-
PDF
- Split View
-
Views
-
Cite
Cite
Marco Falcone, David Paterson, Spotlight on ceftazidime/avibactam: a new option for MDR Gram-negative infections, Journal of Antimicrobial Chemotherapy, Volume 71, Issue 10, October 2016, Pages 2713–2722, https://doi.org/10.1093/jac/dkw239
Close - Share Icon Share
Abstract
During the last decade infections caused by MDR Gram-negative bacteria (GNB) have become increasingly prevalent. Because of their high morbidity and mortality rates, these infections constitute a serious threat to public health worldwide. Ceftazidime/avibactam is a new approved agent combining ceftazidime and a novel β-lactamase inhibitor with activity against various β-lactamases produced by MDR GNB. Avibactam has a spectrum of inhibition of class A and C β-lactamases, including ESBLs, AmpC and Klebsiella pneumoniae carbapenemase (KPC) enzymes. Thus, combination with this inhibitor expands ceftazidime's spectrum of activity to MDR Enterobacteriaceae and Pseudomonas aeruginosa strains. In Phase II clinical trials of patients with complicated intra-abdominal infections and complicated urinary tract infections ceftazidime/avibactam exhibited clinical efficacy comparable to those of meropenem and imipenem/cilastatin, respectively. A Phase III clinical trial confirmed the efficacy of ceftazidime/avibactam in patients with MDR Enterobacteriaceae and P. aeruginosa infections. Microbiological surveillance studies, in vivo animal models of infection and pharmacokinetic/pharmacodynamic target attainment analyses are also discussed, to assess the potential role of this new drug in the treatment of infections caused by MDR GNB.
Introduction
Bacterial resistance to antimicrobial agents is an emergency worldwide. Data from the USA show that at least 2 million people develop infections caused by resistant bacteria and at least 23 000 people die as a direct result of these infections each year.1 The National Healthcare Safety Network (NHSN) declared that 20% of pathogens reported from all hospital-acquired infections (HAIs) were MDR.2 Nosocomial infections caused by MDR Gram-negative bacteria, including Pseudomonas aeruginosa, Acinetobacter baumannii and ESBL-producing or carbapenemase-producing Enterobacteriaceae, have increased steadily over the last decade and are associated with high morbidity and mortality rates.3,4
For many years, carbapenems have been considered as the most effective option to treat infections due to MDR Gram-negative bacilli, including species such as P. aeruginosa and ESBL-producing Enterobacteriaceae. The recent emergence of new types of β-lactamases able to hydrolyse carbapenems, including Klebsiella pneumoniae carbapenemases (KPCs) or various types of metallo-β-lactamases (MBLs), has complicated the therapeutic management of nosocomial Gram-negative infections that nowadays represent a real challenge for physicians.5 A recent meta-analysis found that patients with bacteraemia due to carbapenem-resistant Enterobacteriaceae (CRE) have a mortality rate significantly higher than those with infection caused by a carbapenem-susceptible strain.6 Suggested regimens for treatment of CRE infections include combinations of multiple antibiotics such as colistin, tigecycline, meropenem and gentamicin, or in selected cases a dual-carbapenem therapy.7–10 However, many of these molecules have important limitations related to efficacy data, an unfavourable pharmacokinetic/pharmacodynamic profile and toxicity. Furthermore, there are no published data from randomized controlled trials assessing antimicrobial treatment options for these types of infection, and the optimal regimen for infections caused by CRE has yet to be defined.
Ceftazidime/avibactam is a novel β-lactam/β-lactamase inhibitor combination recently approved by the US FDA for treatment of complicated urinary tract infection (cUTI) and complicated intra-abdominal infection (cIAI) in combination with metronidazole.11–14 A Phase III study for treatment of hospital-acquired pneumonia (HAP), including ventilator-associated pneumonia (VAP), is currently ongoing.15 The FDA has proposed clinical use of ceftazidime/avibactam in the treatment of cUTI, cIAI and also in other bacterial infections, including HAP/VAP, that are susceptible to ceftazidime/avibactam and for which limited or no options are available. Currently, ceftazidime/avibactam is under evaluation by the EMA for final approval. The drug is not under consideration by the Pharmaceuticals and Medical Devices Agency of Japan.
Mechanism of action and spectrum of activity
Ceftazidime is a third-generation cephalosporin administered intravenously or intramuscularly. Like all β-lactam antibiotics, ceftazidime binds to a variety of PBPs. The chemical structure of ceftazidime is such that it is primarily an inhibitor of PBP3 of Gram-negative bacteria, including P. aeruginosa. Binding of ceftazidime to PBPs results in the inhibition of cell wall synthesis. Avibactam is a semi-synthetic, non-β-lactam, β-lactamase inhibitor. Avibactam inactivates susceptible β-lactamases by covalent acylation of the β-lactamase active-site serine residue.16
Avibactam differs from other β-lactamase inhibitors, such as clavulanic acid, sulbactam and tazobactam, in three key aspects. (i) Structurally, avibactam is a [3,2,1]-diazabicyclooctanone derivative that employs a reactive urea rather than a β-lactam to inhibit serine β-lactamases. (ii) The mechanism of β-lactamase inhibition by avibactam is covalent, but reversible, in contrast to clavulanic acid, sulbactam and tazobactam, which are also covalent, but irreversible. These β-lactam-based β-lactamase inhibitors bind to the β-lactamases and irreversibly generate an acyl-enzyme intermediate that undergoes hydrolysis or chemical rearrangement, restoring the activity of the β-lactamases. Conversely, since the acylation reaction of avibactam is reversible, the hydrolysis does not take place and the activity of avibactam is restored.16 (iii) Avibactam has an expanded spectrum of β-lactamase inhibition compared with the other three molecules, which are largely limited to coverage of class A enzymes. As shown in Figure 1, avibactam in vitro inhibits the activity of Ambler class A (ESBL and KPC), class C (AmpC) and some class D (OXA-48) enzymes, but it is not active against metallo-β-lactamases (NDM, VIM, IMP, VEB, PER), due to the absence of the active-site serine residue, or against Acinetobacter OXA-type carbapenemases.17
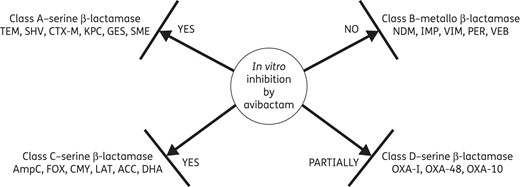
Activity of avibactam against different classes of β-lactamases.
In vitro activity
The in vitro activity of ceftazidime/avibactam has been evaluated against a wide range of microorganisms isolated from patients with different infections.18–28 Tables 1–3 show MIC50, MIC90 and percentage of susceptibility, according to CLSI, of ceftazidime alone and in combination with avibactam against various Enterobacteriaceae, P. aeruginosa and A. baumannii, respectively. As shown, ceftazidime/avibactam has broad activity against Enterobacteriaceae and P. aeruginosa isolates, but lower activity against A. baumannii. As described in Table 3, 72% of ceftazidime-non-susceptible P. aeruginosa isolates reversed to susceptibility when ceftazidime was protected from hydrolysis by the presence of avibactam. Sader et al.24 confirmed these findings in a larger number of strains, but the drug exhibited limited activity against Acinetobacter spp. (MIC50/MIC90, 16/>32 mg/L; 31.2% inhibited at ≤8 mg/L), and colistin and amikacin remained the most active compounds tested against this organism. Activity of avibactam has been confirmed in P. aeruginosa strains isolated from patients with cystic fibrosis: avibactam at a concentration of 4 mg/L was sufficient to bring into the susceptible range P. aeruginosa strains with a ceftazidime MIC ≤256 mg/L.29 Moreover, Dubée et al.30 showed that avibactam is effective in inactivating a broad-spectrum β-lactamase, BlaMab, produced by Mycobacterium abscessus, suggesting a role of this molecule in the treatment of infections caused by this microorganism. Ceftazidime/avibactam has also limited activity against anaerobic Gram-negative microorganisms, although its activity is enhanced by the addition of metronidazole.31
In vitro activity of ceftazidime alone and in combination with avibactam against Enterobacteriaceae isolates
| Study . | Isolates (n) . | Ceftazidime . | Ceftazidime/avibactam . | ||||
|---|---|---|---|---|---|---|---|
| MIC50 . | MIC90 . | %Sa . | MIC50 . | MIC90 . | %Sa . | ||
| Aktaş et al.18 | Klebsiella pneumoniae OXA 48 (25) | 256 | 512 | 8 | 0.25 | 0.5 | 100 |
| Klebsiella pneumoniae CTX-M-15 (12) | 8 | 64 | 25 | 0.06 | 0.25 | 100 | |
| E. coli CTX-M-15 (20) | 32 | 32 | 25 | <0.008 | <0.008 | 100 | |
| E. coli OXA 48 (1) | 4 | — | — | <0.008 | — | — | |
| E. coli IMP-1 (1) | 256 | — | — | 64 | — | — | |
| Sader et al.21 | Enterobacteriaceae (8640) | 0.12 | 8 | 89.3 | 0.12 | 0.25 | 99.8 |
| Klebsiella pneumoniae (1847) | 0.12 | 32 | 85.4 | 0.12 | 0.5 | 99.9 | |
| Klebsiella pneumoniae ESBL (296) | >32 | >32 | 8.8 | 0.5 | 1 | 99.3 | |
| Klebsiella pneumoniae MEM NSb (115) | >32 | >32 | 0 | 0.5 | 2 | 98.3 | |
| Klebsiella oxytoca (442) | 0.12 | 0.5 | 96.8 | 0.06 | 0.25 | 100 | |
| Klebsiella oxytoca ESBL (44) | 1 | >32 | 68.2 | 0.25 | 1 | 100 | |
| E. coli (2767) | 0.12 | 2 | 91.8 | 0.06 | 0.12 | 100 | |
| E. coli ESBL (328) | 16 | >32 | 30.8 | 0.12 | 0.25 | 100 | |
| Wang et al.23 | E. coli (25) | 1 | 64 | — | 0.125 | 0.5 | — |
| E. coli CAZ NS (18) | 2 | 64 | — | 0.25 | 0.5 | — | |
| Klebsiella pneumoniae (25) | 1 | 64 | — | 0.125 | 1 | — | |
| Klebsiella pneumoniae CAZ NS (14) | 4 | 64 | — | 0.125 | 1 | — | |
| Klebsiella oxytoca (25) | 0.25 | 2 | — | 0.25 | 0.5 | — | |
| Keepers et al.19 | Enterobacteriaceae (15) | 64 | 256 | — | 0.25 | 1 | — |
| Flamm et al.25 | E. coli BSI (568) | 0.12 | 4 | 90.3 | 0.06 | 0.12 | — |
| E. coli ESBL BSI (72) | 16 | >32 | 23.6 | 0.12 | 0.5 | — | |
| E. coli PNEU (355) | 0.25 | 16 | 88.2 | 0.12 | 0.25 | — | |
| E. coli ESBL PNEU (62) | 16 | >32 | 32.3 | 0.12 | 0.25 | — | |
| E. coli IAI (164) | 0.12 | 1 | 92.1 | 0.06 | 0.12 | — | |
| E. coli ESBL IAI (17) | 16 | >32 | 23.5 | 0.12 | 0.5 | — | |
| E. coli UTI (913) | 0.12 | 0.5 | 94.6 | 0.06 | 0.12 | — | |
| E. coli ESBL UTI (78) | 8 | 32 | 37.2 | 0.12 | 0.25 | — | |
| Klebsiella pneumoniae BSI (353) | 0.12 | 16 | 88.1 | 0.12 | 0.25 | — | |
| Klebsiella pneumoniae ESBL BSI (49) | 32 | >32 | 14.3 | 0.25 | 1 | — | |
| Klebsiella pneumoniae PNEU (596) | 0.12 | >32 | 84.1 | 0.12 | 0.5 | — | |
| Klebsiella pneumoniae ESBL PNEU (115) | >32 | >32 | 17.4 | 0.25 | 1 | — | |
| Klebsiella pneumoniae IAI (104) | 0.12 | 32 | 86.5 | 0.12 | 0.5 | — | |
| Klebsiella pneumoniae ESBL IAI (17) | >32 | >32 | 17.6 | 0.5 | 2 | — | |
| Klebsiella pneumoniae UTI (501) | 0.12 | 8 | 89.2 | 0.12 | 0.25 | — | |
| Klebsiella pneumoniae ESBL UTI (65) | >32 | >32 | 16.9 | 0.5 | 1 | — | |
| Sader et al.22 | Enterobacter spp. (3970) | 0.25 | >32 | 79.5 | 0.12 | 0.5 | 99.9 |
| Enterobacter CAZ NS (814) | >32 | >32 | 0 | 0.25 | 1 | 99.6 | |
| Serratia marcescens (1541) | 0.25 | 0.5 | 97.1 | 0.12 | 0.5 | 99.8 | |
| Serratia marcescens CAZ NS (44) | 32 | >32 | 0 | 0.5 | 2 | 93.2 | |
| Citrobacter spp (1399) | 0.25 | 16 | 88.3 | 0.12 | 0.25 | 99.9 | |
| Citrobacter CAZ NS (164) | >32 | >32 | 0 | 0.25 | 1 | 99.4 | |
| Study . | Isolates (n) . | Ceftazidime . | Ceftazidime/avibactam . | ||||
|---|---|---|---|---|---|---|---|
| MIC50 . | MIC90 . | %Sa . | MIC50 . | MIC90 . | %Sa . | ||
| Aktaş et al.18 | Klebsiella pneumoniae OXA 48 (25) | 256 | 512 | 8 | 0.25 | 0.5 | 100 |
| Klebsiella pneumoniae CTX-M-15 (12) | 8 | 64 | 25 | 0.06 | 0.25 | 100 | |
| E. coli CTX-M-15 (20) | 32 | 32 | 25 | <0.008 | <0.008 | 100 | |
| E. coli OXA 48 (1) | 4 | — | — | <0.008 | — | — | |
| E. coli IMP-1 (1) | 256 | — | — | 64 | — | — | |
| Sader et al.21 | Enterobacteriaceae (8640) | 0.12 | 8 | 89.3 | 0.12 | 0.25 | 99.8 |
| Klebsiella pneumoniae (1847) | 0.12 | 32 | 85.4 | 0.12 | 0.5 | 99.9 | |
| Klebsiella pneumoniae ESBL (296) | >32 | >32 | 8.8 | 0.5 | 1 | 99.3 | |
| Klebsiella pneumoniae MEM NSb (115) | >32 | >32 | 0 | 0.5 | 2 | 98.3 | |
| Klebsiella oxytoca (442) | 0.12 | 0.5 | 96.8 | 0.06 | 0.25 | 100 | |
| Klebsiella oxytoca ESBL (44) | 1 | >32 | 68.2 | 0.25 | 1 | 100 | |
| E. coli (2767) | 0.12 | 2 | 91.8 | 0.06 | 0.12 | 100 | |
| E. coli ESBL (328) | 16 | >32 | 30.8 | 0.12 | 0.25 | 100 | |
| Wang et al.23 | E. coli (25) | 1 | 64 | — | 0.125 | 0.5 | — |
| E. coli CAZ NS (18) | 2 | 64 | — | 0.25 | 0.5 | — | |
| Klebsiella pneumoniae (25) | 1 | 64 | — | 0.125 | 1 | — | |
| Klebsiella pneumoniae CAZ NS (14) | 4 | 64 | — | 0.125 | 1 | — | |
| Klebsiella oxytoca (25) | 0.25 | 2 | — | 0.25 | 0.5 | — | |
| Keepers et al.19 | Enterobacteriaceae (15) | 64 | 256 | — | 0.25 | 1 | — |
| Flamm et al.25 | E. coli BSI (568) | 0.12 | 4 | 90.3 | 0.06 | 0.12 | — |
| E. coli ESBL BSI (72) | 16 | >32 | 23.6 | 0.12 | 0.5 | — | |
| E. coli PNEU (355) | 0.25 | 16 | 88.2 | 0.12 | 0.25 | — | |
| E. coli ESBL PNEU (62) | 16 | >32 | 32.3 | 0.12 | 0.25 | — | |
| E. coli IAI (164) | 0.12 | 1 | 92.1 | 0.06 | 0.12 | — | |
| E. coli ESBL IAI (17) | 16 | >32 | 23.5 | 0.12 | 0.5 | — | |
| E. coli UTI (913) | 0.12 | 0.5 | 94.6 | 0.06 | 0.12 | — | |
| E. coli ESBL UTI (78) | 8 | 32 | 37.2 | 0.12 | 0.25 | — | |
| Klebsiella pneumoniae BSI (353) | 0.12 | 16 | 88.1 | 0.12 | 0.25 | — | |
| Klebsiella pneumoniae ESBL BSI (49) | 32 | >32 | 14.3 | 0.25 | 1 | — | |
| Klebsiella pneumoniae PNEU (596) | 0.12 | >32 | 84.1 | 0.12 | 0.5 | — | |
| Klebsiella pneumoniae ESBL PNEU (115) | >32 | >32 | 17.4 | 0.25 | 1 | — | |
| Klebsiella pneumoniae IAI (104) | 0.12 | 32 | 86.5 | 0.12 | 0.5 | — | |
| Klebsiella pneumoniae ESBL IAI (17) | >32 | >32 | 17.6 | 0.5 | 2 | — | |
| Klebsiella pneumoniae UTI (501) | 0.12 | 8 | 89.2 | 0.12 | 0.25 | — | |
| Klebsiella pneumoniae ESBL UTI (65) | >32 | >32 | 16.9 | 0.5 | 1 | — | |
| Sader et al.22 | Enterobacter spp. (3970) | 0.25 | >32 | 79.5 | 0.12 | 0.5 | 99.9 |
| Enterobacter CAZ NS (814) | >32 | >32 | 0 | 0.25 | 1 | 99.6 | |
| Serratia marcescens (1541) | 0.25 | 0.5 | 97.1 | 0.12 | 0.5 | 99.8 | |
| Serratia marcescens CAZ NS (44) | 32 | >32 | 0 | 0.5 | 2 | 93.2 | |
| Citrobacter spp (1399) | 0.25 | 16 | 88.3 | 0.12 | 0.25 | 99.9 | |
| Citrobacter CAZ NS (164) | >32 | >32 | 0 | 0.25 | 1 | 99.4 | |
BSI, bloodstream infections; IAI, intra-abdominal infections; PNEU, pneumonia; UTI, urinary tract infections; CAZ, ceftazidime; MEM, meropenem; NS, non-susceptible.
a%S, percentage of strains susceptible according to CLSI criteria.
bKlebsiella pneumoniae meropenem non-susceptible if MIC ≥ 2 mg/L.
In vitro activity of ceftazidime alone and in combination with avibactam against Enterobacteriaceae isolates
| Study . | Isolates (n) . | Ceftazidime . | Ceftazidime/avibactam . | ||||
|---|---|---|---|---|---|---|---|
| MIC50 . | MIC90 . | %Sa . | MIC50 . | MIC90 . | %Sa . | ||
| Aktaş et al.18 | Klebsiella pneumoniae OXA 48 (25) | 256 | 512 | 8 | 0.25 | 0.5 | 100 |
| Klebsiella pneumoniae CTX-M-15 (12) | 8 | 64 | 25 | 0.06 | 0.25 | 100 | |
| E. coli CTX-M-15 (20) | 32 | 32 | 25 | <0.008 | <0.008 | 100 | |
| E. coli OXA 48 (1) | 4 | — | — | <0.008 | — | — | |
| E. coli IMP-1 (1) | 256 | — | — | 64 | — | — | |
| Sader et al.21 | Enterobacteriaceae (8640) | 0.12 | 8 | 89.3 | 0.12 | 0.25 | 99.8 |
| Klebsiella pneumoniae (1847) | 0.12 | 32 | 85.4 | 0.12 | 0.5 | 99.9 | |
| Klebsiella pneumoniae ESBL (296) | >32 | >32 | 8.8 | 0.5 | 1 | 99.3 | |
| Klebsiella pneumoniae MEM NSb (115) | >32 | >32 | 0 | 0.5 | 2 | 98.3 | |
| Klebsiella oxytoca (442) | 0.12 | 0.5 | 96.8 | 0.06 | 0.25 | 100 | |
| Klebsiella oxytoca ESBL (44) | 1 | >32 | 68.2 | 0.25 | 1 | 100 | |
| E. coli (2767) | 0.12 | 2 | 91.8 | 0.06 | 0.12 | 100 | |
| E. coli ESBL (328) | 16 | >32 | 30.8 | 0.12 | 0.25 | 100 | |
| Wang et al.23 | E. coli (25) | 1 | 64 | — | 0.125 | 0.5 | — |
| E. coli CAZ NS (18) | 2 | 64 | — | 0.25 | 0.5 | — | |
| Klebsiella pneumoniae (25) | 1 | 64 | — | 0.125 | 1 | — | |
| Klebsiella pneumoniae CAZ NS (14) | 4 | 64 | — | 0.125 | 1 | — | |
| Klebsiella oxytoca (25) | 0.25 | 2 | — | 0.25 | 0.5 | — | |
| Keepers et al.19 | Enterobacteriaceae (15) | 64 | 256 | — | 0.25 | 1 | — |
| Flamm et al.25 | E. coli BSI (568) | 0.12 | 4 | 90.3 | 0.06 | 0.12 | — |
| E. coli ESBL BSI (72) | 16 | >32 | 23.6 | 0.12 | 0.5 | — | |
| E. coli PNEU (355) | 0.25 | 16 | 88.2 | 0.12 | 0.25 | — | |
| E. coli ESBL PNEU (62) | 16 | >32 | 32.3 | 0.12 | 0.25 | — | |
| E. coli IAI (164) | 0.12 | 1 | 92.1 | 0.06 | 0.12 | — | |
| E. coli ESBL IAI (17) | 16 | >32 | 23.5 | 0.12 | 0.5 | — | |
| E. coli UTI (913) | 0.12 | 0.5 | 94.6 | 0.06 | 0.12 | — | |
| E. coli ESBL UTI (78) | 8 | 32 | 37.2 | 0.12 | 0.25 | — | |
| Klebsiella pneumoniae BSI (353) | 0.12 | 16 | 88.1 | 0.12 | 0.25 | — | |
| Klebsiella pneumoniae ESBL BSI (49) | 32 | >32 | 14.3 | 0.25 | 1 | — | |
| Klebsiella pneumoniae PNEU (596) | 0.12 | >32 | 84.1 | 0.12 | 0.5 | — | |
| Klebsiella pneumoniae ESBL PNEU (115) | >32 | >32 | 17.4 | 0.25 | 1 | — | |
| Klebsiella pneumoniae IAI (104) | 0.12 | 32 | 86.5 | 0.12 | 0.5 | — | |
| Klebsiella pneumoniae ESBL IAI (17) | >32 | >32 | 17.6 | 0.5 | 2 | — | |
| Klebsiella pneumoniae UTI (501) | 0.12 | 8 | 89.2 | 0.12 | 0.25 | — | |
| Klebsiella pneumoniae ESBL UTI (65) | >32 | >32 | 16.9 | 0.5 | 1 | — | |
| Sader et al.22 | Enterobacter spp. (3970) | 0.25 | >32 | 79.5 | 0.12 | 0.5 | 99.9 |
| Enterobacter CAZ NS (814) | >32 | >32 | 0 | 0.25 | 1 | 99.6 | |
| Serratia marcescens (1541) | 0.25 | 0.5 | 97.1 | 0.12 | 0.5 | 99.8 | |
| Serratia marcescens CAZ NS (44) | 32 | >32 | 0 | 0.5 | 2 | 93.2 | |
| Citrobacter spp (1399) | 0.25 | 16 | 88.3 | 0.12 | 0.25 | 99.9 | |
| Citrobacter CAZ NS (164) | >32 | >32 | 0 | 0.25 | 1 | 99.4 | |
| Study . | Isolates (n) . | Ceftazidime . | Ceftazidime/avibactam . | ||||
|---|---|---|---|---|---|---|---|
| MIC50 . | MIC90 . | %Sa . | MIC50 . | MIC90 . | %Sa . | ||
| Aktaş et al.18 | Klebsiella pneumoniae OXA 48 (25) | 256 | 512 | 8 | 0.25 | 0.5 | 100 |
| Klebsiella pneumoniae CTX-M-15 (12) | 8 | 64 | 25 | 0.06 | 0.25 | 100 | |
| E. coli CTX-M-15 (20) | 32 | 32 | 25 | <0.008 | <0.008 | 100 | |
| E. coli OXA 48 (1) | 4 | — | — | <0.008 | — | — | |
| E. coli IMP-1 (1) | 256 | — | — | 64 | — | — | |
| Sader et al.21 | Enterobacteriaceae (8640) | 0.12 | 8 | 89.3 | 0.12 | 0.25 | 99.8 |
| Klebsiella pneumoniae (1847) | 0.12 | 32 | 85.4 | 0.12 | 0.5 | 99.9 | |
| Klebsiella pneumoniae ESBL (296) | >32 | >32 | 8.8 | 0.5 | 1 | 99.3 | |
| Klebsiella pneumoniae MEM NSb (115) | >32 | >32 | 0 | 0.5 | 2 | 98.3 | |
| Klebsiella oxytoca (442) | 0.12 | 0.5 | 96.8 | 0.06 | 0.25 | 100 | |
| Klebsiella oxytoca ESBL (44) | 1 | >32 | 68.2 | 0.25 | 1 | 100 | |
| E. coli (2767) | 0.12 | 2 | 91.8 | 0.06 | 0.12 | 100 | |
| E. coli ESBL (328) | 16 | >32 | 30.8 | 0.12 | 0.25 | 100 | |
| Wang et al.23 | E. coli (25) | 1 | 64 | — | 0.125 | 0.5 | — |
| E. coli CAZ NS (18) | 2 | 64 | — | 0.25 | 0.5 | — | |
| Klebsiella pneumoniae (25) | 1 | 64 | — | 0.125 | 1 | — | |
| Klebsiella pneumoniae CAZ NS (14) | 4 | 64 | — | 0.125 | 1 | — | |
| Klebsiella oxytoca (25) | 0.25 | 2 | — | 0.25 | 0.5 | — | |
| Keepers et al.19 | Enterobacteriaceae (15) | 64 | 256 | — | 0.25 | 1 | — |
| Flamm et al.25 | E. coli BSI (568) | 0.12 | 4 | 90.3 | 0.06 | 0.12 | — |
| E. coli ESBL BSI (72) | 16 | >32 | 23.6 | 0.12 | 0.5 | — | |
| E. coli PNEU (355) | 0.25 | 16 | 88.2 | 0.12 | 0.25 | — | |
| E. coli ESBL PNEU (62) | 16 | >32 | 32.3 | 0.12 | 0.25 | — | |
| E. coli IAI (164) | 0.12 | 1 | 92.1 | 0.06 | 0.12 | — | |
| E. coli ESBL IAI (17) | 16 | >32 | 23.5 | 0.12 | 0.5 | — | |
| E. coli UTI (913) | 0.12 | 0.5 | 94.6 | 0.06 | 0.12 | — | |
| E. coli ESBL UTI (78) | 8 | 32 | 37.2 | 0.12 | 0.25 | — | |
| Klebsiella pneumoniae BSI (353) | 0.12 | 16 | 88.1 | 0.12 | 0.25 | — | |
| Klebsiella pneumoniae ESBL BSI (49) | 32 | >32 | 14.3 | 0.25 | 1 | — | |
| Klebsiella pneumoniae PNEU (596) | 0.12 | >32 | 84.1 | 0.12 | 0.5 | — | |
| Klebsiella pneumoniae ESBL PNEU (115) | >32 | >32 | 17.4 | 0.25 | 1 | — | |
| Klebsiella pneumoniae IAI (104) | 0.12 | 32 | 86.5 | 0.12 | 0.5 | — | |
| Klebsiella pneumoniae ESBL IAI (17) | >32 | >32 | 17.6 | 0.5 | 2 | — | |
| Klebsiella pneumoniae UTI (501) | 0.12 | 8 | 89.2 | 0.12 | 0.25 | — | |
| Klebsiella pneumoniae ESBL UTI (65) | >32 | >32 | 16.9 | 0.5 | 1 | — | |
| Sader et al.22 | Enterobacter spp. (3970) | 0.25 | >32 | 79.5 | 0.12 | 0.5 | 99.9 |
| Enterobacter CAZ NS (814) | >32 | >32 | 0 | 0.25 | 1 | 99.6 | |
| Serratia marcescens (1541) | 0.25 | 0.5 | 97.1 | 0.12 | 0.5 | 99.8 | |
| Serratia marcescens CAZ NS (44) | 32 | >32 | 0 | 0.5 | 2 | 93.2 | |
| Citrobacter spp (1399) | 0.25 | 16 | 88.3 | 0.12 | 0.25 | 99.9 | |
| Citrobacter CAZ NS (164) | >32 | >32 | 0 | 0.25 | 1 | 99.4 | |
BSI, bloodstream infections; IAI, intra-abdominal infections; PNEU, pneumonia; UTI, urinary tract infections; CAZ, ceftazidime; MEM, meropenem; NS, non-susceptible.
a%S, percentage of strains susceptible according to CLSI criteria.
bKlebsiella pneumoniae meropenem non-susceptible if MIC ≥ 2 mg/L.
In vitro activity of ceftazidime alone and in combination with avibactam against Pseudomonas aeruginosa isolates
| Study . | Isolates/infection (n) . | Ceftazidime . | Ceftazidime/avibactam . | ||||
|---|---|---|---|---|---|---|---|
| MIC50 . | MIC90 . | %Sa . | MIC50 . | MIC90 . | %Sa . | ||
| Levasseur et al.20 | 126 | 8 | 64 | 65 | 4 | 8 | 94 |
| Aktaş et al.18 | 14 | 128 | 128 | 14 | 4 | 16 | 86 |
| Sader et al.21 | 1967 | 2 | 32 | 83.2 | 2 | 4 | 96.9 |
| MEM NSb (354) | 16 | >32 | 49.2 | 4 | 16 | 87.3 | |
| CAZ NSc (330) | 32 | >32 | 0 | 4 | 16 | 82.1 | |
| Wang et al.23 | 25 | 4 | 16 | — | 4 | 8 | — |
| Keepers et al.19 | 18 | 32 | 128 | — | 4 | 16 | — |
| Flamm et al.25 | BSI (141) | 2 | 32 | 83 | 2 | 8 | — |
| CAZ NS BSI (24) | >32 | >32 | 0 | 8 | >32 | — | |
| PNEU (881) | 2 | 32 | 79.5 | 2 | 8 | — | |
| CAZ NS PNEU (181) | 32 | >32 | 0 | 4 | 16 | — | |
| IAI (82) | 2 | 82 | 85.4 | 2 | 4 | — | |
| CAZ NS IAI (12) | 32 | >32 | 0 | 4 | 16 | — | |
| UTI (155) | 2 | 16 | 89.7 | 2 | 4 | — | |
| CAZ NS UTI (16) | 32 | >32 | 0 | 2 | 8 | — | |
| Sader et al.22 | 5328 | 2 | 32 | 83.9 | 2 | 4 | 96.8 |
| CAZ, FEP, TZP, MEM NS (396)d | — | — | — | 8 | 32 | 67.4 | |
| Study . | Isolates/infection (n) . | Ceftazidime . | Ceftazidime/avibactam . | ||||
|---|---|---|---|---|---|---|---|
| MIC50 . | MIC90 . | %Sa . | MIC50 . | MIC90 . | %Sa . | ||
| Levasseur et al.20 | 126 | 8 | 64 | 65 | 4 | 8 | 94 |
| Aktaş et al.18 | 14 | 128 | 128 | 14 | 4 | 16 | 86 |
| Sader et al.21 | 1967 | 2 | 32 | 83.2 | 2 | 4 | 96.9 |
| MEM NSb (354) | 16 | >32 | 49.2 | 4 | 16 | 87.3 | |
| CAZ NSc (330) | 32 | >32 | 0 | 4 | 16 | 82.1 | |
| Wang et al.23 | 25 | 4 | 16 | — | 4 | 8 | — |
| Keepers et al.19 | 18 | 32 | 128 | — | 4 | 16 | — |
| Flamm et al.25 | BSI (141) | 2 | 32 | 83 | 2 | 8 | — |
| CAZ NS BSI (24) | >32 | >32 | 0 | 8 | >32 | — | |
| PNEU (881) | 2 | 32 | 79.5 | 2 | 8 | — | |
| CAZ NS PNEU (181) | 32 | >32 | 0 | 4 | 16 | — | |
| IAI (82) | 2 | 82 | 85.4 | 2 | 4 | — | |
| CAZ NS IAI (12) | 32 | >32 | 0 | 4 | 16 | — | |
| UTI (155) | 2 | 16 | 89.7 | 2 | 4 | — | |
| CAZ NS UTI (16) | 32 | >32 | 0 | 2 | 8 | — | |
| Sader et al.22 | 5328 | 2 | 32 | 83.9 | 2 | 4 | 96.8 |
| CAZ, FEP, TZP, MEM NS (396)d | — | — | — | 8 | 32 | 67.4 | |
BSI, bloodstream infections; IAI, intra-abdominal infections; PNEU, pneumonia; UTI, urinary tract infections; CAZ, ceftazidime; FEP, cefepime; MEM, meropenem; TZP, piperacillin/tazobactam; NS, non-susceptible.
a%S, percentage of susceptible strains according to CLSI criteria.
bPseudomonas aeruginosa meropenem non-susceptible if MIC ≥ 4 mg/L.
cPseudomonas aeruginosa ceftazidime non-susceptible if MIC ≥ 16 mg/L.
dPseudomonas aeruginosa isolates non-susceptible to ceftazidime if MIC ≥ 16 mg/L, to cefepime if MIC ≥16 mg/L, to piperacillin/tazobactam if MIC ≥ 32 mg/L and to meropenem if MIC ≥ 4 mg/L.
In vitro activity of ceftazidime alone and in combination with avibactam against Pseudomonas aeruginosa isolates
| Study . | Isolates/infection (n) . | Ceftazidime . | Ceftazidime/avibactam . | ||||
|---|---|---|---|---|---|---|---|
| MIC50 . | MIC90 . | %Sa . | MIC50 . | MIC90 . | %Sa . | ||
| Levasseur et al.20 | 126 | 8 | 64 | 65 | 4 | 8 | 94 |
| Aktaş et al.18 | 14 | 128 | 128 | 14 | 4 | 16 | 86 |
| Sader et al.21 | 1967 | 2 | 32 | 83.2 | 2 | 4 | 96.9 |
| MEM NSb (354) | 16 | >32 | 49.2 | 4 | 16 | 87.3 | |
| CAZ NSc (330) | 32 | >32 | 0 | 4 | 16 | 82.1 | |
| Wang et al.23 | 25 | 4 | 16 | — | 4 | 8 | — |
| Keepers et al.19 | 18 | 32 | 128 | — | 4 | 16 | — |
| Flamm et al.25 | BSI (141) | 2 | 32 | 83 | 2 | 8 | — |
| CAZ NS BSI (24) | >32 | >32 | 0 | 8 | >32 | — | |
| PNEU (881) | 2 | 32 | 79.5 | 2 | 8 | — | |
| CAZ NS PNEU (181) | 32 | >32 | 0 | 4 | 16 | — | |
| IAI (82) | 2 | 82 | 85.4 | 2 | 4 | — | |
| CAZ NS IAI (12) | 32 | >32 | 0 | 4 | 16 | — | |
| UTI (155) | 2 | 16 | 89.7 | 2 | 4 | — | |
| CAZ NS UTI (16) | 32 | >32 | 0 | 2 | 8 | — | |
| Sader et al.22 | 5328 | 2 | 32 | 83.9 | 2 | 4 | 96.8 |
| CAZ, FEP, TZP, MEM NS (396)d | — | — | — | 8 | 32 | 67.4 | |
| Study . | Isolates/infection (n) . | Ceftazidime . | Ceftazidime/avibactam . | ||||
|---|---|---|---|---|---|---|---|
| MIC50 . | MIC90 . | %Sa . | MIC50 . | MIC90 . | %Sa . | ||
| Levasseur et al.20 | 126 | 8 | 64 | 65 | 4 | 8 | 94 |
| Aktaş et al.18 | 14 | 128 | 128 | 14 | 4 | 16 | 86 |
| Sader et al.21 | 1967 | 2 | 32 | 83.2 | 2 | 4 | 96.9 |
| MEM NSb (354) | 16 | >32 | 49.2 | 4 | 16 | 87.3 | |
| CAZ NSc (330) | 32 | >32 | 0 | 4 | 16 | 82.1 | |
| Wang et al.23 | 25 | 4 | 16 | — | 4 | 8 | — |
| Keepers et al.19 | 18 | 32 | 128 | — | 4 | 16 | — |
| Flamm et al.25 | BSI (141) | 2 | 32 | 83 | 2 | 8 | — |
| CAZ NS BSI (24) | >32 | >32 | 0 | 8 | >32 | — | |
| PNEU (881) | 2 | 32 | 79.5 | 2 | 8 | — | |
| CAZ NS PNEU (181) | 32 | >32 | 0 | 4 | 16 | — | |
| IAI (82) | 2 | 82 | 85.4 | 2 | 4 | — | |
| CAZ NS IAI (12) | 32 | >32 | 0 | 4 | 16 | — | |
| UTI (155) | 2 | 16 | 89.7 | 2 | 4 | — | |
| CAZ NS UTI (16) | 32 | >32 | 0 | 2 | 8 | — | |
| Sader et al.22 | 5328 | 2 | 32 | 83.9 | 2 | 4 | 96.8 |
| CAZ, FEP, TZP, MEM NS (396)d | — | — | — | 8 | 32 | 67.4 | |
BSI, bloodstream infections; IAI, intra-abdominal infections; PNEU, pneumonia; UTI, urinary tract infections; CAZ, ceftazidime; FEP, cefepime; MEM, meropenem; TZP, piperacillin/tazobactam; NS, non-susceptible.
a%S, percentage of susceptible strains according to CLSI criteria.
bPseudomonas aeruginosa meropenem non-susceptible if MIC ≥ 4 mg/L.
cPseudomonas aeruginosa ceftazidime non-susceptible if MIC ≥ 16 mg/L.
dPseudomonas aeruginosa isolates non-susceptible to ceftazidime if MIC ≥ 16 mg/L, to cefepime if MIC ≥16 mg/L, to piperacillin/tazobactam if MIC ≥ 32 mg/L and to meropenem if MIC ≥ 4 mg/L.
In vitro activity of ceftazidime alone and in combination with avibactam against Acinetobacter baumannii isolates
| Study . | Isolates/infection (n) . | Ceftazidime . | Ceftazidime/avibactam . | ||||
|---|---|---|---|---|---|---|---|
| MIC50 . | MIC90 . | %S* . | MIC50 . | MIC90 . | %S* . | ||
| Aktaş et al.18 | A. baumannii (20) | 128 | >512 | 0 | 32 | 256 | 20 |
| Sader et al.21 | Acinetobacter spp. (321) | 32 | >32 | 41.7 | 16 | >32 | 31.2 |
| Wang et al.23 | A. baumannii (28) | 8 | >128 | — | 8 | 32 | — |
| Flamm et al.25 | A. baumannii BSI (27) | 8 | >32 | 63 | 16 | >32 | — |
| A. baumannii PNEU (139) | >32 | >32 | 30.2 | 32 | >32 | — | |
| A. baumannii IAI (9) | — | — | — | — | — | — | |
| A. baumanii UTI (13) | 8 | >32 | 69.2 | 8 | 32 | — | |
| Study . | Isolates/infection (n) . | Ceftazidime . | Ceftazidime/avibactam . | ||||
|---|---|---|---|---|---|---|---|
| MIC50 . | MIC90 . | %S* . | MIC50 . | MIC90 . | %S* . | ||
| Aktaş et al.18 | A. baumannii (20) | 128 | >512 | 0 | 32 | 256 | 20 |
| Sader et al.21 | Acinetobacter spp. (321) | 32 | >32 | 41.7 | 16 | >32 | 31.2 |
| Wang et al.23 | A. baumannii (28) | 8 | >128 | — | 8 | 32 | — |
| Flamm et al.25 | A. baumannii BSI (27) | 8 | >32 | 63 | 16 | >32 | — |
| A. baumannii PNEU (139) | >32 | >32 | 30.2 | 32 | >32 | — | |
| A. baumannii IAI (9) | — | — | — | — | — | — | |
| A. baumanii UTI (13) | 8 | >32 | 69.2 | 8 | 32 | — | |
BSI, bloodstream infections; IAI, intra-abdominal infections; PNEU, pneumonia; UTI, urinary tract infections.
a%S, percentage of susceptible strains according to CLSI criteria.
In vitro activity of ceftazidime alone and in combination with avibactam against Acinetobacter baumannii isolates
| Study . | Isolates/infection (n) . | Ceftazidime . | Ceftazidime/avibactam . | ||||
|---|---|---|---|---|---|---|---|
| MIC50 . | MIC90 . | %S* . | MIC50 . | MIC90 . | %S* . | ||
| Aktaş et al.18 | A. baumannii (20) | 128 | >512 | 0 | 32 | 256 | 20 |
| Sader et al.21 | Acinetobacter spp. (321) | 32 | >32 | 41.7 | 16 | >32 | 31.2 |
| Wang et al.23 | A. baumannii (28) | 8 | >128 | — | 8 | 32 | — |
| Flamm et al.25 | A. baumannii BSI (27) | 8 | >32 | 63 | 16 | >32 | — |
| A. baumannii PNEU (139) | >32 | >32 | 30.2 | 32 | >32 | — | |
| A. baumannii IAI (9) | — | — | — | — | — | — | |
| A. baumanii UTI (13) | 8 | >32 | 69.2 | 8 | 32 | — | |
| Study . | Isolates/infection (n) . | Ceftazidime . | Ceftazidime/avibactam . | ||||
|---|---|---|---|---|---|---|---|
| MIC50 . | MIC90 . | %S* . | MIC50 . | MIC90 . | %S* . | ||
| Aktaş et al.18 | A. baumannii (20) | 128 | >512 | 0 | 32 | 256 | 20 |
| Sader et al.21 | Acinetobacter spp. (321) | 32 | >32 | 41.7 | 16 | >32 | 31.2 |
| Wang et al.23 | A. baumannii (28) | 8 | >128 | — | 8 | 32 | — |
| Flamm et al.25 | A. baumannii BSI (27) | 8 | >32 | 63 | 16 | >32 | — |
| A. baumannii PNEU (139) | >32 | >32 | 30.2 | 32 | >32 | — | |
| A. baumannii IAI (9) | — | — | — | — | — | — | |
| A. baumanii UTI (13) | 8 | >32 | 69.2 | 8 | 32 | — | |
BSI, bloodstream infections; IAI, intra-abdominal infections; PNEU, pneumonia; UTI, urinary tract infections.
a%S, percentage of susceptible strains according to CLSI criteria.
Data from experimental models
Ceftazidime/avibactam has been tested in different murine models of infection, including bacteraemia, pyelonephritis, pneumonia and meningitis. Levasseur et al.27 evaluated the efficacy of ceftazidime/avibactam against 5 ceftazidime-susceptible (MICs of 0.06–0.25 mg/L) and 15 ceftazidime-resistant (MICs of 64 to >128 mg/L) Enterobacteriaceae species, bearing either TEM, SHV, CTX-M extended-spectrum or AmpC β-lactamases, in a murine septicaemia model; ceftazidime and ceftazidime/avibactam demonstrated similar efficacies against ceftazidime-susceptible isolates, whereas only ceftazidime/avibactam was effective in ceftazidime-resistant β-lactamase-producing strains. Ceftazidime/avibactam was also compared with ceftazidime alone, ceftazidime/clavulanate and imipenem in a mouse model of pyelonephritis induced by ceftazidime-resistant Klebsiella pneumoniae, Escherichia coli, Enterobacter cloacae, Morganllea morganii or Citrobacter freundii.32 In untreated animals, the bacterial load 48 h post-infection was 105–107 cfu/kidney. Ceftazidime alone was ineffective against all strains, while ceftazidime/avibactam demonstrated efficacy with a significant 2.6–4.5 log10 reduction in kidney bacterial counts 48 h after therapy initiation. Overall, imipenem showed similar efficacy to ceftazidime/avibactam, while the ceftazidime/clavulanate combination was active only against one isolate.32 A further study evaluating ceftazidime/avibactam efficacy in a murine model of pneumonia induced by intratracheal inoculation of AmpC- and SHV-11-producing K. pneumoniae isolates found that the mean lung bacterial counts were reduced to 7.9 log10 cfu by ceftazidime monotherapy and to 4.5 log10 cfu by ceftazidime in combination with avibactam.33
Since cephalosporins have a recognized ability to reach high concentrations in the CSF, the activity of ceftazidime/avibactam has also been evaluated in experimental models of meningitis in rabbits.34 After infection with an AmpC-producing K. pneumoniae isolate showing an MIC of ceftazidime >128 mg/L, animals were treated with intravenous injections of ceftazidime (150 mg/kg) alone or in combination with avibactam (37.5 mg/kg) or meropenem (125 mg/kg). CSF and blood were sampled from 0 to 8 h following initiation of antibacterial therapy and tested for ceftazidime/avibactam and meropenem concentrations; in addition, bacterial titres were measured in CSF. The mean CSF penetration of avibactam in rabbits was 38%. While ceftazidime monotherapy was unable to reduce the CSF bacterial load, bacterial titres in CSF were significantly decreased following treatment with ceftazidime/avibactam (>5 log reduction at 8 h after initiation of therapy).34
More recently, Berkhout et al.35 evaluated penetration of ceftazidime/avibactam in a neutropenic murine thigh infection model to study pharmacokinetics in plasma and epithelial lining fluid (ELF). Mice were infected with P. aeruginosa to cause pneumonia and were given different doses of ceftazidime and avibactam in various combined concentrations. Concomitant samples of serum and bronchoalveolar lavage (BAL) fluid were taken at up to 12 timepoints until 6 h after administration. The pharmacokinetics of both compounds were linear and dose proportional in both plasma and ELF and were independent of the infection type, indicating that similar dose ratios of ceftazidime and avibactam could be used for different types and sites of infection.35
Pharmacokinetics and metabolism
Ceftazidime is a β-lactam antibiotic exerting bactericidal activity by interfering with bacterial cell wall synthesis and inhibiting cross-linking of the peptidoglycan. The pharmacokinetics of ceftazidime have been extensively reviewed elsewhere.36 Ceftazidime demonstrates a time-dependent killing that is maximal at 4 or 5 × MIC for the isolated organism. It is usually administered in intermittent infusion, it is not metabolized and is excreted by glomerular filtration. Data describing the pharmacokinetics of avibactam are available in abstract form,37,38 while a recent Phase I study evaluated the pharmacokinetic parameters of avibactam alone and a ceftazidime/avibactam combination in healthy Japanese volunteers.39 The approved dosing of ceftazidime/avibactam in patients with normal renal function (CLCR >50 mL/min calculated with the Cockcroft–Gault formula) is 2.5 g intravenously every 8 h over a 2 h infusion. Ceftazidime/avibactam is co-formulated in a ratio of 4:1 by weight of ceftazidime:avibactam (2 g ceftazidime, 0.5 g avibactam). The pharmacokinetic parameters of ceftazidime and avibactam40,41 are reported in Table 4. The mean half-life of avibactam is 1.4 h following single or multiple doses alone or in combination with ceftazidime. No plasma accumulation is observed for avibactam or ceftazidime after multiple-dose administration.42 In healthy adults, avibactam has a volume of distribution of 20–24 L and a low protein binding (it is reported to be 8%).38,39 The majority of avibactam is excreted unchanged in the urine within 24 h. Following single-dose administration on day 1, the mean percentage of avibactam excreted in urine was 73.2% for avibactam alone and 86.5% when given in combination with ceftazidime. Following multiple-dose administration, the mean percentage of the avibactam dose excreted in urine on day 7 was 99.5% for avibactam alone, and 95.8% when given in combination with ceftazidime.41 No dose adjustment is required based on age or gender.42
| Parameter . | Ceftazidime, 2 g every 8 h . | Avibactam, 500 mg every 8 h . |
|---|---|---|
| Cmax, mg/L | 90.4 | 14.6 |
| t1/2, h | 2.7 | 2.7 |
| V, L | 17 | 22.2 |
| AUC0–tau, mg·h/L | 291 | 38.2 |
| Protein binding, % | 21 | 8 |
| Elimination (urine), % | 83 | >97 |
| Parameter . | Ceftazidime, 2 g every 8 h . | Avibactam, 500 mg every 8 h . |
|---|---|---|
| Cmax, mg/L | 90.4 | 14.6 |
| t1/2, h | 2.7 | 2.7 |
| V, L | 17 | 22.2 |
| AUC0–tau, mg·h/L | 291 | 38.2 |
| Protein binding, % | 21 | 8 |
| Elimination (urine), % | 83 | >97 |
Cmax, peak serum concentration; t1/2, half-life; V, volume of distribution; AUC0–tau, area under the curve over the dosing interval.
| Parameter . | Ceftazidime, 2 g every 8 h . | Avibactam, 500 mg every 8 h . |
|---|---|---|
| Cmax, mg/L | 90.4 | 14.6 |
| t1/2, h | 2.7 | 2.7 |
| V, L | 17 | 22.2 |
| AUC0–tau, mg·h/L | 291 | 38.2 |
| Protein binding, % | 21 | 8 |
| Elimination (urine), % | 83 | >97 |
| Parameter . | Ceftazidime, 2 g every 8 h . | Avibactam, 500 mg every 8 h . |
|---|---|---|
| Cmax, mg/L | 90.4 | 14.6 |
| t1/2, h | 2.7 | 2.7 |
| V, L | 17 | 22.2 |
| AUC0–tau, mg·h/L | 291 | 38.2 |
| Protein binding, % | 21 | 8 |
| Elimination (urine), % | 83 | >97 |
Cmax, peak serum concentration; t1/2, half-life; V, volume of distribution; AUC0–tau, area under the curve over the dosing interval.
Pharmacokinetic parameters have also been studied after a single 100 mg dose of avibactam (30 min intravenous infusion) in normal subjects, in patients with mild (CLCR 50–79 mL/min), moderate (CLCR 30–49 mL/min) and severe (CLCR <30 mL/min non-anuric) renal impairment and in anuric patients requiring haemodialysis. Since avibactam is almost completely excreted in urine, its clearance declined as a function of CLCR. Therefore, doses should be adjusted to the same proportions in patients with impaired renal function. The recommended doses for patients with varying degrees of renal impairment include: (i) CLCR = 31–50 mL/min, 1.25 g intravenously every 8 h; (ii) CLCR = 16–30 mL/min, 0.94 g intravenously every 12 h; (iii) CLCR = 6–15 mL/min, 0.94 g intravenously every 24 h; and (iv) CLCR ≤ 5 mL/min, 0.94 g intravenously every 48 h. Patients on haemodialysis should receive their dose after dialysis. No adjustment is required for hepatic impairment.
In vitro, avibactam is a substrate of OAT1 and OAT3 transporters, which may contribute to the active uptake from the blood compartment and to its excretion. As a potent OAT inhibitor, probenecid inhibits OAT uptake of avibactam by 56%–70% and can decrease the elimination of avibactam when co-administrated. Because a clinical interaction study of ceftazidime/avibactam or avibactam alone with probenecid has not been conducted, co-administration of the two drugs is not recommended.42
Clinical data
The clinical efficacy of ceftazidime/avibactam has been demonstrated in the setting of cUTI and cIAI and derives principally from Phase II studies,43,44 summarized in Table 5.
Results from the two Phase II, prospective, randomized, double-blind comparative trials on ceftazidime/avibactam
| Study . | Study population . | Study treatments . | Endpoints . | Results . | |
|---|---|---|---|---|---|
| efficacy . | safety and tolerability . | ||||
| Vazquez et al.43 | 137 patients | CAZ/AVI 500/125 mg each iv q8h (n = 69) versus imipenem/cilastatin 500 mg/500 mg iv q6h (n = 68) for 7–14 days (step down to oral ciprofloxacin was permitted) | |||
| Inclusion criteria | Primary endpoint | ||||
| Favourable microbiological response (FMR) at the test-of-cure (TOC) visit (5–9 days after the last dose of study drug)a | 19/27 (70.4%) CAZ/AVI arm 25/35 (71.4%) comparator arm [observed difference −1.1% (95% CI:−27.2%–25%)] | AEb 46/68 (67.6%) CAZ/AVI arm 51/67 (76.1%) comparator arm | ||
| |||||
| Exclusion criteria | Secondary endpoint | ||||
| FMR at the end of iv therapya | 25/26 (96.2%) CAZ/AVI arm 34/34 (100%) comparator arm | SAEc 6/68 (8.8%) CAZ/AVI arm 2/67 (3%) comparator arm | ||
| FMR at the late follow-up (LFU) visit, 4–6 weeks post-therapya | 15/26 (57.7%) CAZ/AVI arm 18/30 (60%) comparator arm | |||
| Clinical response at the end of iv therapyd | 28/28 (100%) CAZ/AVI arm 36/36 (100%) comparator arm | |||
| Clinical response at the TOC visitd | 24/28 (85.7%) CAZ/AVI arm 29/36 (80.5%) comparator arm | |||
| Clinical response at the LFU visitd | 21/28 (75%) CAZ/AVI arm 24/36 (66.7%) comparator arm | |||
| |||||
| |||||
| |||||
| |||||
| |||||
| Lucasti et al.44 | 204 patients | ||||
| Inclusion criteria | CAZ/AVI 2000/500 mg plus metronidazole 500 mg each iv q8h (n = 101) versus meropenem 1000 mg iv q8h (n = 102) for 5–14 days | Clinical response at the TOC visita | 62/68 (91.2%) CAZ/AVI arm 71/76 (93.4%) comparator arm [estimated difference −2.2% (95% CI:−20.4%– 12.2%)] | AEe 65/101 (64.4%) CAZ/AVI arm 59/102 (57.8%) comparator arm | |
| |||||
| |||||
| Exclusion criteria | |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| Clinical response at the end of iv therapya | 66/68 (97.1%) CAZ/AVI arm 74/76 (97.4%) comparator arm [observed difference −0.3% (95% CI:−17.7%–15.4%)] | SAEf 9/101 (8.9%) CAZ/AVI arm 11/102 (10.8%) comparator arm | ||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| Study . | Study population . | Study treatments . | Endpoints . | Results . | |
|---|---|---|---|---|---|
| efficacy . | safety and tolerability . | ||||
| Vazquez et al.43 | 137 patients | CAZ/AVI 500/125 mg each iv q8h (n = 69) versus imipenem/cilastatin 500 mg/500 mg iv q6h (n = 68) for 7–14 days (step down to oral ciprofloxacin was permitted) | |||
| Inclusion criteria | Primary endpoint | ||||
| Favourable microbiological response (FMR) at the test-of-cure (TOC) visit (5–9 days after the last dose of study drug)a | 19/27 (70.4%) CAZ/AVI arm 25/35 (71.4%) comparator arm [observed difference −1.1% (95% CI:−27.2%–25%)] | AEb 46/68 (67.6%) CAZ/AVI arm 51/67 (76.1%) comparator arm | ||
| |||||
| Exclusion criteria | Secondary endpoint | ||||
| FMR at the end of iv therapya | 25/26 (96.2%) CAZ/AVI arm 34/34 (100%) comparator arm | SAEc 6/68 (8.8%) CAZ/AVI arm 2/67 (3%) comparator arm | ||
| FMR at the late follow-up (LFU) visit, 4–6 weeks post-therapya | 15/26 (57.7%) CAZ/AVI arm 18/30 (60%) comparator arm | |||
| Clinical response at the end of iv therapyd | 28/28 (100%) CAZ/AVI arm 36/36 (100%) comparator arm | |||
| Clinical response at the TOC visitd | 24/28 (85.7%) CAZ/AVI arm 29/36 (80.5%) comparator arm | |||
| Clinical response at the LFU visitd | 21/28 (75%) CAZ/AVI arm 24/36 (66.7%) comparator arm | |||
| |||||
| |||||
| |||||
| |||||
| |||||
| Lucasti et al.44 | 204 patients | ||||
| Inclusion criteria | CAZ/AVI 2000/500 mg plus metronidazole 500 mg each iv q8h (n = 101) versus meropenem 1000 mg iv q8h (n = 102) for 5–14 days | Clinical response at the TOC visita | 62/68 (91.2%) CAZ/AVI arm 71/76 (93.4%) comparator arm [estimated difference −2.2% (95% CI:−20.4%– 12.2%)] | AEe 65/101 (64.4%) CAZ/AVI arm 59/102 (57.8%) comparator arm | |
| |||||
| |||||
| Exclusion criteria | |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| Clinical response at the end of iv therapya | 66/68 (97.1%) CAZ/AVI arm 74/76 (97.4%) comparator arm [observed difference −0.3% (95% CI:−17.7%–15.4%)] | SAEf 9/101 (8.9%) CAZ/AVI arm 11/102 (10.8%) comparator arm | ||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
CAZ/AVI, ceftazidime/avibactam; cUTI, complicated urinary tract infections; cIAIs, complicated intra-abdominal infections; AE, adverse events; SAE, serious adverse events.
aIn microbiologically evaluable (ME) population.
bMost common adverse events: constipation, diarrhoea, abdominal pain, headache, anxiety and injection/infusion site reactions.
cThree serious adverse events (renal failure, diarrhoea and accidental overdose of CAZ/AVI) were considered to be drug related in the CAZ/AVI arm and one (increased creatinine levels) in the control arm.
dIn clinically evaluable (CE) population.
eMost common adverse events: nausea, vomiting, abdominal pain, pyrexia, increased transaminase levels.
fThree deaths in the CAZ/AVI group and two in the control group (none considered to be drug related).
Results from the two Phase II, prospective, randomized, double-blind comparative trials on ceftazidime/avibactam
| Study . | Study population . | Study treatments . | Endpoints . | Results . | |
|---|---|---|---|---|---|
| efficacy . | safety and tolerability . | ||||
| Vazquez et al.43 | 137 patients | CAZ/AVI 500/125 mg each iv q8h (n = 69) versus imipenem/cilastatin 500 mg/500 mg iv q6h (n = 68) for 7–14 days (step down to oral ciprofloxacin was permitted) | |||
| Inclusion criteria | Primary endpoint | ||||
| Favourable microbiological response (FMR) at the test-of-cure (TOC) visit (5–9 days after the last dose of study drug)a | 19/27 (70.4%) CAZ/AVI arm 25/35 (71.4%) comparator arm [observed difference −1.1% (95% CI:−27.2%–25%)] | AEb 46/68 (67.6%) CAZ/AVI arm 51/67 (76.1%) comparator arm | ||
| |||||
| Exclusion criteria | Secondary endpoint | ||||
| FMR at the end of iv therapya | 25/26 (96.2%) CAZ/AVI arm 34/34 (100%) comparator arm | SAEc 6/68 (8.8%) CAZ/AVI arm 2/67 (3%) comparator arm | ||
| FMR at the late follow-up (LFU) visit, 4–6 weeks post-therapya | 15/26 (57.7%) CAZ/AVI arm 18/30 (60%) comparator arm | |||
| Clinical response at the end of iv therapyd | 28/28 (100%) CAZ/AVI arm 36/36 (100%) comparator arm | |||
| Clinical response at the TOC visitd | 24/28 (85.7%) CAZ/AVI arm 29/36 (80.5%) comparator arm | |||
| Clinical response at the LFU visitd | 21/28 (75%) CAZ/AVI arm 24/36 (66.7%) comparator arm | |||
| |||||
| |||||
| |||||
| |||||
| |||||
| Lucasti et al.44 | 204 patients | ||||
| Inclusion criteria | CAZ/AVI 2000/500 mg plus metronidazole 500 mg each iv q8h (n = 101) versus meropenem 1000 mg iv q8h (n = 102) for 5–14 days | Clinical response at the TOC visita | 62/68 (91.2%) CAZ/AVI arm 71/76 (93.4%) comparator arm [estimated difference −2.2% (95% CI:−20.4%– 12.2%)] | AEe 65/101 (64.4%) CAZ/AVI arm 59/102 (57.8%) comparator arm | |
| |||||
| |||||
| Exclusion criteria | |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| Clinical response at the end of iv therapya | 66/68 (97.1%) CAZ/AVI arm 74/76 (97.4%) comparator arm [observed difference −0.3% (95% CI:−17.7%–15.4%)] | SAEf 9/101 (8.9%) CAZ/AVI arm 11/102 (10.8%) comparator arm | ||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| Study . | Study population . | Study treatments . | Endpoints . | Results . | |
|---|---|---|---|---|---|
| efficacy . | safety and tolerability . | ||||
| Vazquez et al.43 | 137 patients | CAZ/AVI 500/125 mg each iv q8h (n = 69) versus imipenem/cilastatin 500 mg/500 mg iv q6h (n = 68) for 7–14 days (step down to oral ciprofloxacin was permitted) | |||
| Inclusion criteria | Primary endpoint | ||||
| Favourable microbiological response (FMR) at the test-of-cure (TOC) visit (5–9 days after the last dose of study drug)a | 19/27 (70.4%) CAZ/AVI arm 25/35 (71.4%) comparator arm [observed difference −1.1% (95% CI:−27.2%–25%)] | AEb 46/68 (67.6%) CAZ/AVI arm 51/67 (76.1%) comparator arm | ||
| |||||
| Exclusion criteria | Secondary endpoint | ||||
| FMR at the end of iv therapya | 25/26 (96.2%) CAZ/AVI arm 34/34 (100%) comparator arm | SAEc 6/68 (8.8%) CAZ/AVI arm 2/67 (3%) comparator arm | ||
| FMR at the late follow-up (LFU) visit, 4–6 weeks post-therapya | 15/26 (57.7%) CAZ/AVI arm 18/30 (60%) comparator arm | |||
| Clinical response at the end of iv therapyd | 28/28 (100%) CAZ/AVI arm 36/36 (100%) comparator arm | |||
| Clinical response at the TOC visitd | 24/28 (85.7%) CAZ/AVI arm 29/36 (80.5%) comparator arm | |||
| Clinical response at the LFU visitd | 21/28 (75%) CAZ/AVI arm 24/36 (66.7%) comparator arm | |||
| |||||
| |||||
| |||||
| |||||
| |||||
| Lucasti et al.44 | 204 patients | ||||
| Inclusion criteria | CAZ/AVI 2000/500 mg plus metronidazole 500 mg each iv q8h (n = 101) versus meropenem 1000 mg iv q8h (n = 102) for 5–14 days | Clinical response at the TOC visita | 62/68 (91.2%) CAZ/AVI arm 71/76 (93.4%) comparator arm [estimated difference −2.2% (95% CI:−20.4%– 12.2%)] | AEe 65/101 (64.4%) CAZ/AVI arm 59/102 (57.8%) comparator arm | |
| |||||
| |||||
| Exclusion criteria | |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| Clinical response at the end of iv therapya | 66/68 (97.1%) CAZ/AVI arm 74/76 (97.4%) comparator arm [observed difference −0.3% (95% CI:−17.7%–15.4%)] | SAEf 9/101 (8.9%) CAZ/AVI arm 11/102 (10.8%) comparator arm | ||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
| |||||
CAZ/AVI, ceftazidime/avibactam; cUTI, complicated urinary tract infections; cIAIs, complicated intra-abdominal infections; AE, adverse events; SAE, serious adverse events.
aIn microbiologically evaluable (ME) population.
bMost common adverse events: constipation, diarrhoea, abdominal pain, headache, anxiety and injection/infusion site reactions.
cThree serious adverse events (renal failure, diarrhoea and accidental overdose of CAZ/AVI) were considered to be drug related in the CAZ/AVI arm and one (increased creatinine levels) in the control arm.
dIn clinically evaluable (CE) population.
eMost common adverse events: nausea, vomiting, abdominal pain, pyrexia, increased transaminase levels.
fThree deaths in the CAZ/AVI group and two in the control group (none considered to be drug related).
The FDA has approved ceftazidime/avibactam for the treatment of cUTI. A Phase II study was recently conducted in 135 hospitalized patients with cUTI (pyelonephritis or other cUTI).43 Patients were randomized 1:1 to receive intravenous ceftazidime/avibactam (ceftazidime 500 mg plus avibactam 125 mg every 8 h) or imipenem/cilastatin (500 mg/500 mg every 6 h) for a total of 7–14 days. The investigators established criteria for switch to oral ciprofloxacin after completion of at least 4 days of intravenous study drug therapy. The predominant uropathogen was E. coli. Favourable microbiological response was achieved in 70.4% of patients receiving ceftazidime/avibactam and 71.4% receiving imipenem/cilastatin [observed difference −1.1% (95% CI: −27.2% to 25.0%)]. Among patients with ceftazidime-resistant uropathogens, a response was observed in 6/7 (85.7%) receiving ceftazidime/avibactam.43 Lucasti et al.44 studied the safety and efficacy of ceftazidime/avibactam plus metronidazole compared with meropenem in hospitalized patients with cIAI. Adults with confirmed cIAI requiring surgical intervention and antibiotics were randomized 1:1 to receive intravenously either (i) 2000 mg of ceftazidime plus 500 mg of avibactam plus a separate infusion of 500 mg of metronidazole (n = 101) or (ii) 1000 mg of meropenem plus placebo every 8 h (n = 102) for a minimum of 5 days and a maximum of 14 days. E. coli was the most common pathogen isolated from the site of IAI and all E. coli isolates were susceptible to both ceftazidime/avibactam and meropenem. A favourable clinical response was observed in 91.2% and 93.4% of ceftazidime/avibactam plus metronidazole and meropenem patients, respectively, with an estimated difference in response rates of –2.2% (95% CI: −20.4% to 12.2%). There was no relationship between the APACHE II score at baseline, site of primary infection and type of infection (mono- or polymicrobial) and clinical response.44 A recent Phase III study45 assessed the efficacy of ceftazidime/avibactam compared with the best available therapy (a carbapenem in 97% of cases) in the treatment of cUTI and cIAI caused by ceftazidime-resistant Enterobacteriaceae or P. aeruginosa. Ceftazidime/avibactam was not inferior to carbapenems in terms of clinical efficacy, safety and tolerability.45
The FDA recently outlined that the subgroup of patients with moderate renal impairment at baseline (defined as an estimated CLCR of >30 and ≤50 mL/min) receiving ceftazidime/avibactam + metronidazole had lower clinical cure rates than those receiving meropenem.46 A review of serum creatinine and dosing data suggests that this subgroup of patients may have been underexposed to the study drugs due to rapid improvements in CLCR after enrolment without a similar rapid correction in dosing. Based on a preliminary review of data, approximately 60% of patients with moderate renal impairment in each treatment group had an estimated CLCR >50 mL/min by 48 h post-baseline and thus would require an increase in dose to reflect changing CLCR. Pharmacokinetic/pharmacodynamic data suggest that if the patient's renal function improves to be in the range of normal renal function, target attainment at MICs of 4 and 8 mg/L would be 80.5% and 52.2%, respectively, if the patient continues to receive 1.25 g every 8 h. Moreover, for the majority of patients (10 ceftazidime/avibactam plus metronidazole; 5 meropenem) with moderate renal impairment who experienced failure, this could be classified as a lack of efficacy likely unrelated to the study drug (i.e. inadequate surgical intervention, an inadequate trial of study drug, or a pathogen that was not expected to respond to the treatment received). These observations probably suggest the need for therapeutic drug monitoring to adjust ceftazidime/avibactam dosages in patients with renal failure. A Phase III, randomized, double-blind study evaluating the effects of ceftazidime/avibactam compared with doripenem for treating hospitalized patients with cUTI, including acute pyelonephritis, has been recently completed,11,12 but the results are not yet available.
Although ceftazidime/avibactam has not yet received FDA approval for treating HAP, a Phase III study to assess the efficacy, safety and tolerability of ceftazidime/avibactam versus meropenem in the treatment of HAP including VAP is currently recruiting participants.15 No trials specifically for the treatment of CRE have yet been performed.
Safety
Data about the safety of ceftazidime/avibactam include experience from Phase I and Phase II trials, as well as preliminary data from recently completed Phase III trials. Generally, ceftazidime/avibactam is well tolerated. In the cumulative ceftazidime/avibactam clinical programme, 61 deaths have been reported, including 7 in the Phase II studies (4 ceftazidime/avibactam, 3 comparator) and 54 in the ongoing Phase III studies (3 comparator, 3 ceftazidime/avibactam and 48 treatment blinded), but each appears to be attributable to underlying comorbidities, treatment failure and/or emergent infection. In the Phase II studies, adverse events were similar in both treatment arms.43,44 In the study conducted in patients with cUTI there were no differences in terms of adverse events between ceftazidime/avibactam and imipenem/cilastatin; the most common adverse events reported among patients receiving ceftazidime/avibactam were headache, gastrointestinal symptoms and infusion-site reactions. Two serious adverse events, considered to be drug related, were a case of renal failure and a case of diarrhoea.43 Similarly, Lucasti et al.44 observed similar types and frequencies of adverse events in the two treatment groups (64.4% in the of ceftazidime/avibactam + metronidazole group and 57.8% in the meropenem group). The most common side effects were abdominal pain, vomiting, nausea and constipation. The most common laboratory findings were increases in alkaline phosphatase, ALT and AST. Most of these adverse events were mild to moderate and some of these could be attributable to other comorbidities or metronidazole therapy.44
A double-blind, randomized, placebo-controlled, four-period cross-over Phase I study (NCT01290900) conducted in 51 healthy males studied the potential effects of supratherapeutic doses of intravenous ceftazidime/avibactam (single dose of ceftazidime 3000 mg with avibactam 2000 mg, 30 min infusion) on cardiac repolarization. The pharmacokinetic results confirmed achievement of supratherapeutic plasma concentrations, and supratherapeutic doses of ceftazidime/avibactam were not associated with QT/QTc prolongation.47
Comparison with ceftolozane/tazobactam
Ceftazidime/avibactam has a profile similar to that of another second-generation β-lactam/β-lactamase combination antibiotic, ceftolozane/tazobactam. Ceftazidime/avibactam and ceftolozane/tazobactam have similar spectra of antimicrobial activity, and both have potent in vitro anti-Pseudomonas activity, including meropenem-resistant isolates.48 The main difference in terms of in vitro activity is that, unlike tazobactam, avibactam displays activity against carbapenemases of the KPC family and OXA-48 β-lactamases.49 This suggests that ceftazidime/avibactam could be useful to treat CRE infections. However, variants of KPC-2 β-lactamase resistant to inhibition by avibactam have been recently reported,50 and a few reports of resistance of KPC-producing organisms to ceftazidime/avibactam are now emerging.51,52 The mechanisms of this need further elucidation.
The pharmacokinetics of the two drugs are similar, as well as the clinical indications (cUTI and cIAI). The use of these drugs will probably depend on the epidemiological patterns of each hospital, and knowledge of resistance profiles of the most common pathogens isolated from different areas will be necessary. Furthermore, future pathogen-specific studies (e.g. clinical trials involving CRE or P. aeruginosa in special populations) will be useful to specify the role of ceftazidime/avibactam and ceftolozane/tazobactam in different clinical settings.
Conclusions
Ceftazidime/avibactam is a novel drug combination that could be useful in some cases of difficult-to-treat Gram-negative infections, when there are few or no therapeutic options. It is effective against Enterobacteriaceae and P. aeruginosa, but has no activity against A. baumannii. In vitro studies demonstrated that avibactam (a non-β-lactam β-lactamase inhibitor), in contrast to clavulanic acid, sulbactam and tazobactam (β-lactam β-lactamase inhibitors), is active against a larger range of β-lactamases and exerts a reversible and stronger action against them. A major limitation is its inability to inhibit metallo-β-lactamases, such as IMP or NDM. Animal studies demonstrate that ceftazidime/avibactam is effective in ceftazidime-resistant Gram-negative bacteraemia, meningitis, pyelonephritis and pneumonia. Safety and tolerability of ceftazidime/avibactam in clinical trials has been excellent, with few serious drug-related adverse events.
Approaches to optimize the use of newly developed antibiotics are of critical importance to provide the best care to patients. Since the ceftazidime/avibactam combination represents the last powerful weapon to treat infections caused by carbapenem-resistant bacteria, its use should be prudent, reserving this option for patients with documented difficult-to treat infections or in areas with a high prevalence of CRE. To avoid underdosing of this drug and the selection of resistant strains, future pharmacokinetic studies in critically ill patients will be probably necessary, to optimize dosages in septic patients. Preservation of new expanded-activity antibiotics is crucial to provide effective and safe therapies to patients affected by MDR or pan-resistant Gram-negative bacterial infections. A major omission thus far is the absence of clinical trials of ceftazidime/avibactam specifically for serious infections due to CRE.
Funding
The authors prepared this manuscript as part of their normal daily work.
Transparency declarations
D. P. has received honoraria for advisory boards from Merck, AstraZeneca, Shionogi, Meiji and GlaxoSmithKline. M. F. has served on scientific advisory boards for MSD and Pfizer, and has received funding for speaker honoraria from Pfizer, Novartis, Astellas and MSD. He received grant support from Gilead.



