-
PDF
- Split View
-
Views
-
Cite
Cite
Chung-Chih Lai, Wen-Chun Liu, Chi-Tai Fang, Jyh-Yuan Yang, Lan-Hsin Chang, Pei-Ying Wu, Yu-Zhen Luo, Shu-Fang Chang, Yi-Ching Su, Sui-Yuan Chang, Chien-Ching Hung, Transmitted drug resistance of HIV-1 strains among individuals attending voluntary counselling and testing in Taiwan, Journal of Antimicrobial Chemotherapy, Volume 71, Issue 1, January 2016, Pages 226–234, https://doi.org/10.1093/jac/dkv284
Close - Share Icon Share
Abstract
Genotypic drug resistance testing for HIV-1 has been integrated into voluntary counselling and testing (VCT) programmes to investigate the trends of transmitted drug resistance (TDR), including integrase mutations, among individuals with recent or chronic HIV infections in Taiwan.
Between 2006 and 2014, 745 of 21 886 subjects (3.4%) tested HIV positive in the VCT service. The BED assay was used to identify recent HIV infections. Genotypic resistance mutations were interpreted using the WHO 2009 list. Integrase resistance mutations were analysed using the Stanford HIV Drug Resistance Database.
Three-hundred-and-sixty (48.3%) patients were recently infected with HIV-1. Of 440 patients linked to HIV care with analysable reverse transcriptase and protease genes, 49 (11.1%) were infected with HIV-1 harbouring at least one resistance-associated mutation (RAM). The prevalence of TDR to NRTIs, NNRTIs and PIs was 4.1%, 6.4% and 2.3%, respectively. TDR prevalence did not change significantly during the study period. CD4 counts ≤500 cells/mm3 and hepatitis B surface antigen positivity were independent factors associated with acquiring drug-resistant HIV. The prevalence of integrase mutations was 3.2%. Among the seven major integrase mutations (T66I, E92Q, G140S, Y143C/H/R, S147G, Q148H/K/R and N155H), only one strain harbouring the Q148R mutation was detected. We found no statistically significant difference between patients with chronic infection and those with recent infection in the prevalence of drug-resistant mutations to any of the four classes of antiretroviral agents.
The prevalence of TDR of HIV-1 strains to available antiretroviral agents is moderately high, but transmission of HIV-1 with drug-resistant mutations remains stable in Taiwan.
Introduction
The emergence and spread of HIV with drug resistance seems to be inevitable as coverage of ART continues to grow, despite adequate ART options and optimal adherence.1 This has posed a serious threat to public health globally with the rapid scale-up of ART in low- and middle-income countries, where infrastructures for frequent virological monitoring and testing for drug resistance are lacking.2
Transmitted drug resistance (TDR), which occurs when previously uninfected individuals are infected with a drug-resistant HIV strain, is of particular concern because it will jeopardize the success of first-line combination ART (cART).3–5 The burdens of TDR vary substantially across geographical regions and temporal changes in TDR have been inconsistent in different parts of the world.6–12 Current data from WHO surveys suggest there is an association between higher levels of ART coverage and a higher prevalence of TDR to NNRTIs.1 Regular population-based surveillance of transmission of drug-resistant strains is critical to ensure sustained effectiveness of ART and to provide the basis for selecting first-line regimens, especially in resource-limited countries where routine HIV drug resistance testing prior to ART initiation is not generally available due to cost constraints.
Conventionally, standard genotypic resistance testing involves testing for mutations in the reverse transcriptase (RT) and protease (PR) genes, and surveillance of TDR is limited to NRTIs, NNRTIs and PIs. However, among the five recommended regimens for ART-naive patients in the recently revised guidelines, four are integrase strand transfer inhibitor (INSTI) based.13 Currently, transmission of INSTI-resistant virus has rarely been reported among ART-naive patients with HIV infection.14–16 In addition, integrase resistance mutations may have negative impacts on viral fitness.17,18 Therefore, routine testing for INSTI resistance is not recommended at present.13 However, as the use of INSTIs will increase following physician adherence to the guidelines, the potential for transmission of these HIV-1 strains with resistance to INSTIs may also increase. Data on pre-treatment resistance to INSTIs remain scarce among ART-naive patients, especially outside Europe and the USA.19
Previous studies on trends of TDR prevalence of HIV-1 in Taiwan showed an increased rate during 2003–06 and a decline during 2007–10, with the use of the HIVdb programme of the Stanford University HIV Drug Resistance Database.5,20 However, many patients included in prior studies were probably chronically infected, and the prevalence of TDR may be underestimated because drug-resistant strains might revert to WT virus over time in the absence of ART.21 Anonymous voluntary counselling and testing (VCT) has been demonstrated to reach the target populations most at risk of HIV infection, and patients diagnosed with HIV infection via VCT are more likely to be in an early stage of HIV infection.22,23 In this study, we evaluated the prevalences of TDR to NRTIs, NNRTIs, PIs and INSTIs among VCT subjects testing positive for HIV, including patients with laboratory-confirmed recent infection.
Methods
Setting of the voluntary counselling and testing programme
The National Taiwan University Hospital (NTUH), the largest hospital providing free-of-charge HIV care in Taiwan, has provided VCT services since 1999, which was expanded in 2006. The number of attendees of the VCT programme at NTUH accounted for ∼14% of the total number in Taiwan in recent years. A standardized anonymous, self-administered questionnaire interview (available as Supplementary data at JAC Online) designed by the Taiwan CDC has been performed to obtain the information on the demographics, sexual practices, risk behaviours, history of sexually transmitted infections (STIs), number of sexual partners, HIV serostatus of sexual partners, condom use and use of illicit drugs.22 After interview and counselling by trained counsellors, an 8–10 mL blood sample was collected from each individual for serological tests of HIV infection and other STIs. The results were given to the subjects by mobile phone, using a unique testing code consisting of the first alphabetic character and the last three digits of the national identification card, sex and birth year for identification. Those testing positive would be linked to a clinical care and case management programme that was implemented in 2006. Other details have been described previously.22 The study was approved by the research ethics committee of the hospital and the patients who were linked to HIV care at the hospital gave written informed consent for determination of TDR and the BED-CEIA (capture enzyme immunoassay).
Setting of HIV care in Taiwan
HIV-infected Taiwanese patients receive HIV care according to the national treatment guidelines at designated hospitals around Taiwan. Medical costs related to HIV care, including cART, quantification of CD4 lymphocyte counts and plasma HIV RNA load (PVL), and management for opportunistic illnesses are totally reimbursed by the Taiwan CDC. Drug resistance testing, however, was not routinely available and restricted to patients experiencing treatment failure and to pregnant women. The Taiwan CDC has implemented nationwide regulations on the use of first-line regimens since March 2011 due to substantial financial pressure in providing HIV care, and NNRTI-based regimens have been the preferred cART. Regimens with higher costs require pre-prescription approval by the Taiwan CDC. Since then, use of NNRTIs has increased markedly. Furthermore, generic versions of antiretroviral agents have been introduced since December 2012.
Determination of recent HIV infections, subtypes and coinfections
Anti-HIV antibody was tested using particle agglutination (SFD HIV 1/2 PA; Bio-Rad FUJIREBIO, Japan) and HIV infection was confirmed using western blotting (MP Diagnostics HIV BLOT 2.2; MP Biomedicals Asia Pacific Pte Ltd, Singapore). Hepatitis B surface antigen (HBsAg), anti-HBs antibody and hepatitis B core antibody (anti-HBc antibody) were determined with the use of enzyme immunoassay (Abbott Laboratories, Abbott Park, IL, USA). Antibodies to hepatitis C virus were determined with the use of a third-generation enzyme immunoassay (Ax SYM HCV III; Abbott Laboratories, North Chicago, IL, USA).
The BED assay was performed in the residual samples. The BED-CEIA (Calypte, MD, USA) determines the proportion of anti-HIV-1-specific IgG in relation to total IgG, based on the observation that the ratio of anti-HIV IgG to total IgG increases with time shortly after HIV infection.24 If a specimen is reactive in the standard sensitive enzyme immunoassay and has a normalized optical density of <0.8 in the BED assay, the source patient is considered recently infected. The window period of recent infection is 153 days (95% CI 145–166 days). In addition, we also used age <25 years as an indicator of recent infection, according to WHO TDR survey methods, to validate the classification based on the BED assay.6 The HIV-1 subtypes were determined as described previously.25
Determination of drug resistance mutations
For those with confirmed HIV infection and linked to clinical care at NTUH, genotypic resistance assays were performed retrospectively as described previously.5,20 Resistance-associated mutations (RAMs) were defined by the presence of at least one mutation included in the 2009 WHO surveillance drug resistance mutation list.26 To determine genotypic resistant mutations related to INSTIs, an 884 bp fragment of integrase coding regions was PCR amplified. The PCR primer pair used was pol2950A (5′-TCA KCA CCT GCC ATC TGT TTT CC-3′)/2064A (5′-AYA ARG GRA TTG GAG GAA ATG AAC A-3′). The amplification condition was 35 cycles of 94°C for 15 s, 55°C for 1 min and 72°C for 2 min, and a final extension at 72°C for 7 min. The sequences were submitted to the Stanford HIVdb (http://hivdb.stanford.edu) to determine the mutation pattern and susceptibility.27 Mutations causing low-level resistance or above were considered clinically relevant.
PVL and CD4 lymphocyte count were quantified by the Cobas Amplicor HIV-1 Monitor™ Test, version 1.5 (Roche Diagnostics Corporation, Indianapolis, USA) and FACSFlow (Becton Dickinson), respectively.
Statistical analysis
Data were analysed using SAS 9.2 (SAS Institute, NC, USA). Categorical data were analysed using the χ2 or Fisher's exact test, as appropriate, and continuous variables were compared using the Wilcoxon test. All tests were two-tailed and a P value <0.05 was considered significant.
Results
Characteristics of the study population
From April 2006 to December 2014, 21 886 VCT subjects had HIV tests at NTUH (Figure 1). MSM and heterosexuals accounted for 13 325 (60.9%) and 8434 (38.5%) of the attendance, respectively. Almost 60% (n = 12 978; 59.3%) of VCT subjects were aged 20–29 years. Overall, 745 individuals were newly diagnosed with HIV-1 infection, with an overall HIV seroprevalence of 3.4% (95% CI 3.2%–3.7%) (Figure 1). Among the 745 HIV-positive persons, 360 (48.3%) were considered as having recent infections and 376 (50.5%) long-standing infections based on the BED assay. Three-quarters (n = 557; 74.8%) of those infected with HIV-1 were linked to HIV care at NTUH, including 264 (47.4%) classified as having recent infections and 286 (51.3%) chronic infections. The characteristics of these two groups of patients are shown in Table 1. In multivariate analysis, age ≤25 years (OR 2.50; 95% CI 1.62–3.86, P < 0.001), transactional sex behaviours (OR 2.93; 95% CI 1.23–6.98, P = 0.02), oral sex practices (OR 2.14; 95% CI 1.16–3.96, P = 0.02) and having CD4 lymphocyte counts >500 cells/mm3 (OR 1.82; 95% CI 1.16–2.86, P < 0.01) and PVL >5 log10 copies/mL (OR 2.41; 95% CI 1.55–3.76, P < 0.001) were associated with recent infections.
Comparisons of characteristics between VCT subjects with recent and those with long-term HIV infections who were linked to HIV care
| Characteristic . | All patients,aN = 557 . | Recent infections, N = 264 . | Long-term infections, N = 286 . | P . |
|---|---|---|---|---|
| Male, n (%) | 543 (97.5) | 262 (99.2) | 274 (95.8) | 0.01* |
| Age (years), mean ± SD | 29.5 ± 7.2 | 27.5 ± 5.8 | 31.3 (±7.8) | <0.001* |
| ≤25 years, n (%) | 163 (29.3) | 101 (38.3) | 60 (21.0) | <0.001* |
| Risk, n (%) | 0.10 | |||
| MSM | 527 (94.6) | 255 (96.6) | 267 (93.4) | |
| heterosexual | 26 (4.7) | 7 (2.7) | 18 (6.3) | |
| IDU with needle sharing | 4 (0.7) | 2 (0.8) | 1 (0.4) | |
| Reasons for screening, n (%) | ||||
| having confirmed HIV-infected sexual partners | 102 (18.3) | 34 (12.9) | 67 (23.4) | 0.001* |
| having an IDU partner | 2 (0.4) | 2 (0.8) | 0 (0.0) | 0.23 |
| ever having an STI | 132 (23.7) | 60 (22.7) | 71 (24.8) | 0.62 |
| having transactional sex | 30 (5.4) | 21 (8.0) | 9 (3.2) | 0.01* |
| having a one-night stand | 246 (44.2) | 132 (50.0) | 110 (38.5) | 0.01* |
| having anal sex | 438 (78.6) | 231 (87.5) | 202 (70.6) | <0.001* |
| having oral sex | 432 (77.6) | 225 (85.2) | 201 (70.3) | <0.001* |
| illicit drug use | 147 (26.4) | 75 (28.4) | 70 (24.5) | 0.33 |
| Baseline laboratory results | ||||
| RPR ≥4, n (%) | 70 (12.6) | 27 (10.2) | 42 (14.7) | 0.12 |
| western blot for HIV-1, n (%) | <0.001* | |||
| positive | 510 (91.6) | 222 (84.1) | 283 (99.0) | |
| indeterminate | 37 (6.6) | 35 (13.3) | 0 (0.0) | |
| PVL (log10 copies/mL), mean ± SD | 4.60 ± 0.76 | 4.75 ± 0.82 | 4.47 (±0.66) | <0.001* |
| >5 log10 copies/mL, n (%) | 157 (28.2) | 98 (37.1) | 56 (19.6) | <0.001* |
| CD4 cell count (cells/mm3), mean ± SD | 388.4 ± 209.5 | 436.8 ± 220.7 | 345.8 (±187.5) | <0.001* |
| >500 cells/mm3, n (%) | 138 (24.8) | 83 (31.4) | 52 (18.2) | <0.001* |
| HBsAg positivity, n (%) | 53 (9.5) | 20 (7.6) | 32 (11.2) | 0.19 |
| anti-HCV positivity, n (%) | 18 (3.2) | 5 (1.9) | 11 (3.8) | 0.21 |
| available genotypic resistance results, n (%) | 440 (79.0) | 212 (80.3) | 224 (78.3) | 0.52 |
| Characteristic . | All patients,aN = 557 . | Recent infections, N = 264 . | Long-term infections, N = 286 . | P . |
|---|---|---|---|---|
| Male, n (%) | 543 (97.5) | 262 (99.2) | 274 (95.8) | 0.01* |
| Age (years), mean ± SD | 29.5 ± 7.2 | 27.5 ± 5.8 | 31.3 (±7.8) | <0.001* |
| ≤25 years, n (%) | 163 (29.3) | 101 (38.3) | 60 (21.0) | <0.001* |
| Risk, n (%) | 0.10 | |||
| MSM | 527 (94.6) | 255 (96.6) | 267 (93.4) | |
| heterosexual | 26 (4.7) | 7 (2.7) | 18 (6.3) | |
| IDU with needle sharing | 4 (0.7) | 2 (0.8) | 1 (0.4) | |
| Reasons for screening, n (%) | ||||
| having confirmed HIV-infected sexual partners | 102 (18.3) | 34 (12.9) | 67 (23.4) | 0.001* |
| having an IDU partner | 2 (0.4) | 2 (0.8) | 0 (0.0) | 0.23 |
| ever having an STI | 132 (23.7) | 60 (22.7) | 71 (24.8) | 0.62 |
| having transactional sex | 30 (5.4) | 21 (8.0) | 9 (3.2) | 0.01* |
| having a one-night stand | 246 (44.2) | 132 (50.0) | 110 (38.5) | 0.01* |
| having anal sex | 438 (78.6) | 231 (87.5) | 202 (70.6) | <0.001* |
| having oral sex | 432 (77.6) | 225 (85.2) | 201 (70.3) | <0.001* |
| illicit drug use | 147 (26.4) | 75 (28.4) | 70 (24.5) | 0.33 |
| Baseline laboratory results | ||||
| RPR ≥4, n (%) | 70 (12.6) | 27 (10.2) | 42 (14.7) | 0.12 |
| western blot for HIV-1, n (%) | <0.001* | |||
| positive | 510 (91.6) | 222 (84.1) | 283 (99.0) | |
| indeterminate | 37 (6.6) | 35 (13.3) | 0 (0.0) | |
| PVL (log10 copies/mL), mean ± SD | 4.60 ± 0.76 | 4.75 ± 0.82 | 4.47 (±0.66) | <0.001* |
| >5 log10 copies/mL, n (%) | 157 (28.2) | 98 (37.1) | 56 (19.6) | <0.001* |
| CD4 cell count (cells/mm3), mean ± SD | 388.4 ± 209.5 | 436.8 ± 220.7 | 345.8 (±187.5) | <0.001* |
| >500 cells/mm3, n (%) | 138 (24.8) | 83 (31.4) | 52 (18.2) | <0.001* |
| HBsAg positivity, n (%) | 53 (9.5) | 20 (7.6) | 32 (11.2) | 0.19 |
| anti-HCV positivity, n (%) | 18 (3.2) | 5 (1.9) | 11 (3.8) | 0.21 |
| available genotypic resistance results, n (%) | 440 (79.0) | 212 (80.3) | 224 (78.3) | 0.52 |
HCV, hepatitis C virus; IDU, injecting drug users; RPR, rapid plasma reagin.
*P < 0.05.
aIncluding seven patients with undetermined duration of HIV-1 infection by the BED assay.
Comparisons of characteristics between VCT subjects with recent and those with long-term HIV infections who were linked to HIV care
| Characteristic . | All patients,aN = 557 . | Recent infections, N = 264 . | Long-term infections, N = 286 . | P . |
|---|---|---|---|---|
| Male, n (%) | 543 (97.5) | 262 (99.2) | 274 (95.8) | 0.01* |
| Age (years), mean ± SD | 29.5 ± 7.2 | 27.5 ± 5.8 | 31.3 (±7.8) | <0.001* |
| ≤25 years, n (%) | 163 (29.3) | 101 (38.3) | 60 (21.0) | <0.001* |
| Risk, n (%) | 0.10 | |||
| MSM | 527 (94.6) | 255 (96.6) | 267 (93.4) | |
| heterosexual | 26 (4.7) | 7 (2.7) | 18 (6.3) | |
| IDU with needle sharing | 4 (0.7) | 2 (0.8) | 1 (0.4) | |
| Reasons for screening, n (%) | ||||
| having confirmed HIV-infected sexual partners | 102 (18.3) | 34 (12.9) | 67 (23.4) | 0.001* |
| having an IDU partner | 2 (0.4) | 2 (0.8) | 0 (0.0) | 0.23 |
| ever having an STI | 132 (23.7) | 60 (22.7) | 71 (24.8) | 0.62 |
| having transactional sex | 30 (5.4) | 21 (8.0) | 9 (3.2) | 0.01* |
| having a one-night stand | 246 (44.2) | 132 (50.0) | 110 (38.5) | 0.01* |
| having anal sex | 438 (78.6) | 231 (87.5) | 202 (70.6) | <0.001* |
| having oral sex | 432 (77.6) | 225 (85.2) | 201 (70.3) | <0.001* |
| illicit drug use | 147 (26.4) | 75 (28.4) | 70 (24.5) | 0.33 |
| Baseline laboratory results | ||||
| RPR ≥4, n (%) | 70 (12.6) | 27 (10.2) | 42 (14.7) | 0.12 |
| western blot for HIV-1, n (%) | <0.001* | |||
| positive | 510 (91.6) | 222 (84.1) | 283 (99.0) | |
| indeterminate | 37 (6.6) | 35 (13.3) | 0 (0.0) | |
| PVL (log10 copies/mL), mean ± SD | 4.60 ± 0.76 | 4.75 ± 0.82 | 4.47 (±0.66) | <0.001* |
| >5 log10 copies/mL, n (%) | 157 (28.2) | 98 (37.1) | 56 (19.6) | <0.001* |
| CD4 cell count (cells/mm3), mean ± SD | 388.4 ± 209.5 | 436.8 ± 220.7 | 345.8 (±187.5) | <0.001* |
| >500 cells/mm3, n (%) | 138 (24.8) | 83 (31.4) | 52 (18.2) | <0.001* |
| HBsAg positivity, n (%) | 53 (9.5) | 20 (7.6) | 32 (11.2) | 0.19 |
| anti-HCV positivity, n (%) | 18 (3.2) | 5 (1.9) | 11 (3.8) | 0.21 |
| available genotypic resistance results, n (%) | 440 (79.0) | 212 (80.3) | 224 (78.3) | 0.52 |
| Characteristic . | All patients,aN = 557 . | Recent infections, N = 264 . | Long-term infections, N = 286 . | P . |
|---|---|---|---|---|
| Male, n (%) | 543 (97.5) | 262 (99.2) | 274 (95.8) | 0.01* |
| Age (years), mean ± SD | 29.5 ± 7.2 | 27.5 ± 5.8 | 31.3 (±7.8) | <0.001* |
| ≤25 years, n (%) | 163 (29.3) | 101 (38.3) | 60 (21.0) | <0.001* |
| Risk, n (%) | 0.10 | |||
| MSM | 527 (94.6) | 255 (96.6) | 267 (93.4) | |
| heterosexual | 26 (4.7) | 7 (2.7) | 18 (6.3) | |
| IDU with needle sharing | 4 (0.7) | 2 (0.8) | 1 (0.4) | |
| Reasons for screening, n (%) | ||||
| having confirmed HIV-infected sexual partners | 102 (18.3) | 34 (12.9) | 67 (23.4) | 0.001* |
| having an IDU partner | 2 (0.4) | 2 (0.8) | 0 (0.0) | 0.23 |
| ever having an STI | 132 (23.7) | 60 (22.7) | 71 (24.8) | 0.62 |
| having transactional sex | 30 (5.4) | 21 (8.0) | 9 (3.2) | 0.01* |
| having a one-night stand | 246 (44.2) | 132 (50.0) | 110 (38.5) | 0.01* |
| having anal sex | 438 (78.6) | 231 (87.5) | 202 (70.6) | <0.001* |
| having oral sex | 432 (77.6) | 225 (85.2) | 201 (70.3) | <0.001* |
| illicit drug use | 147 (26.4) | 75 (28.4) | 70 (24.5) | 0.33 |
| Baseline laboratory results | ||||
| RPR ≥4, n (%) | 70 (12.6) | 27 (10.2) | 42 (14.7) | 0.12 |
| western blot for HIV-1, n (%) | <0.001* | |||
| positive | 510 (91.6) | 222 (84.1) | 283 (99.0) | |
| indeterminate | 37 (6.6) | 35 (13.3) | 0 (0.0) | |
| PVL (log10 copies/mL), mean ± SD | 4.60 ± 0.76 | 4.75 ± 0.82 | 4.47 (±0.66) | <0.001* |
| >5 log10 copies/mL, n (%) | 157 (28.2) | 98 (37.1) | 56 (19.6) | <0.001* |
| CD4 cell count (cells/mm3), mean ± SD | 388.4 ± 209.5 | 436.8 ± 220.7 | 345.8 (±187.5) | <0.001* |
| >500 cells/mm3, n (%) | 138 (24.8) | 83 (31.4) | 52 (18.2) | <0.001* |
| HBsAg positivity, n (%) | 53 (9.5) | 20 (7.6) | 32 (11.2) | 0.19 |
| anti-HCV positivity, n (%) | 18 (3.2) | 5 (1.9) | 11 (3.8) | 0.21 |
| available genotypic resistance results, n (%) | 440 (79.0) | 212 (80.3) | 224 (78.3) | 0.52 |
HCV, hepatitis C virus; IDU, injecting drug users; RPR, rapid plasma reagin.
*P < 0.05.
aIncluding seven patients with undetermined duration of HIV-1 infection by the BED assay.
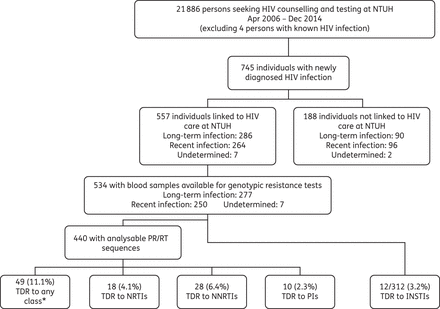
Flow chart of this study. *Any class refers to NRTIs, NNRTIs or PIs.
Approximately 95% of HIV-infected patients were MSM. Overall, 197 (92.9%) of patients with recent infections and 201 (89.7%) of those with chronic infections were infected with HIV-1 subtype B. The second most common subtype was CRF01_AE, which accounted for 3.3% of those with recent infections and 5.8% of those with chronic infections.
TDR to NRTIs, NNRTIs and PIs
Among the 557 HIV-positive patients linked to HIV care at NTUH, 534 (95.9%) had blood specimens available for genotypic resistance tests, and the PR and RT genes were successfully sequenced in 440 of them (82.4%). Patients with analysable RT/PR sequences had significantly higher baseline PVL (4.66 versus 4.46 log10 copies/mL, P = 0.02) compared with those without analysable RT/PR sequences, and other baseline demographic and clinical characteristics were generally similar (Table S1, available as Supplementary data at JAC Online).
Forty-nine HIV-1 strains (11.1%) harboured at least one PR or RT mutation. The overall prevalence of transmitted RAMs to NRTIs, NNRTIs and PIs was 4.1%, 6.4% and 2.3%, respectively (Figure 1). The prevalence of transmitted RAM to any class of antiretroviral agents (NRTIs, NNRTIs or PIs) was 9.0% and 13.4% (P = 0.19) for HIV-1 strains from patients with recent infections and those with chronic infections, respectively. The prevalence of TDR to each class was not statistically significantly different between patients with recent and those with chronic infections, based on the BED assay (Figure 2a). If we used age <25 years as the only criterion of recent infection, the TDR prevalence of patients with recent and those with chronic infections remained comparable (12.6% versus 10.5%, P = 0.65) (data not shown).
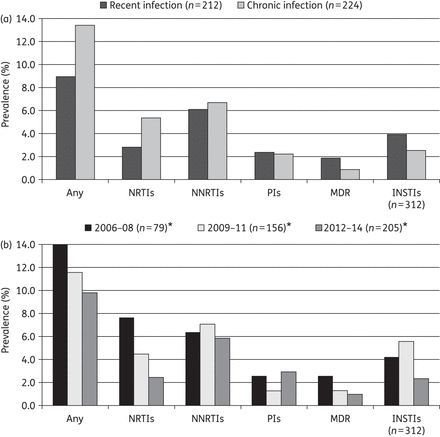
Prevalence of HIV-1 TDR by recent infections (a) and by study periods (b). Any class refers to NRTIs, NNRTIs or PIs. *These numbers did not include genotypic resistance testing of integrase gene.
The overall prevalence of TDR to any antiretroviral agent (NRTIs, NNRTIs or PIs) was 13.9%, 11.5% and 9.8% during 2006–08, 2009–11 and 2012–14, respectively (Figure 2b). The numerically declining trends of TDR to any class of antiretroviral agents did not reach statistical significance. Specific RAMs to NRTIs, NNRTIs and PIs are shown in Figure 3(a). Only K101E/P and K103N/S mutations in RT were observed in >1% of the HIV-1 strains.
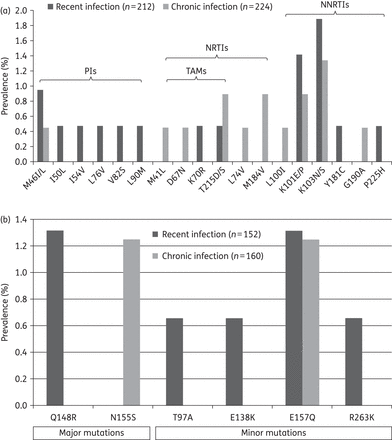
Frequencies of TDR mutations to NRTIs, NNRTIs and PIs (a) and to INSTIs (b). TAM, thymidine analogue mutation.
Comparisons between patients infected with HIV-1 strains harbouring drug-resistant mutations and those without are shown in Table 2. All HIV strains with transmitted RAMs came from MSM, and all but one were subtype B. CD4 lymphocyte counts ≤500 cells/mm3 and HBsAg positivity were independent predictors of acquiring drug-resistant HIV in the multivariate model.
Comparison of characteristics between patients with resistant strains and those without RAMs
| Characteristic . | With RAMs, N = 49 . | Without RAMs, N = 391 . | P . | Multivariate analysis . | |
|---|---|---|---|---|---|
| OR (95% CI) . | P . | ||||
| Year of sampling, n (%) | 0.59 | ||||
| 2006–08 | 11 (22.5) | 68 (17.4) | 1 | ||
| 2009–11 | 18 (36.7) | 138 (35.3) | 1.04 (0.39–2.76) | 0.94 | |
| 2012–14 | 20 (40.8) | 185 (47.3) | 0.88 (0.33–2.33) | 0.80 | |
| Male, n (%) | 49 (100.0) | 380 (97.2) | 0.62 | 0.99 | |
| Age (years), mean ± SD | 29.2 ± 6.3 | 29.5 ± 7.1 | 0.75 | ||
| ≤25 years, n (%) | 16 (32.7) | 111 (28.4) | 0.62 | 1.65 (0.77–3.57) | 0.20 |
| MSM, n (%) | 49 (100.0) | 366 (93.6) | 0.10 | 0.98 | |
| Recent HIV infections, n (%) | 19 (38.8) | 193 (49.4) | 0.17 | 0.64 (0.31–1.31) | 0.22 |
| Reasons for screening, n (%) | |||||
| having confirmed HIV-infected sexual partners | 9 (18.4) | 69 (17.7) | 0.84 | ||
| having an IDU partner | 0 (0.0) | 2 (0.5) | >0.99 | ||
| ever having an STI | 8 (16.3) | 97 (24.8) | 0.22 | ||
| having transactional sex | 1 (2.0) | 23 (5.9) | 0.50 | ||
| having a one-night stand | 21 (42.9) | 176 (45.0) | 0.88 | ||
| having anal sex | 38 (77.6) | 312 (79.8) | 0.71 | ||
| having oral sex | 38 (77.6) | 312 (79.8) | 0.71 | ||
| having unsafe oral sex oncea | 33 (86.8) | 300 (96.2) | 0.05 | 0.22 (0.02–2.62) | 0.23 |
| illicit drug use | 9 (18.4) | 107 (27.4) | 0.23 | ||
| Baseline laboratory results | |||||
| RPR ≥4, n (%) | 8 (16.3) | 51 (13.0) | 0.51 | ||
| western blot for HIV-1, n (%) | 0.23 | ||||
| positive | 46 (93.9) | 356 (91.0) | |||
| indeterminate | 1 (2.0) | 29 (7.4) | |||
| PVL (log10 copies/mL), mean ± SD | 4.52 ± 0.68 | 4.68 ± 0.74 | 0.17 | ||
| >5 log10 copies/mL, n (%) | 12 (24.5) | 120 (30.7) | 0.41 | 0.62 (0.28–1.37) | 0.23 |
| CD4 cell count (cells/mm3), mean ± SD | 336.9 ± 173.5 | 387.6 ± 201.7 | 0.09 | ||
| >500 cells/mm3, n (%) | 5 (10.2) | 97 (24.8) | 0.02* | 0.33 (0.11–0.98) | 0.05* |
| HBsAg positivity, n (%) | 10 (20.4) | 29 (7.4) | 0.01* | 4.82 (1.94–11.9) | <0.001* |
| anti-HCV positivity, n (%) | 0 (0.0) | 14 (3.6) | 0.38 | ||
| subtype B | 48 (98.0) | 354 (90.5) | 0.10 | 3.08 (0.39–24.11) | 0.28 |
| Characteristic . | With RAMs, N = 49 . | Without RAMs, N = 391 . | P . | Multivariate analysis . | |
|---|---|---|---|---|---|
| OR (95% CI) . | P . | ||||
| Year of sampling, n (%) | 0.59 | ||||
| 2006–08 | 11 (22.5) | 68 (17.4) | 1 | ||
| 2009–11 | 18 (36.7) | 138 (35.3) | 1.04 (0.39–2.76) | 0.94 | |
| 2012–14 | 20 (40.8) | 185 (47.3) | 0.88 (0.33–2.33) | 0.80 | |
| Male, n (%) | 49 (100.0) | 380 (97.2) | 0.62 | 0.99 | |
| Age (years), mean ± SD | 29.2 ± 6.3 | 29.5 ± 7.1 | 0.75 | ||
| ≤25 years, n (%) | 16 (32.7) | 111 (28.4) | 0.62 | 1.65 (0.77–3.57) | 0.20 |
| MSM, n (%) | 49 (100.0) | 366 (93.6) | 0.10 | 0.98 | |
| Recent HIV infections, n (%) | 19 (38.8) | 193 (49.4) | 0.17 | 0.64 (0.31–1.31) | 0.22 |
| Reasons for screening, n (%) | |||||
| having confirmed HIV-infected sexual partners | 9 (18.4) | 69 (17.7) | 0.84 | ||
| having an IDU partner | 0 (0.0) | 2 (0.5) | >0.99 | ||
| ever having an STI | 8 (16.3) | 97 (24.8) | 0.22 | ||
| having transactional sex | 1 (2.0) | 23 (5.9) | 0.50 | ||
| having a one-night stand | 21 (42.9) | 176 (45.0) | 0.88 | ||
| having anal sex | 38 (77.6) | 312 (79.8) | 0.71 | ||
| having oral sex | 38 (77.6) | 312 (79.8) | 0.71 | ||
| having unsafe oral sex oncea | 33 (86.8) | 300 (96.2) | 0.05 | 0.22 (0.02–2.62) | 0.23 |
| illicit drug use | 9 (18.4) | 107 (27.4) | 0.23 | ||
| Baseline laboratory results | |||||
| RPR ≥4, n (%) | 8 (16.3) | 51 (13.0) | 0.51 | ||
| western blot for HIV-1, n (%) | 0.23 | ||||
| positive | 46 (93.9) | 356 (91.0) | |||
| indeterminate | 1 (2.0) | 29 (7.4) | |||
| PVL (log10 copies/mL), mean ± SD | 4.52 ± 0.68 | 4.68 ± 0.74 | 0.17 | ||
| >5 log10 copies/mL, n (%) | 12 (24.5) | 120 (30.7) | 0.41 | 0.62 (0.28–1.37) | 0.23 |
| CD4 cell count (cells/mm3), mean ± SD | 336.9 ± 173.5 | 387.6 ± 201.7 | 0.09 | ||
| >500 cells/mm3, n (%) | 5 (10.2) | 97 (24.8) | 0.02* | 0.33 (0.11–0.98) | 0.05* |
| HBsAg positivity, n (%) | 10 (20.4) | 29 (7.4) | 0.01* | 4.82 (1.94–11.9) | <0.001* |
| anti-HCV positivity, n (%) | 0 (0.0) | 14 (3.6) | 0.38 | ||
| subtype B | 48 (98.0) | 354 (90.5) | 0.10 | 3.08 (0.39–24.11) | 0.28 |
HCV, hepatitis C virus; IDU, injecting drug user; RPR, rapid plasma reagin.
*P < 0.05.
aThe denominators were patients having oral sex for each category.
Comparison of characteristics between patients with resistant strains and those without RAMs
| Characteristic . | With RAMs, N = 49 . | Without RAMs, N = 391 . | P . | Multivariate analysis . | |
|---|---|---|---|---|---|
| OR (95% CI) . | P . | ||||
| Year of sampling, n (%) | 0.59 | ||||
| 2006–08 | 11 (22.5) | 68 (17.4) | 1 | ||
| 2009–11 | 18 (36.7) | 138 (35.3) | 1.04 (0.39–2.76) | 0.94 | |
| 2012–14 | 20 (40.8) | 185 (47.3) | 0.88 (0.33–2.33) | 0.80 | |
| Male, n (%) | 49 (100.0) | 380 (97.2) | 0.62 | 0.99 | |
| Age (years), mean ± SD | 29.2 ± 6.3 | 29.5 ± 7.1 | 0.75 | ||
| ≤25 years, n (%) | 16 (32.7) | 111 (28.4) | 0.62 | 1.65 (0.77–3.57) | 0.20 |
| MSM, n (%) | 49 (100.0) | 366 (93.6) | 0.10 | 0.98 | |
| Recent HIV infections, n (%) | 19 (38.8) | 193 (49.4) | 0.17 | 0.64 (0.31–1.31) | 0.22 |
| Reasons for screening, n (%) | |||||
| having confirmed HIV-infected sexual partners | 9 (18.4) | 69 (17.7) | 0.84 | ||
| having an IDU partner | 0 (0.0) | 2 (0.5) | >0.99 | ||
| ever having an STI | 8 (16.3) | 97 (24.8) | 0.22 | ||
| having transactional sex | 1 (2.0) | 23 (5.9) | 0.50 | ||
| having a one-night stand | 21 (42.9) | 176 (45.0) | 0.88 | ||
| having anal sex | 38 (77.6) | 312 (79.8) | 0.71 | ||
| having oral sex | 38 (77.6) | 312 (79.8) | 0.71 | ||
| having unsafe oral sex oncea | 33 (86.8) | 300 (96.2) | 0.05 | 0.22 (0.02–2.62) | 0.23 |
| illicit drug use | 9 (18.4) | 107 (27.4) | 0.23 | ||
| Baseline laboratory results | |||||
| RPR ≥4, n (%) | 8 (16.3) | 51 (13.0) | 0.51 | ||
| western blot for HIV-1, n (%) | 0.23 | ||||
| positive | 46 (93.9) | 356 (91.0) | |||
| indeterminate | 1 (2.0) | 29 (7.4) | |||
| PVL (log10 copies/mL), mean ± SD | 4.52 ± 0.68 | 4.68 ± 0.74 | 0.17 | ||
| >5 log10 copies/mL, n (%) | 12 (24.5) | 120 (30.7) | 0.41 | 0.62 (0.28–1.37) | 0.23 |
| CD4 cell count (cells/mm3), mean ± SD | 336.9 ± 173.5 | 387.6 ± 201.7 | 0.09 | ||
| >500 cells/mm3, n (%) | 5 (10.2) | 97 (24.8) | 0.02* | 0.33 (0.11–0.98) | 0.05* |
| HBsAg positivity, n (%) | 10 (20.4) | 29 (7.4) | 0.01* | 4.82 (1.94–11.9) | <0.001* |
| anti-HCV positivity, n (%) | 0 (0.0) | 14 (3.6) | 0.38 | ||
| subtype B | 48 (98.0) | 354 (90.5) | 0.10 | 3.08 (0.39–24.11) | 0.28 |
| Characteristic . | With RAMs, N = 49 . | Without RAMs, N = 391 . | P . | Multivariate analysis . | |
|---|---|---|---|---|---|
| OR (95% CI) . | P . | ||||
| Year of sampling, n (%) | 0.59 | ||||
| 2006–08 | 11 (22.5) | 68 (17.4) | 1 | ||
| 2009–11 | 18 (36.7) | 138 (35.3) | 1.04 (0.39–2.76) | 0.94 | |
| 2012–14 | 20 (40.8) | 185 (47.3) | 0.88 (0.33–2.33) | 0.80 | |
| Male, n (%) | 49 (100.0) | 380 (97.2) | 0.62 | 0.99 | |
| Age (years), mean ± SD | 29.2 ± 6.3 | 29.5 ± 7.1 | 0.75 | ||
| ≤25 years, n (%) | 16 (32.7) | 111 (28.4) | 0.62 | 1.65 (0.77–3.57) | 0.20 |
| MSM, n (%) | 49 (100.0) | 366 (93.6) | 0.10 | 0.98 | |
| Recent HIV infections, n (%) | 19 (38.8) | 193 (49.4) | 0.17 | 0.64 (0.31–1.31) | 0.22 |
| Reasons for screening, n (%) | |||||
| having confirmed HIV-infected sexual partners | 9 (18.4) | 69 (17.7) | 0.84 | ||
| having an IDU partner | 0 (0.0) | 2 (0.5) | >0.99 | ||
| ever having an STI | 8 (16.3) | 97 (24.8) | 0.22 | ||
| having transactional sex | 1 (2.0) | 23 (5.9) | 0.50 | ||
| having a one-night stand | 21 (42.9) | 176 (45.0) | 0.88 | ||
| having anal sex | 38 (77.6) | 312 (79.8) | 0.71 | ||
| having oral sex | 38 (77.6) | 312 (79.8) | 0.71 | ||
| having unsafe oral sex oncea | 33 (86.8) | 300 (96.2) | 0.05 | 0.22 (0.02–2.62) | 0.23 |
| illicit drug use | 9 (18.4) | 107 (27.4) | 0.23 | ||
| Baseline laboratory results | |||||
| RPR ≥4, n (%) | 8 (16.3) | 51 (13.0) | 0.51 | ||
| western blot for HIV-1, n (%) | 0.23 | ||||
| positive | 46 (93.9) | 356 (91.0) | |||
| indeterminate | 1 (2.0) | 29 (7.4) | |||
| PVL (log10 copies/mL), mean ± SD | 4.52 ± 0.68 | 4.68 ± 0.74 | 0.17 | ||
| >5 log10 copies/mL, n (%) | 12 (24.5) | 120 (30.7) | 0.41 | 0.62 (0.28–1.37) | 0.23 |
| CD4 cell count (cells/mm3), mean ± SD | 336.9 ± 173.5 | 387.6 ± 201.7 | 0.09 | ||
| >500 cells/mm3, n (%) | 5 (10.2) | 97 (24.8) | 0.02* | 0.33 (0.11–0.98) | 0.05* |
| HBsAg positivity, n (%) | 10 (20.4) | 29 (7.4) | 0.01* | 4.82 (1.94–11.9) | <0.001* |
| anti-HCV positivity, n (%) | 0 (0.0) | 14 (3.6) | 0.38 | ||
| subtype B | 48 (98.0) | 354 (90.5) | 0.10 | 3.08 (0.39–24.11) | 0.28 |
HCV, hepatitis C virus; IDU, injecting drug user; RPR, rapid plasma reagin.
*P < 0.05.
aThe denominators were patients having oral sex for each category.
TDR-associated mutations to INSTIs
Among 312 HIV-positive patients with genotypic resistance testing of the HIV-1 integrase gene, 216 (69.2%) were enrolled during the period 2012–14. Overall, 10 (3.2%) HIV-1 strains harboured integrase RAMs (Figure 1). The prevalence was 4.3% (1/23) during 2006–08, before the introduction of INSTIs in Taiwan (Figure 2b). The frequencies of individual RAMs are shown in Figure 3(b). If we limited RAMs to major INSTI mutations (T66I, E92Q, G140S, Y143C/H/R, S147G, Q148H/K/R, N155H),14 only one strain harbouring the Q148R mutation was detected, however.
Discussion
Our study, conducted among VCT subjects testing positive for HIV-1 in Taiwan during 2006–14, showed that 11.1% of the patients were infected with HIV-1 strains harbouring at least one PR or RT RAM. Consequently, the prevalence of TDR of HIV-1 in Taiwan was estimated as moderate (5%–15%) according to the WHO categorization method. The prevalence of TDR seemed to be stable throughout the 9 years of the study. We also identified transmission of HIV strains with RAMs to INSTIs for the first time in Taiwan, at a prevalence of 3.2%. In only one patient, a major mutation (Q148R) conferring high-level resistance to two INSTIs in clinical use (raltegravir and elvitegravir) was found.
Our prior surveillance in Taiwan during 2000–10 showed that the overall prevalence of RAMs was 8.0%, and many patients were probably chronically infected due to their low CD4 lymphocyte counts.5,20 Since transmitted RAMs are more likely to be detected in persons with recent infection compared with those with established infection,28 it is not surprising that the prevalence in this study seems to be slightly higher. Our HIV-infected patients in the current study were predominately MSM with subtype B, and nearly half of them had recently acquired HIV (within the past 153 days) according to the BED assay. Therefore, this study may be more accurate in estimating the current rate of transmission of RAMs when access to VCT services have been further improved to detect HIV infection early. However, no statistically significant difference was found between chronic and recent infections in the prevalence of one or more drug resistance mutations. In our study, even patients with chronic infections beyond the 153 day window had relatively high CD4 lymphocyte counts (mean 346 cells/mm3) at baseline, which suggests that the interval between infection and testing was not too extended. Our findings were consistent with a similar surveillance among VCT subjects in southern Taiwan, which reported a 10.6% prevalence of TDR.29 This prevalence is also in line with those reported in Australia, Europe and North America, in the range of 8%–17%.8,9,12,23
To ameliorate the surging costs of antiretroviral drugs, Taiwan CDC, which started to provide free-of-charge ART in the 1990s, has implemented regulations for the prescription of more expensive INSTI- or PI-based regimens. These measures have raised concerns about the emergence of drug resistance, particularly in the absence of routine baseline genotypic resistance testing before cART initiation. In this surveillance, we did not detect any rising trends of TDR to any of the three classes of antiretroviral agents (NRTIs, NNRTIs and PIs). However, mutations conferring resistance to NNRTIs were most common (6.4%), accounting for more than half of the RAMs found. This finding is worrisome, as NNRTI-based regimens are the preferred first-line cART in Taiwan. Vigilant national monitoring in the following years is needed to determine the long-term impacts of the ART policy on the transmission of RAMs and its clinical implications.
In this study, CD4 lymphocyte counts ≤500 cells/mm3 and positive HBsAg were independent predictors of acquisition of HIV with RAMs. To our knowledge, hepatitis B virus (HBV) infection has not been reported to be associated with TDR acquisition. Many of our patients were born after 1986, when universal neonatal HBV vaccination and catch-up vaccination were implemented in Taiwan.30 In the era of nationwide HBV vaccination for newborns, HBsAg positivity has also been identified to be significantly associated with syphilis and hepatitis C virus antibody (anti-hepatitis C virus) positivity, which implies that HBV might still be transmitted through high-risk sexual behaviours when immunity against HBV wanes and booster vaccination may not be administered.30 An outbreak of HBV infection with transmission of drug resistance via sexual contacts among HIV-infected patients has also been reported.31 One possibility of our finding is that drug-resistant HIV might be co-transmitted with HBV through high-risk behaviours. However, this point may not be supported due to similarly high-risk behaviours between patients with and without TDR. Another explanation is HBV/HIV-coinfected patients might be exposed to lamivudine as HBV treatment before HIV diagnosis. Indeed, in our study, two HBsAg-positive patients had received lamivudine monotherapy for HBV before, with their HIV-1 strains harbouring the M184V mutation (data not shown). However, among 18 patients acquiring NRTI-resistant strains, the frequencies of the M184V mutation in HBsAg-positive and HBsAg-negative patients were not significantly different [2/5 (40%) versus 3/13 (23.1%), P = 0.53] (data not shown). Further studies are needed to elucidate the correlation of HBV coinfection and TDR.
INSTI resistance is potentially influenced by exposure to antiretroviral drugs and HIV-1 subtypes. In clinical trials of ART-naive patients, rates of virological failure with INSTI resistance were low, ranging from 0% to 3%.17 Raltegravir has been licensed in Taiwan since 2009 and remains the only INSTI in the Taiwanese market. Despite its established efficacy and safety, raltegravir is reserved for patients for whom NNRTI-based regimens are not indicated because of tolerance or resistance issues according to the Taiwanese regulations on cART. In this setting, a prevalence of 3.2% RAMs in integrase, though low, is a point of concern. Among the INSTI-related major mutations we detected, Q148R can cause 30- to 100-fold reduction of drug susceptibility,32 while N155S is a rare non-polymorphic mutation which was selected in vitro and can reduce raltegravir efficacy less efficiently than N155H.33 Among the four minor mutations detected in this study, T97A and E157Q are polymorphic accessory mutations that can be selected in patients receiving raltegravir,34 while E138K and R263K are non-polymorphic INSTI-resistance mutations selected in patients receiving INSTIs.35 At present, none of these minor mutations occurs in combination with INSTI-related major mutations in our study subjects. Further investigations are needed to determine to what extent these RAMs impact the clinical effectiveness of INSTIs. Moreover, additional research is required to determine the source of these mutations. Continuous monitoring is warranted to prevent the spread of strains resistant to INSTIs in the community.
Some questions about INSTI resistance remain unanswered, however. Major INSTI resistance mutations listed in the 2014 edition of the IAS-USA panel and the Stanford University HIV Drug Resistance database are different.36 Some minor integrase mutations do not reduce susceptibility to INSTIs on their own, but require the presence of other mutations, and minor RAMs have been observed in up to 22.5% of INSTI-naive patients.27
The findings of this study should be interpreted with the necessary caution. First, the study was based on data from persons seeking VCT at only one hospital, although this site provides the largest VCT programme and HIV care in Taiwan. Second, the number of cases included in this study was small. Third, our participants were predominately MSM infected with subtype B. While MSM account for >85% of HIV infections reported to Taiwan CDC annually after 2008, when the HIV outbreak among injecting drug users was brought under control, this sampling bias precludes generalization of our findings to injecting drug users and heterosexuals. Furthermore, it has been recognized that TDR is most prevalent among MSM worldwide.12 Thus, TDR epidemics in Taiwan in at-risk populations other than MSM or in subtypes other than B remain to be investigated.
In conclusion, the prevalence of TDR-associated mutations among persons seeking VCT services in Taiwan remains stable and is comparable to the range found in high-income countries. Consequently, baseline genotypic resistance testing should be considered for routine use in newly diagnosed HIV-infected individuals according to the treatment guidelines.13
Funding
This study was supported by grants from the Taiwan Centers for Disease Control (grant numbers DOH102-DC-1401 and MOHW103-CDC-C-114-000405 to C.-C. H.). The funding source played no role in study design and conduct, data collection, analysis or interpretation, writing of the manuscript or the decision to submit the manuscript for publication.
Transparency declarations
C.-C. H. has received research support from Janssen, has received speaker honoraria from Bristol-Myers Squibb, ViiV, Abbvie and Gilead, and has served on advisory boards for Gilead and Abbvie. All other authors: none to declare.
Author contributions
Conceived and designed the experiments: C.-C. L., W.-C. L., C.-T. F., J.-Y. Y., C.-C. H. and S.-Y. C. Performed the experiments: W.-C. L., J.-Y. Y., L.-H. C., P.-Y. W., Y.-Z. L., S.-F. C., Y.-C. S. and S.-Y. C. Analysed the data: C.-C. L., W.-C. L., C.-T. F., J.-Y. Y., C.-C. H. and S.-Y. C. Wrote the paper: C.-C. L., W.-C. L., C.-C. H. and S.-Y. C.
Acknowledgements
Preliminary analyses of these data were presented at the Twelfth International Congress on Drug Therapy in HIV Infection, Glasgow, 2014 (Abstract P226).
We would like to thank the patients for participation in this study; the Taiwan CDC for research grant support; and Professor Charles Boucher (Department of Virology, Erasmus Medical Center, University Medical Center, Rotterdam, the Netherlands) for review of this manuscript prior to submission.
References
Author notes
C.-C. L. and W.-C. L. contributed equally to the work.
S.-Y. C. and C.-C. H. contributed equally to the work.



