-
PDF
- Split View
-
Views
-
Cite
Cite
Rodrigo E. Mendes, Mariana Castanheira, David J. Farrell, Robert K. Flamm, Helio S. Sader, Ronald N. Jones, Longitudinal (2001–14) analysis of enterococci and VRE causing invasive infections in European and US hospitals, including a contemporary (2010–13) analysis of oritavancin in vitro potency, Journal of Antimicrobial Chemotherapy, Volume 71, Issue 12, 1 December 2016, Pages 3453–3458, https://doi.org/10.1093/jac/dkw319
Close - Share Icon Share
The objective of this study was to evaluate the prevalence and in vitro susceptibility of enterococci and VRE among bloodstream infections in European and US hospitals over time.
Isolates recovered from the blood of infected patients in Europe (72 996) and the USA (67 725) between 2001 and 2014 were included in the prevalence analysis. A subset (2349) collected during 2011–13 was used for the in vitro activity analysis.
Enterococcus faecium rates increased in Europe (from 1.4% in 2001 to 4.3% in 2014). These rates also increased in the USA (from 3.0% in 2001 to 5.4% in 2010), with decreasing prevalence (4.6% in 2011 to 3.6% in 2014) in later years. Enterococcus faecalis rates remained stable in Europe, but rose in the USA from 6.9% in 2001 to 8.8% in 2009, declining later (from 7.4% to 5.0%). VRE rates among E. faecalis did not vary in either region, while VRE rates among E. faecium increased in Europe (from 4.7% to 20.3%). US VRE rates among E. faecium increased until 2010 (60.0% in 2001 to 80.7% in 2010), decreasing from 75.1% in 2011 to 68.4% in 2013. Oritavancin demonstrated activity against vancomycin-susceptible E. faecalis (MIC50/90, 0.015/0.06 mg/L; 99.5% susceptible) and vancomycin-resistant E. faecalis (MIC50/90, 0.25/0.5 mg/L). Oritavancin showed MIC50, MIC90 and MIC100 values of 0.03, 0.12 and 0.25 mg/L, respectively, for VanA E. faecium.
Rates of E. faecium and VRE increased in Europe. Although still elevated, VRE rates appeared to show a decreasing trend in the USA since 2010. Oritavancin demonstrated activity against enterococci, including VRE.
Introduction
Enterococci currently represent the second and third most frequently observed pathogens responsible for healthcare-associated infections (HAIs) in the USA and Europe, respectively.1,2 The vast majority of enterococcal infections are caused by Enterococcus faecalis and Enterococcus faecium, and until a few decades ago E. faecalis accounted for 80%–90% of the isolates.3 More recent reports have described the emergence and dissemination of E. faecium isolates resistant to both vancomycin (VRE) and aminoglycosides, which precludes use of this combination as a standard therapy.4
Oritavancin is a semisynthetic bactericidal lipoglycopeptide approved by the FDA in the USA (2014) and by the EMA (2015) for the treatment of adults with acute bacterial skin and skin structure infections (ABSSSIs).5 This study was carried out: (i) to evaluate the prevalence of enterococci and VRE causing bloodstream infections (BSIs) in US and European hospitals (2001–14); and (ii) to determine the comparative in vitro activity of oritavancin against a subset of recently (2010–13) isolated enterococci.
Methods
Clinical isolates for the enterococcal prevalence and VRE rate analysis
Isolates recovered from blood cultures in Europe (72 996) and the USA (67 725) collected during 2001–14 were included in the prevalence analysis for enterococci (6215 and 8286 isolates from Europe and the USA, respectively) and the VRE rates over time. These isolates were part of the SENTRY programme and deemed to be the cause of the BSI, per local guidelines. Participating sites were instructed to only include consecutive and unique isolates (one per patient episode).
Clinical isolates for the in vitro antimicrobial analysis
The antimicrobial activity analysis utilized a subset of the isolates described above (those from 2010–13), consisting of 1349 E. faecalis and 1000 E. faecium recovered from blood specimens in the USA (28 centres) and Europe (38 centres in 16 countries). All non-US isolates were analysed in aggregate and labelled as European isolates.
Antimicrobial susceptibility testing
Isolates were tested for susceptibility by the broth microdilution method using panels manufactured by ThermoFisher Scientific (Cleveland, OH, USA).6 Quality assurance was performed by concurrent testing of CLSI-recommended strains, Staphylococcus aureus ATCC 29213 and E. faecalis ATCC 29212.7 MIC results were interpreted using the CLSI (US isolates) and EUCAST (Europe isolates) breakpoint criteria, as available.7,8 The in vitro activities of oritavancin and certain comparator agents were evaluated according to the vancomycin resistance phenotypes (CLSI criteria),7 as follows: VanA phenotype (vancomycin MIC >4 mg/L and teicoplanin MIC >8 mg/L) and VanB phenotype (vancomycin MIC >4 mg/L and teicoplanin MIC ≤8 mg/L).
Results
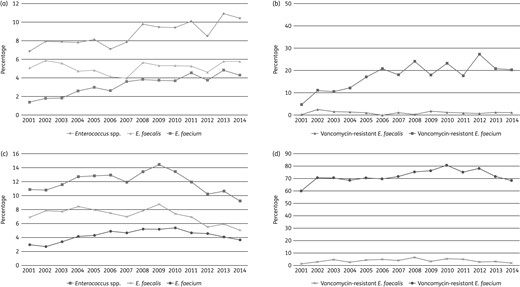
Occurrence rates of Enterococcus spp., E. faecalis and E. faecium isolates causing BSIs in European (a) and US (c) hospitals during the SENTRY Antimicrobial Surveillance Program from 2001 to 2014. The respective vancomycin resistance rates for E. faecalis and E. faecium during the same period in Europe (b) and the USA (d) are also displayed.
In the USA, the enterococci frequency peaked in 2009 (14.4% of all BSI isolates) and then decreased to 9.2% in 2014 (Figure 1c). In the USA in 2001, E. faecalis was encountered more often than E. faecium (6.9% versus 3.0%, respectively); however, the gap between these rates has since decreased due to a rise in prevalence of E. faecium (to 5.4% in 2010). The E. faecalis rates remained between 6.9% in 2001 and 8.8% in 2009, after which rates for both organisms began to decline. A subtle increase in the rates for VRE among E. faecalis in the USA appears to have occurred until 2008 (up to 6.5%), but the rate decreased to 1.9% in the subsequent years (Figure 1d). In contrast, VRE rates among E. faecium from the USA were 60.0% in 2001 and rose to 80.7% by 2010; a decline to 68.4% was observed in 2014.
Oritavancin had equal MIC50 values for vancomycin-susceptible E. faecalis from Europe (MIC50/MIC90, 0.015/0.03 mg/L) and the USA (MIC50/MIC90, 0.015/0.06 mg/L), inhibiting 99.8% and 99.3% of isolates at the breakpoint for susceptibility, respectively (Table 1 and Table S2). High susceptibility rates (99.9%–100.0%) among vancomycin-susceptible E. faecalis for both regions were observed for teicoplanin, daptomycin and linezolid (Table 1). Overall, the oritavancin MIC50 and MIC90 values for VanA-phenotype E. faecalis (MIC50/MIC90, 0.25/0.5 mg/L) were higher than those obtained for vancomycin-susceptible isolates (MIC50/MIC90, 0.015/0.06 mg/L) (Table 1). However, oritavancin inhibited all VanA isolates at ≤0.5 mg/L and displayed an MIC50 result that was at least 2-fold lower than values for active comparator agents, such as ampicillin, daptomycin and linezolid, which showed 100.0% susceptibility rates regardless of geographical region (Table 1).
Antimicrobial activity of oritavancin and comparator agents against vancomycin-susceptible and -resistant enterococcal clinical isolates causing BSIs in US and European hospitals (2010–13)
| Organism (no. tested Europe/USA)/antimicrobial agent . | MIC50 . | MIC90 . | MIC50 . | MIC90 . | %Sa . | %I . | %R . | %S . | %I . | %R . |
|---|---|---|---|---|---|---|---|---|---|---|
| Europe . | USA . | Europe . | USA . | |||||||
| E. faecalis | ||||||||||
| vancomycin susceptible (592/719) | ||||||||||
| oritavancin | 0.015 | 0.03 | 0.015 | 0.06 | 99.3 | — | — | 99.3 | — | — |
| ampicillin | ≤1 | 2 | ≤1 | 2 | 100.0 | 0.0 | 0.0 | 100.0 | — | 0.0 |
| vancomycin | 1 | 2 | 1 | 2 | 100.0 | — | 0.0 | 100.0 | 0.0 | 0.0 |
| teicoplanin | ≤2 | ≤2 | ≤2 | ≤2 | 100.0 | — | 0.0 | 100.0 | 0.0 | 0.0 |
| erythromycin | >4 | >4 | >4 | >4 | — | — | — | 9.5 | 35.6 | 54.9 |
| tetracycline | >8 | >8 | >8 | >8 | — | — | — | 24.1 | 0.4 | 75.5 |
| levofloxacinb | 1 | >4 | 1 | >4 | — | — | — | 70.2 | 0.7 | 29.1 |
| daptomycin | 1 | 1 | 1 | 1 | — | — | — | 100.0 | — | — |
| linezolid | 1 | 2 | 1 | 1 | 99.9 | — | 0.1 | 99.9 | 0.0 | 0.1 |
| vancomycin resistant, VanA mediated (4/27) | ||||||||||
| oritavancin | 0.25 | — | 0.25 | 0.5 | — | — | — | — | — | — |
| ampicillin | 1 | — | ≤1 | 2 | 100.0 | 0.0 | 0.0 | 100.0 | — | 0.0 |
| vancomycin | >16 | — | >16 | >16 | 0.0 | — | 100.0 | 0.0 | 0.0 | 100.0 |
| teicoplanin | >16 | — | >8 | >8 | 0.0 | — | 100.0 | 0.0 | 0.0 | 100.0 |
| erythromycin | >16 | — | >4 | >4 | — | — | — | 3.7 | 7.4 | 88.9 |
| tetracycline | >8 | — | >8 | >8 | — | — | — | 7.4 | 0.0 | 92.6 |
| levofloxacinb | 0.5 | — | >4 | >4 | — | — | — | 3.7 | 0.0 | 96.3 |
| daptomycin | 0.5 | — | 1 | 1 | — | — | — | 100.0 | — | — |
| linezolid | 1 | — | 1 | 1 | 100.0 | — | 0.0 | 100.0 | 0.0 | 0.0 |
| E. faecium | ||||||||||
| vancomycin susceptible (367/111) | ||||||||||
| oritavancin | ≤0.008 | ≤0.008 | ≤0.008 | ≤0.008 | — | — | — | — | — | — |
| ampicillin | >8 | >8 | >8 | >8 | 7.9 | 0.0 | 92.1 | 36.9 | — | 63.1 |
| vancomycin | 1 | 1 | 1 | 1 | 100.0 | — | 0.0 | 100.0 | 0.0 | 0.0 |
| teicoplanin | ≤2 | ≤2 | ≤2 | ≤2 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 |
| erythromycin | >4 | >4 | 2 | >4 | — | — | — | 12.6 | 47.7 | 39.6 |
| tetracycline | ≤0.25 | >8 | >8 | >8 | — | — | — | 35.1 | 0.0 | 64.9 |
| levofloxacinb | >4 | >4 | >4 | >4 | — | — | — | 33.3 | 7.2 | 59.5 |
| daptomycin | 2 | 2 | 2 | 4 | — | — | — | 98.2 | — | — |
| linezolid | 1 | 1 | 1 | 2 | 98.2 | — | 1.8 | 98.2 | 0.0 | 1.8 |
| vancomycin resistant, VanA mediated (94/406) | ||||||||||
| oritavancin | 0.015 | 0.06 | 0.03 | 0.12 | — | — | — | — | — | — |
| ampicillin | >8 | >8 | >8 | >8 | 0.0 | 0.0 | 100.0 | 0.2 | — | 99.8 |
| vancomycin | >16 | >16 | >16 | >16 | 0.0 | — | 100.0 | 0.0 | 0.0 | 100.0 |
| teicoplanin | >8 | >8 | >8 | >8 | 0.0 | — | 100.0 | 0.0 | 2.8 | 97.2 |
| erythromycin | >4 | >4 | >4 | >4 | — | — | — | 1.2 | 6.7 | 92.1 |
| tetracycline | >8 | >8 | >8 | >8 | — | — | — | 21.9 | 1.2 | 76.8 |
| levofloxacinb | >4 | >4 | >4 | >4 | — | — | — | 0.0 | 0.0 | 100.0 |
| daptomycin | 2 | 4 | 2 | 2 | — | — | — | 100.0 | — | — |
| linezolid | 1 | 2 | 1 | 1 | 99.7 | — | 0.2 | 99.0 | 0.7 | 0.2 |
| vancomycin resistant, VanB mediated (9/13) | ||||||||||
| oritavancin | ≤0.008 | — | ≤0.008 | ≤0.008 | — | — | — | — | — | — |
| ampicillin | >8 | — | >8 | >8 | 0.0 | 0.0 | 100.0 | 0.0 | — | 100.0 |
| vancomycin | >16 | — | >16 | >16 | 0.0 | — | 66.7 | 0.0 | 15.4 | 84.6 |
| teicoplanin | ≤2 | — | ≤2 | 8 | 100.0 | — | 0.0 | 100.0 | 0.0 | 0.0 |
| erythromycin | >4 | — | >4 | >4 | — | — | — | 0.0 | 38.5 | 61.5 |
| tetracycline | ≤0.25 | — | >8 | >8 | — | — | — | 23.1 | 0.0 | 76.9 |
| levofloxacinb | >4 | — | >4 | >4 | — | — | — | 0.0 | 0.0 | 100.0 |
| daptomycin | 2 | — | 1 | 2 | — | — | — | 100.0 | — | — |
| linezolid | 1 | — | 1 | 1 | 100.0 | — | 0.0 | 92.3 | 7.7 | 0.0 |
| Organism (no. tested Europe/USA)/antimicrobial agent . | MIC50 . | MIC90 . | MIC50 . | MIC90 . | %Sa . | %I . | %R . | %S . | %I . | %R . |
|---|---|---|---|---|---|---|---|---|---|---|
| Europe . | USA . | Europe . | USA . | |||||||
| E. faecalis | ||||||||||
| vancomycin susceptible (592/719) | ||||||||||
| oritavancin | 0.015 | 0.03 | 0.015 | 0.06 | 99.3 | — | — | 99.3 | — | — |
| ampicillin | ≤1 | 2 | ≤1 | 2 | 100.0 | 0.0 | 0.0 | 100.0 | — | 0.0 |
| vancomycin | 1 | 2 | 1 | 2 | 100.0 | — | 0.0 | 100.0 | 0.0 | 0.0 |
| teicoplanin | ≤2 | ≤2 | ≤2 | ≤2 | 100.0 | — | 0.0 | 100.0 | 0.0 | 0.0 |
| erythromycin | >4 | >4 | >4 | >4 | — | — | — | 9.5 | 35.6 | 54.9 |
| tetracycline | >8 | >8 | >8 | >8 | — | — | — | 24.1 | 0.4 | 75.5 |
| levofloxacinb | 1 | >4 | 1 | >4 | — | — | — | 70.2 | 0.7 | 29.1 |
| daptomycin | 1 | 1 | 1 | 1 | — | — | — | 100.0 | — | — |
| linezolid | 1 | 2 | 1 | 1 | 99.9 | — | 0.1 | 99.9 | 0.0 | 0.1 |
| vancomycin resistant, VanA mediated (4/27) | ||||||||||
| oritavancin | 0.25 | — | 0.25 | 0.5 | — | — | — | — | — | — |
| ampicillin | 1 | — | ≤1 | 2 | 100.0 | 0.0 | 0.0 | 100.0 | — | 0.0 |
| vancomycin | >16 | — | >16 | >16 | 0.0 | — | 100.0 | 0.0 | 0.0 | 100.0 |
| teicoplanin | >16 | — | >8 | >8 | 0.0 | — | 100.0 | 0.0 | 0.0 | 100.0 |
| erythromycin | >16 | — | >4 | >4 | — | — | — | 3.7 | 7.4 | 88.9 |
| tetracycline | >8 | — | >8 | >8 | — | — | — | 7.4 | 0.0 | 92.6 |
| levofloxacinb | 0.5 | — | >4 | >4 | — | — | — | 3.7 | 0.0 | 96.3 |
| daptomycin | 0.5 | — | 1 | 1 | — | — | — | 100.0 | — | — |
| linezolid | 1 | — | 1 | 1 | 100.0 | — | 0.0 | 100.0 | 0.0 | 0.0 |
| E. faecium | ||||||||||
| vancomycin susceptible (367/111) | ||||||||||
| oritavancin | ≤0.008 | ≤0.008 | ≤0.008 | ≤0.008 | — | — | — | — | — | — |
| ampicillin | >8 | >8 | >8 | >8 | 7.9 | 0.0 | 92.1 | 36.9 | — | 63.1 |
| vancomycin | 1 | 1 | 1 | 1 | 100.0 | — | 0.0 | 100.0 | 0.0 | 0.0 |
| teicoplanin | ≤2 | ≤2 | ≤2 | ≤2 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 |
| erythromycin | >4 | >4 | 2 | >4 | — | — | — | 12.6 | 47.7 | 39.6 |
| tetracycline | ≤0.25 | >8 | >8 | >8 | — | — | — | 35.1 | 0.0 | 64.9 |
| levofloxacinb | >4 | >4 | >4 | >4 | — | — | — | 33.3 | 7.2 | 59.5 |
| daptomycin | 2 | 2 | 2 | 4 | — | — | — | 98.2 | — | — |
| linezolid | 1 | 1 | 1 | 2 | 98.2 | — | 1.8 | 98.2 | 0.0 | 1.8 |
| vancomycin resistant, VanA mediated (94/406) | ||||||||||
| oritavancin | 0.015 | 0.06 | 0.03 | 0.12 | — | — | — | — | — | — |
| ampicillin | >8 | >8 | >8 | >8 | 0.0 | 0.0 | 100.0 | 0.2 | — | 99.8 |
| vancomycin | >16 | >16 | >16 | >16 | 0.0 | — | 100.0 | 0.0 | 0.0 | 100.0 |
| teicoplanin | >8 | >8 | >8 | >8 | 0.0 | — | 100.0 | 0.0 | 2.8 | 97.2 |
| erythromycin | >4 | >4 | >4 | >4 | — | — | — | 1.2 | 6.7 | 92.1 |
| tetracycline | >8 | >8 | >8 | >8 | — | — | — | 21.9 | 1.2 | 76.8 |
| levofloxacinb | >4 | >4 | >4 | >4 | — | — | — | 0.0 | 0.0 | 100.0 |
| daptomycin | 2 | 4 | 2 | 2 | — | — | — | 100.0 | — | — |
| linezolid | 1 | 2 | 1 | 1 | 99.7 | — | 0.2 | 99.0 | 0.7 | 0.2 |
| vancomycin resistant, VanB mediated (9/13) | ||||||||||
| oritavancin | ≤0.008 | — | ≤0.008 | ≤0.008 | — | — | — | — | — | — |
| ampicillin | >8 | — | >8 | >8 | 0.0 | 0.0 | 100.0 | 0.0 | — | 100.0 |
| vancomycin | >16 | — | >16 | >16 | 0.0 | — | 66.7 | 0.0 | 15.4 | 84.6 |
| teicoplanin | ≤2 | — | ≤2 | 8 | 100.0 | — | 0.0 | 100.0 | 0.0 | 0.0 |
| erythromycin | >4 | — | >4 | >4 | — | — | — | 0.0 | 38.5 | 61.5 |
| tetracycline | ≤0.25 | — | >8 | >8 | — | — | — | 23.1 | 0.0 | 76.9 |
| levofloxacinb | >4 | — | >4 | >4 | — | — | — | 0.0 | 0.0 | 100.0 |
| daptomycin | 2 | — | 1 | 2 | — | — | — | 100.0 | — | — |
| linezolid | 1 | — | 1 | 1 | 100.0 | — | 0.0 | 92.3 | 7.7 | 0.0 |
S, susceptible; I, intermediate; R, resistant.
—, results not available due to small number of isolates (i.e. MIC90 values) or no breakpoint available (i.e. % of susceptible, intermediate and resistant).
aOritavancin breakpoint for vancomycin-susceptible E. faecalis was that approved by CLSI/EUCAST. Breakpoint criteria for comparator agents were those from CLSI (US isolates) and EUCAST (Europe isolates).
bFor urinary tract infection isolates only.
Antimicrobial activity of oritavancin and comparator agents against vancomycin-susceptible and -resistant enterococcal clinical isolates causing BSIs in US and European hospitals (2010–13)
| Organism (no. tested Europe/USA)/antimicrobial agent . | MIC50 . | MIC90 . | MIC50 . | MIC90 . | %Sa . | %I . | %R . | %S . | %I . | %R . |
|---|---|---|---|---|---|---|---|---|---|---|
| Europe . | USA . | Europe . | USA . | |||||||
| E. faecalis | ||||||||||
| vancomycin susceptible (592/719) | ||||||||||
| oritavancin | 0.015 | 0.03 | 0.015 | 0.06 | 99.3 | — | — | 99.3 | — | — |
| ampicillin | ≤1 | 2 | ≤1 | 2 | 100.0 | 0.0 | 0.0 | 100.0 | — | 0.0 |
| vancomycin | 1 | 2 | 1 | 2 | 100.0 | — | 0.0 | 100.0 | 0.0 | 0.0 |
| teicoplanin | ≤2 | ≤2 | ≤2 | ≤2 | 100.0 | — | 0.0 | 100.0 | 0.0 | 0.0 |
| erythromycin | >4 | >4 | >4 | >4 | — | — | — | 9.5 | 35.6 | 54.9 |
| tetracycline | >8 | >8 | >8 | >8 | — | — | — | 24.1 | 0.4 | 75.5 |
| levofloxacinb | 1 | >4 | 1 | >4 | — | — | — | 70.2 | 0.7 | 29.1 |
| daptomycin | 1 | 1 | 1 | 1 | — | — | — | 100.0 | — | — |
| linezolid | 1 | 2 | 1 | 1 | 99.9 | — | 0.1 | 99.9 | 0.0 | 0.1 |
| vancomycin resistant, VanA mediated (4/27) | ||||||||||
| oritavancin | 0.25 | — | 0.25 | 0.5 | — | — | — | — | — | — |
| ampicillin | 1 | — | ≤1 | 2 | 100.0 | 0.0 | 0.0 | 100.0 | — | 0.0 |
| vancomycin | >16 | — | >16 | >16 | 0.0 | — | 100.0 | 0.0 | 0.0 | 100.0 |
| teicoplanin | >16 | — | >8 | >8 | 0.0 | — | 100.0 | 0.0 | 0.0 | 100.0 |
| erythromycin | >16 | — | >4 | >4 | — | — | — | 3.7 | 7.4 | 88.9 |
| tetracycline | >8 | — | >8 | >8 | — | — | — | 7.4 | 0.0 | 92.6 |
| levofloxacinb | 0.5 | — | >4 | >4 | — | — | — | 3.7 | 0.0 | 96.3 |
| daptomycin | 0.5 | — | 1 | 1 | — | — | — | 100.0 | — | — |
| linezolid | 1 | — | 1 | 1 | 100.0 | — | 0.0 | 100.0 | 0.0 | 0.0 |
| E. faecium | ||||||||||
| vancomycin susceptible (367/111) | ||||||||||
| oritavancin | ≤0.008 | ≤0.008 | ≤0.008 | ≤0.008 | — | — | — | — | — | — |
| ampicillin | >8 | >8 | >8 | >8 | 7.9 | 0.0 | 92.1 | 36.9 | — | 63.1 |
| vancomycin | 1 | 1 | 1 | 1 | 100.0 | — | 0.0 | 100.0 | 0.0 | 0.0 |
| teicoplanin | ≤2 | ≤2 | ≤2 | ≤2 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 |
| erythromycin | >4 | >4 | 2 | >4 | — | — | — | 12.6 | 47.7 | 39.6 |
| tetracycline | ≤0.25 | >8 | >8 | >8 | — | — | — | 35.1 | 0.0 | 64.9 |
| levofloxacinb | >4 | >4 | >4 | >4 | — | — | — | 33.3 | 7.2 | 59.5 |
| daptomycin | 2 | 2 | 2 | 4 | — | — | — | 98.2 | — | — |
| linezolid | 1 | 1 | 1 | 2 | 98.2 | — | 1.8 | 98.2 | 0.0 | 1.8 |
| vancomycin resistant, VanA mediated (94/406) | ||||||||||
| oritavancin | 0.015 | 0.06 | 0.03 | 0.12 | — | — | — | — | — | — |
| ampicillin | >8 | >8 | >8 | >8 | 0.0 | 0.0 | 100.0 | 0.2 | — | 99.8 |
| vancomycin | >16 | >16 | >16 | >16 | 0.0 | — | 100.0 | 0.0 | 0.0 | 100.0 |
| teicoplanin | >8 | >8 | >8 | >8 | 0.0 | — | 100.0 | 0.0 | 2.8 | 97.2 |
| erythromycin | >4 | >4 | >4 | >4 | — | — | — | 1.2 | 6.7 | 92.1 |
| tetracycline | >8 | >8 | >8 | >8 | — | — | — | 21.9 | 1.2 | 76.8 |
| levofloxacinb | >4 | >4 | >4 | >4 | — | — | — | 0.0 | 0.0 | 100.0 |
| daptomycin | 2 | 4 | 2 | 2 | — | — | — | 100.0 | — | — |
| linezolid | 1 | 2 | 1 | 1 | 99.7 | — | 0.2 | 99.0 | 0.7 | 0.2 |
| vancomycin resistant, VanB mediated (9/13) | ||||||||||
| oritavancin | ≤0.008 | — | ≤0.008 | ≤0.008 | — | — | — | — | — | — |
| ampicillin | >8 | — | >8 | >8 | 0.0 | 0.0 | 100.0 | 0.0 | — | 100.0 |
| vancomycin | >16 | — | >16 | >16 | 0.0 | — | 66.7 | 0.0 | 15.4 | 84.6 |
| teicoplanin | ≤2 | — | ≤2 | 8 | 100.0 | — | 0.0 | 100.0 | 0.0 | 0.0 |
| erythromycin | >4 | — | >4 | >4 | — | — | — | 0.0 | 38.5 | 61.5 |
| tetracycline | ≤0.25 | — | >8 | >8 | — | — | — | 23.1 | 0.0 | 76.9 |
| levofloxacinb | >4 | — | >4 | >4 | — | — | — | 0.0 | 0.0 | 100.0 |
| daptomycin | 2 | — | 1 | 2 | — | — | — | 100.0 | — | — |
| linezolid | 1 | — | 1 | 1 | 100.0 | — | 0.0 | 92.3 | 7.7 | 0.0 |
| Organism (no. tested Europe/USA)/antimicrobial agent . | MIC50 . | MIC90 . | MIC50 . | MIC90 . | %Sa . | %I . | %R . | %S . | %I . | %R . |
|---|---|---|---|---|---|---|---|---|---|---|
| Europe . | USA . | Europe . | USA . | |||||||
| E. faecalis | ||||||||||
| vancomycin susceptible (592/719) | ||||||||||
| oritavancin | 0.015 | 0.03 | 0.015 | 0.06 | 99.3 | — | — | 99.3 | — | — |
| ampicillin | ≤1 | 2 | ≤1 | 2 | 100.0 | 0.0 | 0.0 | 100.0 | — | 0.0 |
| vancomycin | 1 | 2 | 1 | 2 | 100.0 | — | 0.0 | 100.0 | 0.0 | 0.0 |
| teicoplanin | ≤2 | ≤2 | ≤2 | ≤2 | 100.0 | — | 0.0 | 100.0 | 0.0 | 0.0 |
| erythromycin | >4 | >4 | >4 | >4 | — | — | — | 9.5 | 35.6 | 54.9 |
| tetracycline | >8 | >8 | >8 | >8 | — | — | — | 24.1 | 0.4 | 75.5 |
| levofloxacinb | 1 | >4 | 1 | >4 | — | — | — | 70.2 | 0.7 | 29.1 |
| daptomycin | 1 | 1 | 1 | 1 | — | — | — | 100.0 | — | — |
| linezolid | 1 | 2 | 1 | 1 | 99.9 | — | 0.1 | 99.9 | 0.0 | 0.1 |
| vancomycin resistant, VanA mediated (4/27) | ||||||||||
| oritavancin | 0.25 | — | 0.25 | 0.5 | — | — | — | — | — | — |
| ampicillin | 1 | — | ≤1 | 2 | 100.0 | 0.0 | 0.0 | 100.0 | — | 0.0 |
| vancomycin | >16 | — | >16 | >16 | 0.0 | — | 100.0 | 0.0 | 0.0 | 100.0 |
| teicoplanin | >16 | — | >8 | >8 | 0.0 | — | 100.0 | 0.0 | 0.0 | 100.0 |
| erythromycin | >16 | — | >4 | >4 | — | — | — | 3.7 | 7.4 | 88.9 |
| tetracycline | >8 | — | >8 | >8 | — | — | — | 7.4 | 0.0 | 92.6 |
| levofloxacinb | 0.5 | — | >4 | >4 | — | — | — | 3.7 | 0.0 | 96.3 |
| daptomycin | 0.5 | — | 1 | 1 | — | — | — | 100.0 | — | — |
| linezolid | 1 | — | 1 | 1 | 100.0 | — | 0.0 | 100.0 | 0.0 | 0.0 |
| E. faecium | ||||||||||
| vancomycin susceptible (367/111) | ||||||||||
| oritavancin | ≤0.008 | ≤0.008 | ≤0.008 | ≤0.008 | — | — | — | — | — | — |
| ampicillin | >8 | >8 | >8 | >8 | 7.9 | 0.0 | 92.1 | 36.9 | — | 63.1 |
| vancomycin | 1 | 1 | 1 | 1 | 100.0 | — | 0.0 | 100.0 | 0.0 | 0.0 |
| teicoplanin | ≤2 | ≤2 | ≤2 | ≤2 | 100.0 | 0.0 | 0.0 | 100.0 | 0.0 | 0.0 |
| erythromycin | >4 | >4 | 2 | >4 | — | — | — | 12.6 | 47.7 | 39.6 |
| tetracycline | ≤0.25 | >8 | >8 | >8 | — | — | — | 35.1 | 0.0 | 64.9 |
| levofloxacinb | >4 | >4 | >4 | >4 | — | — | — | 33.3 | 7.2 | 59.5 |
| daptomycin | 2 | 2 | 2 | 4 | — | — | — | 98.2 | — | — |
| linezolid | 1 | 1 | 1 | 2 | 98.2 | — | 1.8 | 98.2 | 0.0 | 1.8 |
| vancomycin resistant, VanA mediated (94/406) | ||||||||||
| oritavancin | 0.015 | 0.06 | 0.03 | 0.12 | — | — | — | — | — | — |
| ampicillin | >8 | >8 | >8 | >8 | 0.0 | 0.0 | 100.0 | 0.2 | — | 99.8 |
| vancomycin | >16 | >16 | >16 | >16 | 0.0 | — | 100.0 | 0.0 | 0.0 | 100.0 |
| teicoplanin | >8 | >8 | >8 | >8 | 0.0 | — | 100.0 | 0.0 | 2.8 | 97.2 |
| erythromycin | >4 | >4 | >4 | >4 | — | — | — | 1.2 | 6.7 | 92.1 |
| tetracycline | >8 | >8 | >8 | >8 | — | — | — | 21.9 | 1.2 | 76.8 |
| levofloxacinb | >4 | >4 | >4 | >4 | — | — | — | 0.0 | 0.0 | 100.0 |
| daptomycin | 2 | 4 | 2 | 2 | — | — | — | 100.0 | — | — |
| linezolid | 1 | 2 | 1 | 1 | 99.7 | — | 0.2 | 99.0 | 0.7 | 0.2 |
| vancomycin resistant, VanB mediated (9/13) | ||||||||||
| oritavancin | ≤0.008 | — | ≤0.008 | ≤0.008 | — | — | — | — | — | — |
| ampicillin | >8 | — | >8 | >8 | 0.0 | 0.0 | 100.0 | 0.0 | — | 100.0 |
| vancomycin | >16 | — | >16 | >16 | 0.0 | — | 66.7 | 0.0 | 15.4 | 84.6 |
| teicoplanin | ≤2 | — | ≤2 | 8 | 100.0 | — | 0.0 | 100.0 | 0.0 | 0.0 |
| erythromycin | >4 | — | >4 | >4 | — | — | — | 0.0 | 38.5 | 61.5 |
| tetracycline | ≤0.25 | — | >8 | >8 | — | — | — | 23.1 | 0.0 | 76.9 |
| levofloxacinb | >4 | — | >4 | >4 | — | — | — | 0.0 | 0.0 | 100.0 |
| daptomycin | 2 | — | 1 | 2 | — | — | — | 100.0 | — | — |
| linezolid | 1 | — | 1 | 1 | 100.0 | — | 0.0 | 92.3 | 7.7 | 0.0 |
S, susceptible; I, intermediate; R, resistant.
—, results not available due to small number of isolates (i.e. MIC90 values) or no breakpoint available (i.e. % of susceptible, intermediate and resistant).
aOritavancin breakpoint for vancomycin-susceptible E. faecalis was that approved by CLSI/EUCAST. Breakpoint criteria for comparator agents were those from CLSI (US isolates) and EUCAST (Europe isolates).
bFor urinary tract infection isolates only.
All vancomycin-susceptible and -resistant (VanB-phenotype) E. faecium were inhibited by oritavancin at ≤0.015 mg/L, except for one isolate from the USA (MIC 0.03 mg/L; Table 1 and Table S2). Vancomycin, teicoplanin, daptomycin and linezolid were also active (98.2%–100.0% susceptible) against vancomycin-susceptible E. faecium (Table 1). Higher MICs of oritavancin were noted for VanA E. faecium from Europe (MIC50/MIC90, 0.015/0.06 mg/L) and the USA (MIC50/MIC90, 0.03/0.12 mg/L; Table 1). Daptomycin (MIC50, 2 mg/L) and linezolid (MIC50, 1 mg/L) were also active against vancomycin-resistant (VanA) isolates (Table 1).
Discussion
The European CDC report described an enterococcal prevalence rate of 12.5% among organisms causing BSIs in intensive care patients in 2012.9 This rate was similar to that observed during the last 4 years of the present study (8.5%–10.9%). The European Antimicrobial Resistance Surveillance Network (EARS-Net) reported significant variability of VRE E. faecium rates among European countries in 2013 (from 0% in Estonia to 42.7% in Ireland);10 the high rate in Ireland was also observed in the present study (Table S1). Moreover, significantly increasing trends were reported for VRE E. faecium (from 5.6% in 2010 to 8.9% in 2013).10 Although these latter rates are lower than those observed here for the same period (17.6%–27.3%), both reports show trends to increasing prevalence.
During 2001–02 in the USA, enterococci constituted ∼11% of all isolates causing BSIs. This rate reached 14.4% in 2009 and declined to 9.2% by 2014. These data are consistent with those of Wisplinghoff et al.,11 who reported an overall rate of 9.4% during 1995–2002. The increasing trend of US enterococcal bacteraemia noted here appears to be largely due to the increasing prevalence of E. faecium (from 3.0% in 2001 to 5.4% in 2010). In contrast, rates for E. faecalis remained stable until 2009, after which both E. faecalis and E. faecium rates began to decline. The prevalence rates of US E. faecalis and E. faecium recovered from blood in this study in 2009 were very similar to the enterococcal rates from central line-associated BSIs (CLABSIs) reported for 2009–10 elsewhere (8.8% and 7.0%, respectively).1
The VRE rate among E. faecalis from the USA reached its highest level (6.5%) in 2008 and remained between 1.4% (2001) and 5.3% (2010). Wisplinghoff et al.11 and Sievert et al.1 reported VRE rates among E. faecalis isolates causing BSIs (1995–2002) and CLABSIs (2009–10) of 2.0% and 9.5%, respectively. The VRE rates among E. faecium in the USA seem to have followed the prevalence of this organism, and both rates of E. faecium recovered among bacteraemia isolates and the rate of VRE increased until 2010, when these rates began to decrease. The VRE rate among E. faecium observed in 2001 in the present study (60.0%) was equivalent to that reported previously for the USA for a similar period (1995–2002).11 In addition, a later investigation reported a VRE rate of 82.6% among CLABSIs in the USA during 2009–10, which approximates the rate noted here (76.3%–80.7%) for the same years.1
It is interesting to note that the incidence of nosocomial MRSA infections in the USA also peaked around 2008–09 and then started to decline.12,13 This decrease may be associated with broader screening practices and the implementation of contact precaution measures within the US healthcare system, especially to control MRSA and VRE.14 We speculate that the decline in enterococcal rates observed here among US bacteraemia isolates may somewhat parallel that of nosocomial MRSA, and both could be a consequence of such control practices. Other factors, such as epidemiology changes in circulating clones, could also potentially play a role in the changing rates of nosocomial invasive infections.
The small number of sites representing each European country, the lack of longitudinal data for some hospital sites and consequently the modest number of isolates submitted by some hospitals represent study limitations, especially when attempting to obtain trends for VRE rates within countries. However, a robust analysis was obtained for the US and combined European data, and the latter showed a concerning upward trend for E. faecium and VRE rates, which were also documented by EARS-Net.10 The VRE rates among E. faecium remained markedly elevated (∼70%) in the USA. These results for E. faecium in both regions investigated confirm a worrisome scenario, which has been designated as a serious public health threat by the US CDC,15 and led to the inclusion of this organism among the so-called ESKAPE pathogens.16 This study also demonstrates the MDR nature of vancomycin-resistant E. faecium, which shows high susceptibility to linezolid and daptomycin. In contrast, E. faecalis remain very susceptible to ampicillin, regardless of vancomycin phenotype. When oritavancin was tested against a European and US collection of enterococci, vancomycin-susceptible E. faecalis demonstrated high rates of susceptibility to oritavancin and potent MICs of oritavancin for vancomycin-susceptible and -resistant E. faecium were determined. These in vitro results corroborate previous reports,17 and indicate that oritavancin remains active against invasive, vancomycin-susceptible and -resistant enterococci.
Funding
This surveillance study was sponsored by an educational/research grant from The Medicines Company (Parsippany, NJ, USA) via the SENTRY Antimicrobial Surveillance Program platform. JMI Laboratories also received compensation fees from The Medicines Company for services with regards to manuscript preparation. The Medicines Company had no involvement in the collection, analysis or interpretation of data.
Transparency declarations
JMI Laboratories, Inc. has received research and educational grants in 2014–15 from Achaogen, Actavis, Actelion, Allergan, American Proficiency Institute (API), AmpliPhi, Anacor, Astellas, AstraZeneca, Basilea, Bayer, BD, Cardeas, Cellceutix, CEM-102 Pharmaceuticals, Cempra, Cerexa, Cidara, Cormedix, Cubist, Debiopharm, Dipexium, Dong Wha, Durata, Enteris, Exela, Forest Research Institute, Furiex, Genentech, GSK, Helperby, ICPD, Janssen, Lannett, Longitude, Medpace, Meiji Seika Kasha, Melinta, Merck, Motif, Nabriva, Novartis, Paratek, Pfizer, Pocared, PTC Therapeutics, Rempex, Roche, Salvat, Scynexis, Seachaid, Shionogi, Tetraphase, The Medicines Company, Theravance, ThermoFisher, VenatoRX, Vertex, Wockhardt and Zavante. Some JMI employees are advisors/consultants for Allergan, Astellas, Cubist, Pfizer, Cempra and Theravance. With regards to speakers' bureaus and stock options: none to declare.
Acknowledgements
We wish to thank the following staff members at JMI Laboratories (North Liberty, IA, USA): L. Duncan, M. Janechek, M. Huband, J. Oberholser, P. Rhomberg, J. Ross, J. Schuchert, J. Streit and L. Woosley (for technical support and/or assistance with manuscript preparation). In addition, we would like to thank all SENTRY participating sites contributing clinical isolates during the study period.
References



