received no therapy. Azole treatment failure was defined as a switch from an azole to any other antifungal drug class. Data of the patient population with haematological diseases of the previous study (n = 10) were pooled with the data of the current study for the specific analysis of azole treatment failure and 6 week mortality.15 The pooling of data was deemed necessary and appropriate because of: (i) the rarity of patients infected with RAMs; and (ii) the identical methodology and same study site in both studies.
One BAL sample per patient was included in the study. If for a given patient multiple BAL samples were available, the BAL sample of the period with the highest IA classification was selected. In case of multiple BAL samples for a given patient with the same IA classification, 1 BAL sample was randomly selected.
PathoNostics tested the BAL samples blindly and was not involved in the analysis of the results. G. M. C. and B. J. A. R. analysed the data.
Ethics
The medical ethics committees approved the study under the reference numbers MEC-2014-628, P14.337, UC UZG 2014/1217 and S57319. For one Dutch centre, local approval was not necessary as approval given by another Dutch medical ethics committee also implied approval for that centre. In centres with an opt-out system, all included patients were cross-checked with the list of patients that had objected to the opt-out system. In one centre, opt-out forms were sent to the surviving patients to give them the opportunity to refuse the use of their clinical data.
Categorization of BAL samples
BAL samples with a positive GM (≥1.0) and/or a positive Aspergillus culture of the BAL, sputum or lung biopsy (<6 days after the date of the BAL) were considered positive controls for the presence of Aspergillus in BAL samples. Negative controls were BAL samples with a negative BAL GM in combination with a negative culture from BAL, sputum or biopsy. BAL samples from patients with only a positive serum GM (≥0.5), but a negative BAL GM, were considered as negative controls as there was no microbiological evidence of the presence of Aspergillus in the BAL sample itself on which the PCR was performed.
Definitions of invasive fungal disease
Patients were categorized as having proven, probable or possible invasive fungal disease according to the revised European Organization for Research and Treatment of Cancer/Invasive Infectious Diseases Study Mycoses Group (EORTC/MSG) consensus criteria (available as Supplementary data at JAC Online).16 In addition, patients with appropriate host criteria and positive microbiological findings, but with non-specific radiological features, were classified as having non-classifiable disease. Although this category is not included in the EORTC/MSG definitions, in clinical practice these patients are treated similarly to those with probable IA given their similar outcomes.17
Processing of BAL samples
One millilitre of the BAL sample was used for DNA extraction. Samples were processed as described previously,15 with the exception that DNA was extracted from the BAL supernatant and pellet by using a NucliSENS® easyMag® system (bioMérieux). The onboard lysis protocol and 50 μL elution was selected for this purpose before the DNA eluate was used in the AsperGenius® assay. The AsperGenius® assay was performed on the BAL supernatant and pellet separately. A LightCycler 480 II PCR system (Roche) was used to perform the AsperGenius® assay. For the determination of the Ct values, the second derivative function of the LightCycler 480 software (v. 1.5.62) was applied.
AsperGenius® multiplex real-time PCR assay
The AsperGenius® multiplex real-time PCR assay (PathoNostics, Maastricht, The Netherlands) was used to detect Aspergillus species and Cyp51A gene mutations. The species PCR allows for detection of A. fumigatus complex, A. terreus and Aspergillus species by targeting the 28S rRNA multicopy gene. The A. fumigatus probe detects the most relevant species of the Fumigati complex: A. fumigatus, Aspergillus lentulus and Aspergillus felis. The Aspergillus species probe specifically detects A. fumigatus, A. terreus, Aspergillus flavus and Aspergillus niger. An internal control is included to monitor for inhibition or manual handling errors. The resistance PCR targets the single-copy Cyp51A gene of A. fumigatus and detects TR34/L98H/Y121F/T289A mutations to differentiate WT from mutant A. fumigatus via melting curve analysis.
Each extracted BAL sample was tested in duplicate and a no template control (blank) was included in each run to exclude contamination. A sample was considered positive when one of the duplicates showed increased fluorescence above the threshold. The positive control from the assay was used as a standard for the melting peaks and was tested simultaneously with the BAL samples to determine whether the melting peak represents WT or A. fumigatus with RAM. The resistance PCR was deemed successful when the supernatant or pellet showed melting peaks for (i) at least one of TR34 or L98H, together with (ii) at least one of T289A or Y121F resistance markers.
Statistical analysis
The optimal Ct cut-off and diagnostic performance was determined for the species probe of the PCR using the earlier Ct value of the supernatant or pellet. Using the controls as described above, the receiver operator characteristic (ROC) curve and its area under the curve (AUC) were determined (IBM® SPSS® statistics, v. 21). The closest point of the curve to the (0,1) point and the Youden index were used to further assess the optimal Ct cut-off.18 The sensitivity, specificity, PPV and NPV were calculated for all BAL samples in total and per hospital. The positive and negative likelihoods were calculated for the optimal Ct value.
As an additional sensitivity analysis, we determined the ROC curves, AUC and diagnostic performance when using patients with EORTC/MSG-proven, probable IA versus patients without IA. This was thought to be appropriate because the EORTC/MSG criteria are often used for antifungal therapy studies. Because clinicians tend to treat patients with non-classifiable IA in the same way as proven or probable IA,17 we performed a second sensitivity analysis in which patients with proven, probable or non-classifiable IA were compared with patients without IA.
For the azole treatment failure and 6 week mortality analysis, the two-sided Fisher's exact test was used, with a P value <0.05 considered statistically significant.
Results
In total, 228 BAL samples from 201 patients were available. Samples were obtained between December 2007 and May 2015. No patients refused to have their clinical data used for the purpose of this study. As only one BAL per patient was used, 201 BAL samples were available for the analysis. Seven patients with proven, probable or non-classifiable IA were counted as negative controls since there was no evidence of Aspergillus in the BAL itself from culture or GM (5 positive serum GM, 1 positive sinus culture and 1 positive lung biopsy culture obtained 16 days after the BAL). The clinical characteristics of the 201 patients are summarized in Table 1.
Clinical characteristics of the 201 haematology patients who contributed BAL fluid samples
| Age (years), mean (range) | 56.6 (17.5–82.6) |
| Male, n (%) | 132 (65.7) |
| Diagnosis, n (%) | |
| AML | 78 (38.8) |
| ALL | 16 (8.0) |
| CLL | 13 (6.5) |
| myelodysplastic syndrome | 17 (8.5) |
| Hodgkin's lymphoma | 10 (5.0) |
| non-Hodgkin's lymphoma | 38 (18.9) |
| myeloproliferative disorders | 8 (4.0) |
| plasma cell disorders | 11 (5.5) |
| aplastic anaemia | 3 (1.5) |
| othera | 7 (3.5) |
| Allogeneic stem cell transplantation, n (%) | 82 (40.8) |
| IA, n (%) | |
| proven | 9 (4.5) |
| probable | 43 (21.4) |
| non-classifiable | 43 (21.4) |
| possible | 32 (15.9) |
| no IA | 74 (36.8) |
| BAL GM and/or culture positive or negative, n (%) | |
| GM and culture positive | 28 (13.9) |
| GM positive and culture negative | 56 (27.9) |
| GM negative and culture positive | 4 (2.0) |
| GM and culture negative | 113 (56.2) |
| Treated with following antifungal therapy, n (%)b | |
| amphotericin B lipid complex | 6 (3.0) |
| liposomal amphotericin B | 40 (19.9) |
| conventional amphotericin B deoxycholate | 4 (2.0) |
| caspofungin | 26 (12.9) |
| itraconazole | 3 (1.5) |
| posaconazole | 18 (9.0) |
| voriconazole | 112 (55.7) |
| study anidulafungin versus placebo | 3 (1.5) |
| study voriconazole versus isavuconazole | 3 (1.5) |
| study voriconazole versus posaconazole | 6 (3.0) |
| no antifungal therapy | 56 (27.9) |
| Mortality after IA diagnosis, n (%) | |
| at 6 weeks | 50 (24.9) |
| at 12 weeks | 65 (32.3) |
| Hospital admission duration (days), mean (range) | 40.7 (2–236) |
| Age (years), mean (range) | 56.6 (17.5–82.6) |
| Male, n (%) | 132 (65.7) |
| Diagnosis, n (%) | |
| AML | 78 (38.8) |
| ALL | 16 (8.0) |
| CLL | 13 (6.5) |
| myelodysplastic syndrome | 17 (8.5) |
| Hodgkin's lymphoma | 10 (5.0) |
| non-Hodgkin's lymphoma | 38 (18.9) |
| myeloproliferative disorders | 8 (4.0) |
| plasma cell disorders | 11 (5.5) |
| aplastic anaemia | 3 (1.5) |
| othera | 7 (3.5) |
| Allogeneic stem cell transplantation, n (%) | 82 (40.8) |
| IA, n (%) | |
| proven | 9 (4.5) |
| probable | 43 (21.4) |
| non-classifiable | 43 (21.4) |
| possible | 32 (15.9) |
| no IA | 74 (36.8) |
| BAL GM and/or culture positive or negative, n (%) | |
| GM and culture positive | 28 (13.9) |
| GM positive and culture negative | 56 (27.9) |
| GM negative and culture positive | 4 (2.0) |
| GM and culture negative | 113 (56.2) |
| Treated with following antifungal therapy, n (%)b | |
| amphotericin B lipid complex | 6 (3.0) |
| liposomal amphotericin B | 40 (19.9) |
| conventional amphotericin B deoxycholate | 4 (2.0) |
| caspofungin | 26 (12.9) |
| itraconazole | 3 (1.5) |
| posaconazole | 18 (9.0) |
| voriconazole | 112 (55.7) |
| study anidulafungin versus placebo | 3 (1.5) |
| study voriconazole versus isavuconazole | 3 (1.5) |
| study voriconazole versus posaconazole | 6 (3.0) |
| no antifungal therapy | 56 (27.9) |
| Mortality after IA diagnosis, n (%) | |
| at 6 weeks | 50 (24.9) |
| at 12 weeks | 65 (32.3) |
| Hospital admission duration (days), mean (range) | 40.7 (2–236) |
aMonoclonal B cell lymphocytosis, auto-immune haemolytic anaemia, sickle cell disease, haemophagocytic lymphohistiocytosis, T cell prolymphocytic leukaemia.
bSome patients were treated with more than one antifungal therapy, and some patients were treated within a clinical trial (indicated by the ‘study’ prefix) in which the prescribed antifungal therapy was unknown to the physicians.
Clinical characteristics of the 201 haematology patients who contributed BAL fluid samples
| Age (years), mean (range) | 56.6 (17.5–82.6) |
| Male, n (%) | 132 (65.7) |
| Diagnosis, n (%) | |
| AML | 78 (38.8) |
| ALL | 16 (8.0) |
| CLL | 13 (6.5) |
| myelodysplastic syndrome | 17 (8.5) |
| Hodgkin's lymphoma | 10 (5.0) |
| non-Hodgkin's lymphoma | 38 (18.9) |
| myeloproliferative disorders | 8 (4.0) |
| plasma cell disorders | 11 (5.5) |
| aplastic anaemia | 3 (1.5) |
| othera | 7 (3.5) |
| Allogeneic stem cell transplantation, n (%) | 82 (40.8) |
| IA, n (%) | |
| proven | 9 (4.5) |
| probable | 43 (21.4) |
| non-classifiable | 43 (21.4) |
| possible | 32 (15.9) |
| no IA | 74 (36.8) |
| BAL GM and/or culture positive or negative, n (%) | |
| GM and culture positive | 28 (13.9) |
| GM positive and culture negative | 56 (27.9) |
| GM negative and culture positive | 4 (2.0) |
| GM and culture negative | 113 (56.2) |
| Treated with following antifungal therapy, n (%)b | |
| amphotericin B lipid complex | 6 (3.0) |
| liposomal amphotericin B | 40 (19.9) |
| conventional amphotericin B deoxycholate | 4 (2.0) |
| caspofungin | 26 (12.9) |
| itraconazole | 3 (1.5) |
| posaconazole | 18 (9.0) |
| voriconazole | 112 (55.7) |
| study anidulafungin versus placebo | 3 (1.5) |
| study voriconazole versus isavuconazole | 3 (1.5) |
| study voriconazole versus posaconazole | 6 (3.0) |
| no antifungal therapy | 56 (27.9) |
| Mortality after IA diagnosis, n (%) | |
| at 6 weeks | 50 (24.9) |
| at 12 weeks | 65 (32.3) |
| Hospital admission duration (days), mean (range) | 40.7 (2–236) |
| Age (years), mean (range) | 56.6 (17.5–82.6) |
| Male, n (%) | 132 (65.7) |
| Diagnosis, n (%) | |
| AML | 78 (38.8) |
| ALL | 16 (8.0) |
| CLL | 13 (6.5) |
| myelodysplastic syndrome | 17 (8.5) |
| Hodgkin's lymphoma | 10 (5.0) |
| non-Hodgkin's lymphoma | 38 (18.9) |
| myeloproliferative disorders | 8 (4.0) |
| plasma cell disorders | 11 (5.5) |
| aplastic anaemia | 3 (1.5) |
| othera | 7 (3.5) |
| Allogeneic stem cell transplantation, n (%) | 82 (40.8) |
| IA, n (%) | |
| proven | 9 (4.5) |
| probable | 43 (21.4) |
| non-classifiable | 43 (21.4) |
| possible | 32 (15.9) |
| no IA | 74 (36.8) |
| BAL GM and/or culture positive or negative, n (%) | |
| GM and culture positive | 28 (13.9) |
| GM positive and culture negative | 56 (27.9) |
| GM negative and culture positive | 4 (2.0) |
| GM and culture negative | 113 (56.2) |
| Treated with following antifungal therapy, n (%)b | |
| amphotericin B lipid complex | 6 (3.0) |
| liposomal amphotericin B | 40 (19.9) |
| conventional amphotericin B deoxycholate | 4 (2.0) |
| caspofungin | 26 (12.9) |
| itraconazole | 3 (1.5) |
| posaconazole | 18 (9.0) |
| voriconazole | 112 (55.7) |
| study anidulafungin versus placebo | 3 (1.5) |
| study voriconazole versus isavuconazole | 3 (1.5) |
| study voriconazole versus posaconazole | 6 (3.0) |
| no antifungal therapy | 56 (27.9) |
| Mortality after IA diagnosis, n (%) | |
| at 6 weeks | 50 (24.9) |
| at 12 weeks | 65 (32.3) |
| Hospital admission duration (days), mean (range) | 40.7 (2–236) |
aMonoclonal B cell lymphocytosis, auto-immune haemolytic anaemia, sickle cell disease, haemophagocytic lymphohistiocytosis, T cell prolymphocytic leukaemia.
bSome patients were treated with more than one antifungal therapy, and some patients were treated within a clinical trial (indicated by the ‘study’ prefix) in which the prescribed antifungal therapy was unknown to the physicians.
Eighty-eight BAL samples were positive controls, of which 74 (84.1%) were positive for the species PCR (58 positive in supernatant and pellet, 10 only positive in supernatant and 6 only positive in pellet). The species PCR detected 66 A. fumigatus, 2 A. fumigatus combined with A. terreus, 2 A. terreus and 4 Aspergillus species. Thirty-two of these 74 (43.2%) BAL samples were culture negative and only GM positive.
Twenty-three BAL samples from negative controls were species PCR positive. Five of these 23 BAL samples were from patients with proven, probable or non-classifiable IA (3 diagnosed on positive serum GM, 1 on positive sinus culture and 1 on positive lung biopsy pathology plus culture). Eleven BAL samples were from patients without IA and 7 from patients with possible IA.
Diagnostic performance of the species probe of the AsperGenius® PCR according to different Ct cut-offs and positive/negative controls
| Ct value cut-off of the AsperGenius® species PCR . | Diagnostic performance . | Positive control versus negative control BAL samples as defined in this study,an = 201 . | Proven, probable or non-classifiable IA versus no IA,bn = 169 . | Proven or probable IA versus no IA, n = 126 . |
|---|---|---|---|---|
| <36 | sensitivity (%) | 70.45 | 68.42 | 76.92 |
| specificity (%) | 95.58 | 98.65 | 98.65 | |
| PPV (%) | 92.54 | 98.48 | 97.56 | |
| NPV (%) | 80.60 | 70.87 | 85.88 | |
| <37 | sensitivity (%) | 73.86 | 71.58 | 78.85 |
| specificity (%) | 90.27 | 94.59 | 94.59 | |
| PPV (%) | 85.53 | 94.44 | 91.11 | |
| NPV (%) | 81.60 | 72.16 | 86.42 | |
| <38 | sensitivity (%) | 84.09 | 83.16 | 88.46 |
| specificity (%) | 79.65 | 85.14 | 85.14 | |
| PPV (%) | 76.29 | 87.78 | 80.70 | |
| NPV (%) | 86.54 | 79.75 | 91.30 | |
| <39 | sensitivity (%) | 88.64 | 87.37 | 90.38 |
| specificity (%) | 62.83 | 72.97 | 72.97 | |
| PPV (%) | 65.00 | 80.58 | 70.15 | |
| NPV (%) | 87.65 | 81.82 | 91.53 |
| Ct value cut-off of the AsperGenius® species PCR . | Diagnostic performance . | Positive control versus negative control BAL samples as defined in this study,an = 201 . | Proven, probable or non-classifiable IA versus no IA,bn = 169 . | Proven or probable IA versus no IA, n = 126 . |
|---|---|---|---|---|
| <36 | sensitivity (%) | 70.45 | 68.42 | 76.92 |
| specificity (%) | 95.58 | 98.65 | 98.65 | |
| PPV (%) | 92.54 | 98.48 | 97.56 | |
| NPV (%) | 80.60 | 70.87 | 85.88 | |
| <37 | sensitivity (%) | 73.86 | 71.58 | 78.85 |
| specificity (%) | 90.27 | 94.59 | 94.59 | |
| PPV (%) | 85.53 | 94.44 | 91.11 | |
| NPV (%) | 81.60 | 72.16 | 86.42 | |
| <38 | sensitivity (%) | 84.09 | 83.16 | 88.46 |
| specificity (%) | 79.65 | 85.14 | 85.14 | |
| PPV (%) | 76.29 | 87.78 | 80.70 | |
| NPV (%) | 86.54 | 79.75 | 91.30 | |
| <39 | sensitivity (%) | 88.64 | 87.37 | 90.38 |
| specificity (%) | 62.83 | 72.97 | 72.97 | |
| PPV (%) | 65.00 | 80.58 | 70.15 | |
| NPV (%) | 87.65 | 81.82 | 91.53 |
aBAL samples with a positive GM (≥1.0) and/or positive culture for Aspergillus of BAL, sputum or lung biopsy at most 5 days from date of the BAL were considered positive controls. BAL samples with a negative BAL GM in combination with a negative culture from BAL, sputum or lung biopsy were considered negative controls.
bProven IA and probable IA were defined according to the revised EORTC/MSG consensus criteria. Non-classifiable was defined as a patient with EORTC/MSG host and microbiological criteria fulfilled and a pulmonary infiltrate without a halo or air-crescent or well-defined nodule. No IA was defined as no proven IA, no probable IA, no non-classifiable IA or no possible invasive fungal disease.
Diagnostic performance of the species probe of the AsperGenius® PCR according to different Ct cut-offs and positive/negative controls
| Ct value cut-off of the AsperGenius® species PCR . | Diagnostic performance . | Positive control versus negative control BAL samples as defined in this study,an = 201 . | Proven, probable or non-classifiable IA versus no IA,bn = 169 . | Proven or probable IA versus no IA, n = 126 . |
|---|---|---|---|---|
| <36 | sensitivity (%) | 70.45 | 68.42 | 76.92 |
| specificity (%) | 95.58 | 98.65 | 98.65 | |
| PPV (%) | 92.54 | 98.48 | 97.56 | |
| NPV (%) | 80.60 | 70.87 | 85.88 | |
| <37 | sensitivity (%) | 73.86 | 71.58 | 78.85 |
| specificity (%) | 90.27 | 94.59 | 94.59 | |
| PPV (%) | 85.53 | 94.44 | 91.11 | |
| NPV (%) | 81.60 | 72.16 | 86.42 | |
| <38 | sensitivity (%) | 84.09 | 83.16 | 88.46 |
| specificity (%) | 79.65 | 85.14 | 85.14 | |
| PPV (%) | 76.29 | 87.78 | 80.70 | |
| NPV (%) | 86.54 | 79.75 | 91.30 | |
| <39 | sensitivity (%) | 88.64 | 87.37 | 90.38 |
| specificity (%) | 62.83 | 72.97 | 72.97 | |
| PPV (%) | 65.00 | 80.58 | 70.15 | |
| NPV (%) | 87.65 | 81.82 | 91.53 |
| Ct value cut-off of the AsperGenius® species PCR . | Diagnostic performance . | Positive control versus negative control BAL samples as defined in this study,an = 201 . | Proven, probable or non-classifiable IA versus no IA,bn = 169 . | Proven or probable IA versus no IA, n = 126 . |
|---|---|---|---|---|
| <36 | sensitivity (%) | 70.45 | 68.42 | 76.92 |
| specificity (%) | 95.58 | 98.65 | 98.65 | |
| PPV (%) | 92.54 | 98.48 | 97.56 | |
| NPV (%) | 80.60 | 70.87 | 85.88 | |
| <37 | sensitivity (%) | 73.86 | 71.58 | 78.85 |
| specificity (%) | 90.27 | 94.59 | 94.59 | |
| PPV (%) | 85.53 | 94.44 | 91.11 | |
| NPV (%) | 81.60 | 72.16 | 86.42 | |
| <38 | sensitivity (%) | 84.09 | 83.16 | 88.46 |
| specificity (%) | 79.65 | 85.14 | 85.14 | |
| PPV (%) | 76.29 | 87.78 | 80.70 | |
| NPV (%) | 86.54 | 79.75 | 91.30 | |
| <39 | sensitivity (%) | 88.64 | 87.37 | 90.38 |
| specificity (%) | 62.83 | 72.97 | 72.97 | |
| PPV (%) | 65.00 | 80.58 | 70.15 | |
| NPV (%) | 87.65 | 81.82 | 91.53 |
aBAL samples with a positive GM (≥1.0) and/or positive culture for Aspergillus of BAL, sputum or lung biopsy at most 5 days from date of the BAL were considered positive controls. BAL samples with a negative BAL GM in combination with a negative culture from BAL, sputum or lung biopsy were considered negative controls.
bProven IA and probable IA were defined according to the revised EORTC/MSG consensus criteria. Non-classifiable was defined as a patient with EORTC/MSG host and microbiological criteria fulfilled and a pulmonary infiltrate without a halo or air-crescent or well-defined nodule. No IA was defined as no proven IA, no probable IA, no non-classifiable IA or no possible invasive fungal disease.
Distribution of the BAL samples according to their IA classification and species probe of the AsperGenius® PCR using a Ct value of <38 as the cut-off
| Classification of IA . | BAL samples, n = 201 . | ||
|---|---|---|---|
| Ct <38 . | Ct ≥38 . | total . | |
| Proven | 9 | 0 | 9 |
| GM and culture positive | 5 | 0 | |
| only GM positive | 3 | 0 | |
| only culture positive | 1 | 0 | |
| Probable | 37 | 6 | 43 |
| GM and culture positive | 11 | 0 | |
| only GM positive | 22 | 6 | |
| only culture positive | 4 | 0 | |
| Non-classifiable | 33 | 10 | 43 |
| GM and culture positive | 12 | 0 | |
| only GM positive | 20 | 10 | |
| only culture positive | 1 | 0 | |
| Possible | 7 | 25 | 32 |
| No IA | 11 | 63 | 74 |
| Total | 97 | 104 | 201 |
| Classification of IA . | BAL samples, n = 201 . | ||
|---|---|---|---|
| Ct <38 . | Ct ≥38 . | total . | |
| Proven | 9 | 0 | 9 |
| GM and culture positive | 5 | 0 | |
| only GM positive | 3 | 0 | |
| only culture positive | 1 | 0 | |
| Probable | 37 | 6 | 43 |
| GM and culture positive | 11 | 0 | |
| only GM positive | 22 | 6 | |
| only culture positive | 4 | 0 | |
| Non-classifiable | 33 | 10 | 43 |
| GM and culture positive | 12 | 0 | |
| only GM positive | 20 | 10 | |
| only culture positive | 1 | 0 | |
| Possible | 7 | 25 | 32 |
| No IA | 11 | 63 | 74 |
| Total | 97 | 104 | 201 |
GM in BAL and serum. Culture in BAL and elsewhere in the body.
Distribution of the BAL samples according to their IA classification and species probe of the AsperGenius® PCR using a Ct value of <38 as the cut-off
| Classification of IA . | BAL samples, n = 201 . | ||
|---|---|---|---|
| Ct <38 . | Ct ≥38 . | total . | |
| Proven | 9 | 0 | 9 |
| GM and culture positive | 5 | 0 | |
| only GM positive | 3 | 0 | |
| only culture positive | 1 | 0 | |
| Probable | 37 | 6 | 43 |
| GM and culture positive | 11 | 0 | |
| only GM positive | 22 | 6 | |
| only culture positive | 4 | 0 | |
| Non-classifiable | 33 | 10 | 43 |
| GM and culture positive | 12 | 0 | |
| only GM positive | 20 | 10 | |
| only culture positive | 1 | 0 | |
| Possible | 7 | 25 | 32 |
| No IA | 11 | 63 | 74 |
| Total | 97 | 104 | 201 |
| Classification of IA . | BAL samples, n = 201 . | ||
|---|---|---|---|
| Ct <38 . | Ct ≥38 . | total . | |
| Proven | 9 | 0 | 9 |
| GM and culture positive | 5 | 0 | |
| only GM positive | 3 | 0 | |
| only culture positive | 1 | 0 | |
| Probable | 37 | 6 | 43 |
| GM and culture positive | 11 | 0 | |
| only GM positive | 22 | 6 | |
| only culture positive | 4 | 0 | |
| Non-classifiable | 33 | 10 | 43 |
| GM and culture positive | 12 | 0 | |
| only GM positive | 20 | 10 | |
| only culture positive | 1 | 0 | |
| Possible | 7 | 25 | 32 |
| No IA | 11 | 63 | 74 |
| Total | 97 | 104 | 201 |
GM in BAL and serum. Culture in BAL and elsewhere in the body.
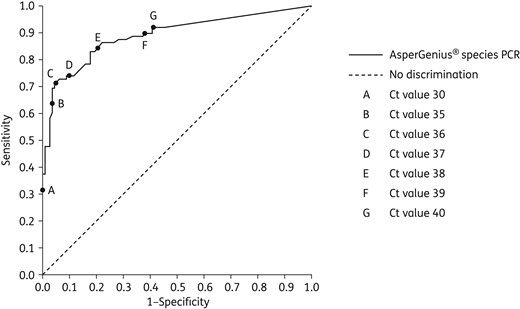
ROC curve of species probe of the AsperGenius® PCR in the BAL fluid samples of the 201 patients with haematological diseases.
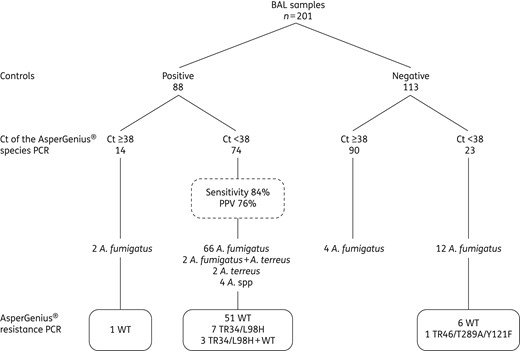
BAL samples divided according to positive/negative controls and species probe of the AsperGenius® PCR and resistance PCR. Two patients had a coinfection with an A. fumigatus and A. terreus.
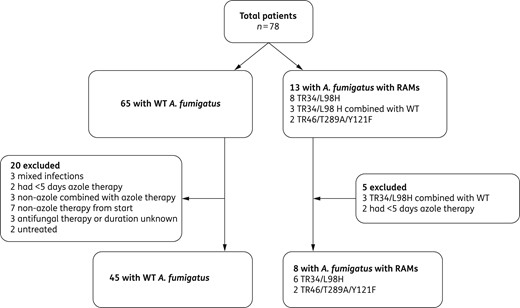
Inclusion for azole treatment failure and 6 week mortality analysis.
Discussion
This retrospective multicentre study showed that the AsperGenius® species PCR has a good diagnostic performance on BAL samples of patients with haematological diseases. The sensitivity, specificity, PPV and NPV were 84%, 80%, 76% and 87%, respectively, when a Ct cut-off of <38 was used.
The two most frequently used statistical methods to analyse a ROC curve are selecting the point closest to (0,1) and the Youden index. In this study, these methods led to contradictory results as to the most appropriate Ct cut-off values (36 and 38, respectively). Given the important clinical consequences of a missed IA diagnosis, a later Ct cut-off of 38 that results in a better sensitivity may be preferred by the clinician if the resulting loss in specificity is acceptable. Therefore, we favour the use of the Ct cut-off of <38, which is later than the Ct cut-off of <36 that we reported in the previous smaller single-centre study.15 The current sensitivity was somewhat lower than in the previous single-centre study (89%),15 and may be explained by the fact that a standard volume of 1 mL of BAL was used in this study versus 1–2 mL in the previous study. The lower volume may have decreased the sensitivity. The difference in sensitivity observed between the study centres may be explained by differences in the way the BAL is performed in each centre. For example, centres may differ in the volume used to perform the BAL or a bronchoscopist may lavage two different parts of the lung, but send it in one container to the laboratory, which may result in a diluted DNA content if Aspergillus is present in only one part of the lung. Unfortunately, the way the BAL procedure was done in each patient was not recorded. The difference in sensitivity could not be explained by the differences in BAL storage methods. One hospital stored its BAL samples at −80°C and had a sensitivity of 75%, while the other four hospitals stored at −20°C and had a sensitivity of 71%–100%. Furthermore, the difference could not be explained by the duration of BAL storage before the PCR was performed because two hospitals contributed BAL samples from the period 2014–2015 and had different sensitivities of 88% and 100%.
As with all diagnostic tests, a test should be interpreted within the context of the prevalence of the disease. When azole resistance is low in a certain area, it is expected that the PPV of the PCR will probably drop. Based on the positive and negative likelihood ratios of 4.13 and 0.20, respectively, one can determine the post-test probability in a Fagan nomogram to take the prevalence in the patient population into account.
On top of the detection of Aspergillus, the AsperGenius® resistance PCR was able to differentiate A. fumigatus without a RAM (=WT) from RAM-positive A. fumigatus, even in culture-negative BAL samples. Most importantly, patients infected with RAM-positive A. fumigatus failed significantly more often on azole treatment than those infected with a WT A. fumigatus (75% versus 27%; P = 0.01). Therefore, this study is the first to show that PCR-detected resistance is clinically relevant. The incidence of azole treatment failure and the 6 week mortality was determined in the pooled data of the current and previous study.15 The sole reason to pool the data of both studies is the fact that azole-resistant IA is still a rare disease with only 13 cases detected in the 78 PCR-positive patients of the 251 patients included in both studies. Given the small numbers, it was not possible to perform a multivariate analysis to investigate other predictors of azole treatment failure.
To date, the AsperGenius® assay has been studied on serum samples of patients with haematological diseases in which the species PCR had a sensitivity and specificity of 79% and 100%, respectively, when a Ct cut-off of 39 was used.19 In addition to the AsperGenius® assay, other Aspergillus PCRs have been tested on BAL samples and sensitivities varied between 38% and 94%.20–22 Therefore, the sensitivity of the species PCR found in this study is comparable, but with the added advantage that RAMs are detected simultaneously. There are other PCRs like the AsperGenius® assay that detect Cyp51A mutations directly in BAL samples.23–25 Spiess et al.23,24 described the detection of Cyp51A mutations on 189 clinical samples in their first and second studies combined and found the TR34/L98H mutation on two BAL samples and one cerebral biopsy. Zhao et al.25 found the Cyp51A mutations M220 and PL216, which are also associated with azole resistance, in 2/94 BAL samples. These studies, along with the current study, show that detection of the Cyp51A mutation in BAL samples is possible, as well as in culture-negative BAL samples. The current study is the first to show that the detection of RAMs is clinically associated with azole treatment failure.
The current study has limitations. First, only Cyp51A mutations included in the assay can be detected. To date, more than 15 Cyp51A gene-mediated resistance mechanisms have been described.26 The included mutations TR34/L98H and TR46/T298A/Y121F originate from the environment, in contrast to Cyp51A mutations that are patient acquired after prolonged azole treatment.7,27,28 The prevalence for the TR34/L98H mutation can account for up to 90.2% of the azole-resistant A. fumigatus, and up to 26.9% for the TR46/T289A/Y121F.6,9–12 However, the prevalence varies by region. For example, a study from the UK found only two TR34/L98H mutations among the 45 azole-resistant A. fumigatus isolates.7 The assay should therefore be interpreted within the context of the local prevalence of the Cyp51A mutations. In addition to the Cyp51A mutations, non-Cyp51A mechanisms that confer azole resistance have been reported.4,6,8–11 Therefore, PCR testing does not replace culture-based susceptibility testing, which should be performed as well. Second, we studied the BAL samples from December 2007 to May 2015. Azole resistance has increased over the past decade.4–11 Eleven of the 201 (5.5%) patients were infected with an A. fumigatus containing a RAM, which may be an underestimate of RAMs in the current population. Lastly, the retrospective nature of the study is another limitation. Antifungal susceptibility testing was not performed routinely in the past. Therefore, the PCR results could only be correlated with the phenotypical resistance in a small portion of the patients. However, in a prospective study, it would be unacceptable to test BAL samples in real time without reporting the detected Cyp51A mutations back to the clinician, which obviously would lead to a switch from an azole to a non-azole therapy. Therefore, this retrospective study had the advantage that it became possible to report on eight patients treated with azoles despite the fact that they had been infected with RAM-positive and therefore azole-resistant A. fumigatus.
Conclusions
The AsperGenius® assay showed a good diagnostic performance in detecting IA in patients with haematological diseases, and the detection of RAM-positive A. fumigatus was associated with azole treatment failure, even when patients were culture negative. Therefore, early detection of RAMs by PCR can lead to prompt adaptation of the antifungal regimen, and hopefully contribute to a more favourable outcome of azole-resistant A. fumigatus in future patients.
Funding
This study was carried out as part of our routine work.
Transparency declarations
L. F. R. S. reports speaker fees from Pfizer, Gilead and MSD, conference fees from Pfizer and Gilead, and personal fees from Pfizer.
K. L. reports grants and personal fees from MSD and travel support to conferences and speaker's honorarium from Pfizer and Gilead.
J. A. M. reports grants and personal fees from MSD, Pfizer Inc., Astellas Pharma, Gilead Sciences and Bio-Rad, and personal fees from Basilea and F2G.
G. J. H. D., G. R. G. and D. W. E. v. T. are employees of PathoNostics B.V.
B. J. A. R. reports grants and personal fees from Gilead.
All other authors: none to declare.
Acknowledgements
Part of this work was presented at the following conference: 7th Trends in Medical Mycology (TIMM), Lisbon, Portugal, 2015 (Title: ‘PCR-based detection of A. fumigatus cyp51A mutations on bronchoalveolar lavage can readily predict azole treatment failure. A multicentre validation study in 201 haematology patients with suspected invasive aspergillosis.’; Number: P060).
We would like to thank M. F. Beaumont and M. van der Weg for their assistance on this project.
References



