-
PDF
- Split View
-
Views
-
Cite
Cite
Alexis Tabah, Jan De Waele, Jeffrey Lipman, Jean Ralph Zahar, Menino Osbert Cotta, Greg Barton, Jean-Francois Timsit, Jason A. Roberts, on behalf of the Working Group for Antimicrobial Use in the ICU within the Infection Section of the European Society of Intensive Care Medicine (ESICM), The ADMIN-ICU survey: a survey on antimicrobial dosing and monitoring in ICUs, Journal of Antimicrobial Chemotherapy, Volume 70, Issue 9, September 2015, Pages 2671–2677, https://doi.org/10.1093/jac/dkv165
Close - Share Icon Share
Abstract
There is little evidence and few guidelines to inform the most appropriate dosing and monitoring for antimicrobials in the ICU. We aimed to survey current practices around the world.
An online structured questionnaire was developed and sent by e-mail to obtain information on local antimicrobial prescribing practices for glycopeptides, piperacillin/tazobactam, carbapenems, aminoglycosides and colistin.
A total of 402 professionals from 328 hospitals in 53 countries responded, of whom 78% were specialists in intensive care medicine (41% intensive care, 30% anaesthesiology, 14% internal medicine) and 12% were pharmacists. Vancomycin was used as a continuous infusion in 31% of units at a median (IQR) daily dose of 25 (25–30) mg/kg. Piperacillin/tazobactam was used as an extended infusion by 22% and as a continuous infusion by 7%. An extended infusion of carbapenem (meropenem or imipenem) was used by 27% and a continuous infusion by 5%. Colistin was used at a daily dose of 7.5 (3.9–9) million IU (MIU)/day, predominantly as a short infusion. The most commonly used aminoglycosides were gentamicin (55%) followed by amikacin (40%), with administration as a single daily dose reported in 94% of the cases. Gentamicin was used at a daily dose of 5 (5–6) mg/day and amikacin at a daily dose of 15 (15–20) mg/day. Therapeutic drug monitoring of vancomycin, piperacillin/tazobactam and meropenem was used by 74%, 1% and 2% of the respondents, respectively. Peak aminoglycoside concentrations were sampled daily by 28% and trough concentrations in all patients by 61% of the respondents.
We found wide variability in reported practices for antibiotic dosing and monitoring. Research is required to develop evidence-based guidelines to standardize practices.
Introduction
The poor patient outcomes of infections in patients in the ICU remain of significant concern. High morbidity and mortality rates as well as very high rates of antibiotic prescription1 suggest that our current approaches to antimicrobial use are not achieving the desired results for many of our patients.2 Further to this, the ICU is a leading area for the development of antimicrobial resistance, suggesting that current approaches to antimicrobial prescription are not sustainable.3
Antimicrobial selection for therapy in a critically ill patient may vary based on whether it is empirical or directed, the source of infection and/or likely pathogens and susceptibility. Regional and institutional factors and available resources may also influence the choice.
The available literature on antimicrobial pharmacokinetics and critically ill patients is vast, albeit incomplete. Indeed, these patients may develop a spectrum of pathophysiological changes that result in an equally wide range of antimicrobial concentrations.2,4 Critically ill patients may develop enhanced clearances of renally cleared drugs through a phenomenon known as augmented renal clearance. In these cases much higher than standard dosing requirements are needed to achieve the pharmacokinetic exposures seen in non-critically ill patients.5,6 Conversely, other patients may develop end-organ dysfunction, leading to greatly reduced drug clearances and therefore lower dosing requirements to avoid overexposure and decrease the risk of toxicity and adverse effects.7 Finally, when the severity of illness progresses to organ failure, use of extracorporeal therapies such as renal replacement therapy (RRT) and/or extracorporeal membrane oxygenation (ECMO) may be needed, and these treatments may also significantly affect antimicrobial pharmacokinetics.8
With this background, we aimed to survey a large sample of clinicians working in ICUs to describe current practices in dosing, administration and monitoring for commonly prescribed antimicrobials, including glycopeptides, piperacillin/tazobactam, carbapenems, aminoglycosides and colistin.
Methods
A panel of experts from the Working Group for Antimicrobial Use in the ICU within the Infection Section of the European Society of Intensive Care Medicine (ESICM) developed a case-based questionnaire to obtain information on different practices used for critically ill patients on dosing, administration and monitoring of five major antimicrobials and antimicrobial classes. The full text of the survey is available as Supplementary data at JAC Online.
The survey was designed to describe the professional characteristics of the respondent, including the role, experience, primary specialty and the possession of any qualifications in infectious diseases. It also included questions relating to the characteristics of the clinical centre where the respondent worked. Five commonly used antimicrobials and antimicrobial classes for which there is contrasting literature on the optimal dosing and/or administration strategies were selected for the survey: glycopeptides, piperacillin/tazobactam, carbapenems (meropenem, imipenem and doripenem; ertapenem was not included because it is rarely used as empirical therapy in this setting due to a lack of Pseudomonas aeruginosa activity), aminoglycosides and colistin. The patient that was the subject of the case within the survey was a 35-year-old critically ill patient, weighing 80 kg, height 1.78 m, with normal renal function who was being treated for an infection.
For glycopeptides, piperacillin/tazobactam, carbapenems and colistin, we surveyed the use of a loading dose, total daily dosing, infusion duration and the use of therapeutic drug monitoring (TDM). For aminoglycosides, we surveyed the agent selected, total dose and number of daily infusions, the use of peak and trough concentration TDM and the dosing response that would be given to resulting concentrations. The survey was made available using SurveyMonkey Internet platform software by the ESICM research team. It required 10–15 min to complete online.
For vancomycin and β-lactam antimicrobials, we defined three modes of administration: intermittent infusions (duration <2 h); extended infusions (duration 2–4 h); and continuous infusions (continuously over 24 h).
The survey was endorsed by the European Critical Care Research Network (ECCRN). The project was exempted from full ethics review as a low- and negligible-risk research project by the Royal Brisbane & Women's Hospital Human Research Ethics Committee (EC00172).
From June to September 2013 an open invitation to answer the survey was sent to the members of the ESICM and the Australian and New Zealand Intensive Care Society (ANZICS) research networks. Other clinicians known to the investigators, but not part of these networks, were also invited to participate and were encouraged to invite their colleagues and local networks to also undertake the survey. A reminder was sent after 1 month.
The data were exported from the SurveyMonkey into a Microsoft Excel file. The file was anonymized and any personal data were removed. All records were reviewed by hand by two investigators (A. T. and J. A. R.). As the survey software recorded any attempt at starting the questionnaire as an entry, if no or very few answers were entered we removed these responses.
When a range rather than a single value was entered, we calculated the mean of the range and used this in the analysis. The exceptions were for trough concentrations (the highest value mentioned was used) and peak concentrations (the lowest value was used).
For colistin we used a conversion factor of 1 000 000 IU (MIU) = 80 mg to transform all doses into MIU.9
Statistical analysis was performed using IBM® SPSS® Statistics 20.0. Data are expressed as median values with IQR for continuous variables and as numbers and/or percentages for categorical variables.
Results
In total, 402 respondents from 328 hospitals in 252 cities in 53 different countries completed the questionnaire. Detail on the origin of the respondents is shown in Table S1 (available as Supplementary data at JAC Online). The characteristics of the respondents are reported in Table 1. Most were specialists in intensive care medicine without any formal qualification in infectious diseases.
| . | . | n (%) . |
|---|---|---|
| Position | doctor in training | 28 (7) |
| other | 12 (3) | |
| pharmacist | 48 (11.9) | |
| specialist in intensive care medicine | 314 (78.1) | |
| Experience in ICU | <5years | 74 (18.4) |
| 5–15 years | 186 (46.3) | |
| >15 years | 142 (35.3) | |
| Primary specialty | anaesthesiology | 121 (30.1) |
| intensive care | 165 (41) | |
| internal medicine | 55 (13.7) | |
| other | 51 (12.7) | |
| infectious diseases | 10 (2.5) | |
| Qualifications in infectious diseases | specialist in infectious diseases | 13 (3.2) |
| university degree in that field | 45 (11.2) | |
| none | 310 (77.1) | |
| other | 34 (8.5) | |
| Type of hospital | general | 132 (32.8) |
| university | 150 (37.3) | |
| university affiliated | 120 (29.9) | |
| Type of ICU | cardiac | 8 (2) |
| medical | 37 (9.2) | |
| medical–surgical | 318 (79.1) | |
| other | 17 (4.2) | |
| surgical | 22 (5.5) | |
| Open or closed ICU | closed | 269 (66.9) |
| open | 133 (33.1) | |
| Availability of a pharmacist | available in the ICU at least once a week | 39 (9.7) |
| available in the ICU every day | 169 (42) | |
| none | 111 (27.6) | |
| phone consultation | 83 (20.6) | |
| Written guidelines for antibiotic dosing? | no | 166 (41.4) |
| yes and scrupulously followed | 88 (21.9) | |
| yes, but not strictly followed | 147 (36.7) |
| . | . | n (%) . |
|---|---|---|
| Position | doctor in training | 28 (7) |
| other | 12 (3) | |
| pharmacist | 48 (11.9) | |
| specialist in intensive care medicine | 314 (78.1) | |
| Experience in ICU | <5years | 74 (18.4) |
| 5–15 years | 186 (46.3) | |
| >15 years | 142 (35.3) | |
| Primary specialty | anaesthesiology | 121 (30.1) |
| intensive care | 165 (41) | |
| internal medicine | 55 (13.7) | |
| other | 51 (12.7) | |
| infectious diseases | 10 (2.5) | |
| Qualifications in infectious diseases | specialist in infectious diseases | 13 (3.2) |
| university degree in that field | 45 (11.2) | |
| none | 310 (77.1) | |
| other | 34 (8.5) | |
| Type of hospital | general | 132 (32.8) |
| university | 150 (37.3) | |
| university affiliated | 120 (29.9) | |
| Type of ICU | cardiac | 8 (2) |
| medical | 37 (9.2) | |
| medical–surgical | 318 (79.1) | |
| other | 17 (4.2) | |
| surgical | 22 (5.5) | |
| Open or closed ICU | closed | 269 (66.9) |
| open | 133 (33.1) | |
| Availability of a pharmacist | available in the ICU at least once a week | 39 (9.7) |
| available in the ICU every day | 169 (42) | |
| none | 111 (27.6) | |
| phone consultation | 83 (20.6) | |
| Written guidelines for antibiotic dosing? | no | 166 (41.4) |
| yes and scrupulously followed | 88 (21.9) | |
| yes, but not strictly followed | 147 (36.7) |
| . | . | n (%) . |
|---|---|---|
| Position | doctor in training | 28 (7) |
| other | 12 (3) | |
| pharmacist | 48 (11.9) | |
| specialist in intensive care medicine | 314 (78.1) | |
| Experience in ICU | <5years | 74 (18.4) |
| 5–15 years | 186 (46.3) | |
| >15 years | 142 (35.3) | |
| Primary specialty | anaesthesiology | 121 (30.1) |
| intensive care | 165 (41) | |
| internal medicine | 55 (13.7) | |
| other | 51 (12.7) | |
| infectious diseases | 10 (2.5) | |
| Qualifications in infectious diseases | specialist in infectious diseases | 13 (3.2) |
| university degree in that field | 45 (11.2) | |
| none | 310 (77.1) | |
| other | 34 (8.5) | |
| Type of hospital | general | 132 (32.8) |
| university | 150 (37.3) | |
| university affiliated | 120 (29.9) | |
| Type of ICU | cardiac | 8 (2) |
| medical | 37 (9.2) | |
| medical–surgical | 318 (79.1) | |
| other | 17 (4.2) | |
| surgical | 22 (5.5) | |
| Open or closed ICU | closed | 269 (66.9) |
| open | 133 (33.1) | |
| Availability of a pharmacist | available in the ICU at least once a week | 39 (9.7) |
| available in the ICU every day | 169 (42) | |
| none | 111 (27.6) | |
| phone consultation | 83 (20.6) | |
| Written guidelines for antibiotic dosing? | no | 166 (41.4) |
| yes and scrupulously followed | 88 (21.9) | |
| yes, but not strictly followed | 147 (36.7) |
| . | . | n (%) . |
|---|---|---|
| Position | doctor in training | 28 (7) |
| other | 12 (3) | |
| pharmacist | 48 (11.9) | |
| specialist in intensive care medicine | 314 (78.1) | |
| Experience in ICU | <5years | 74 (18.4) |
| 5–15 years | 186 (46.3) | |
| >15 years | 142 (35.3) | |
| Primary specialty | anaesthesiology | 121 (30.1) |
| intensive care | 165 (41) | |
| internal medicine | 55 (13.7) | |
| other | 51 (12.7) | |
| infectious diseases | 10 (2.5) | |
| Qualifications in infectious diseases | specialist in infectious diseases | 13 (3.2) |
| university degree in that field | 45 (11.2) | |
| none | 310 (77.1) | |
| other | 34 (8.5) | |
| Type of hospital | general | 132 (32.8) |
| university | 150 (37.3) | |
| university affiliated | 120 (29.9) | |
| Type of ICU | cardiac | 8 (2) |
| medical | 37 (9.2) | |
| medical–surgical | 318 (79.1) | |
| other | 17 (4.2) | |
| surgical | 22 (5.5) | |
| Open or closed ICU | closed | 269 (66.9) |
| open | 133 (33.1) | |
| Availability of a pharmacist | available in the ICU at least once a week | 39 (9.7) |
| available in the ICU every day | 169 (42) | |
| none | 111 (27.6) | |
| phone consultation | 83 (20.6) | |
| Written guidelines for antibiotic dosing? | no | 166 (41.4) |
| yes and scrupulously followed | 88 (21.9) | |
| yes, but not strictly followed | 147 (36.7) |
Glycopeptides
Vancomycin was by far the preferred glycopeptide (88.8%). As shown in Figure 1, it was most often administered as an intermittent infusion (68.7%) as opposed to a continuous infusion. About one-third did not use a loading dose. The loading doses used, as well as daily doses of vancomycin, are presented in Table 2.
Dosing (loading and maintenance) for preferred antibiotics according to type of infusion used (intermittent, extended or continuous)
| . | Used by . | Loading dosea (when administered) . | Missingb . | Maintenance dose per 24 ha . | Missingb . |
|---|---|---|---|---|---|
| Vancomycin II | 276 | 23 (19–25) | 2 | 25 (15–25) | 9 |
| Vancomycin CI | 126 | 15 (13–20) | 6 | 25 (25–30) | 11 |
| Piperacillin/tazobactam II | 291 | 4.5 (4.5–4.5) | 0 | 18 (14.6–18) | 3 |
| Piperacillin/tazobactam EI | 83 | 4.5 (4.5–4.5) | 0 | 18 (13.5–18) | 2 |
| Piperacillin/tazobactam CI | 28 | 4.5 (4.5–4.5) | 0 | 18 (15.8–18) | 0 |
| Meropenem II | 268 | 2 (1–2) | 2 | 3 (3–3) | 7 |
| Meropenem EI | 111 | 2 (1–2) | 0 | 3 (3–6) | 1 |
| Meropenem CI | 17 | 1 (1–2) | 0 | 4 (3–5) | 0 |
| . | Used by . | Loading dosea (when administered) . | Missingb . | Maintenance dose per 24 ha . | Missingb . |
|---|---|---|---|---|---|
| Vancomycin II | 276 | 23 (19–25) | 2 | 25 (15–25) | 9 |
| Vancomycin CI | 126 | 15 (13–20) | 6 | 25 (25–30) | 11 |
| Piperacillin/tazobactam II | 291 | 4.5 (4.5–4.5) | 0 | 18 (14.6–18) | 3 |
| Piperacillin/tazobactam EI | 83 | 4.5 (4.5–4.5) | 0 | 18 (13.5–18) | 2 |
| Piperacillin/tazobactam CI | 28 | 4.5 (4.5–4.5) | 0 | 18 (15.8–18) | 0 |
| Meropenem II | 268 | 2 (1–2) | 2 | 3 (3–3) | 7 |
| Meropenem EI | 111 | 2 (1–2) | 0 | 3 (3–6) | 1 |
| Meropenem CI | 17 | 1 (1–2) | 0 | 4 (3–5) | 0 |
II, intermittent infusion (duration <2 h); EI, extended infusion (duration 2–4 h); CI, continuous infusion (24 h infusion).
Percentages were calculated on the number of respondents for each antibiotic. Dosing values are shown as median (IQR).
aVancomycin doses are in mg/kg; piperacillin/tazobactam and meropenem doses are in g.
bNumber of missing values for each dosing category.
Dosing (loading and maintenance) for preferred antibiotics according to type of infusion used (intermittent, extended or continuous)
| . | Used by . | Loading dosea (when administered) . | Missingb . | Maintenance dose per 24 ha . | Missingb . |
|---|---|---|---|---|---|
| Vancomycin II | 276 | 23 (19–25) | 2 | 25 (15–25) | 9 |
| Vancomycin CI | 126 | 15 (13–20) | 6 | 25 (25–30) | 11 |
| Piperacillin/tazobactam II | 291 | 4.5 (4.5–4.5) | 0 | 18 (14.6–18) | 3 |
| Piperacillin/tazobactam EI | 83 | 4.5 (4.5–4.5) | 0 | 18 (13.5–18) | 2 |
| Piperacillin/tazobactam CI | 28 | 4.5 (4.5–4.5) | 0 | 18 (15.8–18) | 0 |
| Meropenem II | 268 | 2 (1–2) | 2 | 3 (3–3) | 7 |
| Meropenem EI | 111 | 2 (1–2) | 0 | 3 (3–6) | 1 |
| Meropenem CI | 17 | 1 (1–2) | 0 | 4 (3–5) | 0 |
| . | Used by . | Loading dosea (when administered) . | Missingb . | Maintenance dose per 24 ha . | Missingb . |
|---|---|---|---|---|---|
| Vancomycin II | 276 | 23 (19–25) | 2 | 25 (15–25) | 9 |
| Vancomycin CI | 126 | 15 (13–20) | 6 | 25 (25–30) | 11 |
| Piperacillin/tazobactam II | 291 | 4.5 (4.5–4.5) | 0 | 18 (14.6–18) | 3 |
| Piperacillin/tazobactam EI | 83 | 4.5 (4.5–4.5) | 0 | 18 (13.5–18) | 2 |
| Piperacillin/tazobactam CI | 28 | 4.5 (4.5–4.5) | 0 | 18 (15.8–18) | 0 |
| Meropenem II | 268 | 2 (1–2) | 2 | 3 (3–3) | 7 |
| Meropenem EI | 111 | 2 (1–2) | 0 | 3 (3–6) | 1 |
| Meropenem CI | 17 | 1 (1–2) | 0 | 4 (3–5) | 0 |
II, intermittent infusion (duration <2 h); EI, extended infusion (duration 2–4 h); CI, continuous infusion (24 h infusion).
Percentages were calculated on the number of respondents for each antibiotic. Dosing values are shown as median (IQR).
aVancomycin doses are in mg/kg; piperacillin/tazobactam and meropenem doses are in g.
bNumber of missing values for each dosing category.
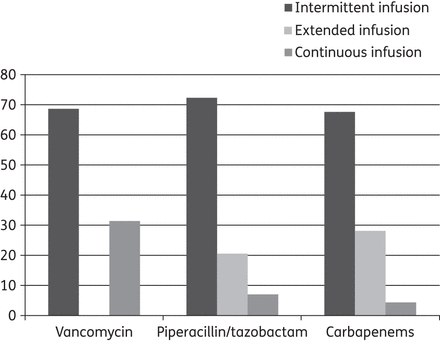
Infusion strategies for vancomycin, piperacillin/tazobactam and carbapenems. Proportion (%) of respondents using an intermittent, extended or continuous infusion. Intermittent infusion is defined as a duration <2 h, extended infusion is defined as a duration of 2–4 h and continuous infusion is defined as a 24 h infusion. Carbapenems include meropenem, imipenem and doripenem.
TDM was used in all patients by 73.6% of respondents (Figure 2). Among these, TDM was sampled every day in almost all patients by 40.4%, every day only in unstable or renally impaired patients by 36.1% and only once or every few days by 19.6%. The minimum target concentration was 23 (20–25) mg/L for continuous infusions and 18 (15–20) mg/L for intermittent infusions.
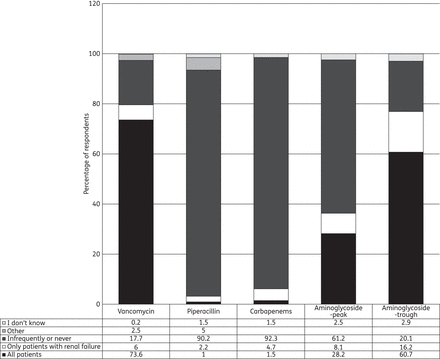
Use of TDM according to antibiotic. Proportion (%) of respondents sampling peak (only for aminoglycosides) and trough concentrations in different clinical situations.
Piperacillin/tazobactam
Piperacillin/tazobactam was used as a short fractionated infusion by 72.4%, with extended and continuous infusions used by 20.6% and 7% of respondents, respectively. A loading dose was used by 82% and 33% of respondents using continuous or extended infusions, respectively (see Table 2 for details regarding dosing).
TDM was infrequently or never used by the majority of respondents (90%). It was used by 1% in all patients and by 2% only in patients with renal failure. One percent measured concentrations on a daily basis, and 5% only once or every few days. In those who measured concentrations, target trough concentrations ranged from 3 to 64 mg/L, or were based on the MIC for the pathogen.
Carbapenems
Meropenem was the most widely used carbapenem (80.5%), followed by imipenem (18.7%) and doripenem (0.8%). TDM for carbapenems was used by 7% of the respondents.
Meropenem was used as a short fractionated infusion by 67% over 30 (30–60) min. Of these respondents using the short fractionated infusion, 5% used a loading dose. An extended infusion over 180 (180–240) min was used by 28% of respondents. Of these respondents using an extended infusion, 38% used a loading dose. Meropenem was administered as a continuous infusion by 4.7% of respondents, of which 73% used a loading dose. Responses regarding loading and maintenance doses are summarized in Table 2.
Imipenem was used as a short fractionated infusion by 73% over 38 (30–60) min, at a median daily dose of 3 (2–3) g/day. An extended infusion over 180 (120–180) min was used by 24% of respondents at a median daily dose of 2.5 (2–3) g/day. Of the respondents using an extended infusion, 22% used a loading dose of 0.5–1 g. A minority of respondents (2.7%) administered imipenem as a continuous infusion with a loading dose of 0.5 or 1 g and a daily dose of 3 g.
Colistin
Colistin was used at least once every week by 46% and at least once a month by 25% of the respondents. About half of the respondents (55%) used a loading dose of 6 (4–9) MIU, and a median daily dose of 7.5 (3.9–9) MIU, divided into 3 (2–3) doses per day. Colistin was administered as a fractionated short infusion by 85% of respondents over 60 (30–60) min or as an extended infusion over 180 (120–180) min by 15% of respondents.
Aminoglycosides
Gentamicin was the preferred aminoglycoside for 55% of respondents, amikacin for 40% and tobramycin for 5%. Aminoglycosides were administered as a single daily dose by 94% of respondents. The median duration of infusion was 60 (30–60) min for amikacin and 30 (30–60) min for gentamicin.
Dosing was variable for all aminoglycosides: 15 (15–20) mg/kg for amikacin, 5 (5–6) mg/kg for gentamicin and 6 (5–7) mg/kg for tobramycin.
Peak concentrations were routinely sampled 30 (30–60) min after the end of the drug infusion by 37.9% of respondents, on a daily basis in almost all patients by 20%, only in patients that are clinically unstable or with an impaired renal function by 36.2%, and only once or every few days by 32.3% of respondents. Target peak concentrations varied considerably and are summarized in Table 3. If the peak was below the target, 80% of respondents would increase the next daily dose, 10% would not change anything and 10% would re-administer a supplementary dose as soon as that result was available.
TDM concentration targets (when used) for once-daily dosed aminoglycosides used by the respondents
| . | Peak concentration (mg/L) . | Respondentsa . | Trough concentration (mg/L) . | Respondentsa . |
|---|---|---|---|---|
| Amikacin | 41 (26–60) | 47 | 3 (2–5) | 59 |
| Gentamicin | 12 (10–17.5) | 37 | 1 (0.5–1.5) | 122 |
| Tobramycin | 12 (10–23.8) | 7 | 2 (1.3–2) | 12 |
| . | Peak concentration (mg/L) . | Respondentsa . | Trough concentration (mg/L) . | Respondentsa . |
|---|---|---|---|---|
| Amikacin | 41 (26–60) | 47 | 3 (2–5) | 59 |
| Gentamicin | 12 (10–17.5) | 37 | 1 (0.5–1.5) | 122 |
| Tobramycin | 12 (10–23.8) | 7 | 2 (1.3–2) | 12 |
The preferred aminoglycoside was gentamicin for 55%, amikacin for 40% and tobramycin for 5% of those who provided data on aminoglycoside use. TDM values are shown as median (IQR).
aNumber of persons that gave a target value for TDM.
TDM concentration targets (when used) for once-daily dosed aminoglycosides used by the respondents
| . | Peak concentration (mg/L) . | Respondentsa . | Trough concentration (mg/L) . | Respondentsa . |
|---|---|---|---|---|
| Amikacin | 41 (26–60) | 47 | 3 (2–5) | 59 |
| Gentamicin | 12 (10–17.5) | 37 | 1 (0.5–1.5) | 122 |
| Tobramycin | 12 (10–23.8) | 7 | 2 (1.3–2) | 12 |
| . | Peak concentration (mg/L) . | Respondentsa . | Trough concentration (mg/L) . | Respondentsa . |
|---|---|---|---|---|
| Amikacin | 41 (26–60) | 47 | 3 (2–5) | 59 |
| Gentamicin | 12 (10–17.5) | 37 | 1 (0.5–1.5) | 122 |
| Tobramycin | 12 (10–23.8) | 7 | 2 (1.3–2) | 12 |
The preferred aminoglycoside was gentamicin for 55%, amikacin for 40% and tobramycin for 5% of those who provided data on aminoglycoside use. TDM values are shown as median (IQR).
aNumber of persons that gave a target value for TDM.
Trough aminoglycoside concentrations were routinely sampled at 23 (18–23) h after administration by the majority of respondents (79.2%). Samples were taken most often on a daily basis by 41.8% of respondents, every day in patients that are clinically unstable or with an impaired renal function by 35.7%, or only once or every few days by 24.3%. Target trough concentrations were 2.5 (2–5) mg/L or below for amikacin and 0.5 (0.5–1) mg/L or below for gentamicin. If concentrations were higher than the stated target, 41.8% of respondents would decrease the next daily dose and 54.5% would sample trough concentrations again and not re-administer until below the target, whereas 3.8% would not make any changes.
Discussion
In this survey, we found wide variability in prescribing practices for several antibiotics that are commonly administered to patients with severe infections. This variability extends to dosing, drug administration and the use of TDM. To the best of our knowledge, this is the first study investigating this variability at a global level. We found the lack of consistency of responses interesting. Consistency and standardization of clinical practice can ensure that minimal quality standards are met and that all patients benefit from new knowledge and improvements as they are being put into practice. Although standardization has been shown to improve outcomes,10 the present data suggest that at this time the benefits of consistent and appropriate antibiotic dosing are unlikely to occur in critically ill patients. Little support is available for the clinician, given that guidelines for empirical antibiotic therapy consistently stress the importance of spectrum and timing of administration,11 but not dosing.
Furthermore, our data also indicate that current information regarding appropriate dosing in these patients is either not easily accessible or variably interpreted by practising clinicians. Classically, dosing provided in the drug-product information is derived from data available at the time of product registration, and these data are mostly based on the pharmacokinetics in healthy volunteers and non-critically ill patients.12 Recent data clearly show that the altered pharmacokinetics in critically ill patients result in highly variable antibiotic concentrations, demonstrating that the original dosing schemes are not adequate in many critically ill patients.2 We observed in this survey that most of the dosing strategies used reflect product-information dosing, which suggests it is the most available resource, but we would contend that the variable results suggest that many respondents do not agree with this resource and consequently that improved access to evidence-based guidelines is urgently required.
The variability in responses in this survey was most pronounced in certain antibiotic classes, such as the aminoglycosides. Several studies have found that standard dosing (e.g. 15 mg/kg amikacin) is not effective in reaching optimal concentrations for antibiotic killing, and higher doses are recommended (>20 mg/kg for amikacin and >8 mg/kg for gentamicin).13,14 Based on the data in this analysis, it seems that some centres have adopted a higher dosing strategy based on the recent literature, whereas others continue to use product-information dosing recommendations. We observed that some centres still use aminoglycosides by dosing two or three times daily, despite the overwhelming evidence favouring once-daily dosing.15
TDM is routinely applied by many of the respondents, but mostly for glycopeptides and aminoglycosides. We noted that most respondents report only monitoring trough concentrations for aminoglycosides, which we would interpret is for minimizing drug toxicity. Monitoring aminoglycoside peak concentrations, which is more likely to assist dosing efficacy, is clearly not yet an established practice. Still, it should be noted that for glycopeptides TDM is not or infrequently used by as many as one out of six respondents. These are surprising findings as TDM for glycopeptides and aminoglycosides is readily available in most centres and standard dosing may unpredictably result in drug concentrations that are either too low or too high. Furthermore, TDM has been shown to be cost-effective in some scenarios.16 TDM can be helpful to detect underdosing as well as overdosing, to improve efficacy and minimize toxicity. Obstacles to implementation of TDM may include infrastructure, cost and lack of clinical outcome data. Despite these obstacles, we are unaware of any international guidelines that do not recommend TDM of glycopeptides and aminoglycosides. For β-lactams the lack of a linear dose–concentration relationship in critically ill patients17 and the clinical implications of insufficient concentrations is a relatively new concept.2 Additionally to communication of this knowledge, availability of the assay, cost of implementation and resources to appropriately interpret the results might also be barriers to its use. Contrary to aminoglycosides, the lack of cost-effectiveness data might be an additional barrier in resource-limited settings.
Although there are many data supporting the use of loading doses to rapidly achieve adequate concentrations, this is infrequently applied, unless antibiotics are administered as a continuous infusion. Loading doses are used by about three in four respondents that use continuous infusions compared with one in three respondents using extended infusions. When a loading dose is used, there is wide variability in the dosing of vancomycin and meropenem. For piperacillin/tazobactam, a loading dose of 4.5 g was used by most of the respondents, which likely corresponds to the amount of drug contained in the product. Again, this variability in practice reflects the uncertainty of clinicians at the bedside and the lack of guidance on this issue.
Prolonged infusions are increasingly being used in the treatment of severe infections.18 The use of continuous infusions for vancomycin has become more common and roughly one out of three respondents reported using this mode of delivery. When aiming at optimizing the pharmacokinetics/pharmacodynamics for β-lactam antibiotics, extended infusions are clearly more popular than continuous infusions; concerns regarding the stability of these drugs may have contributed to this.
Most respondents only infrequently use colistin. There is important variability in the use of a loading dose as well as the dosing itself. Despite recent pharmacokinetic studies,19,20 variability of dosing reflects uncertainty of how to dose, given the very complex pharmacokinetics of this drug as well as concerns regarding potential toxicity.
There are a number of important limitations to this work. Firstly, when data are obtained using a survey it might not represent the reality and complexity of the decisions at the bedside that would be taken when deciding the treatment strategy for a similar patient. Clinical scenarios were presented as having a normal renal function. The aim of this was to simplify the case, but it did not address the question of dosing in augmented renal clearance. No data were collected on the use of RRT and ECMO in each unit. No specific pathogen, antibiogram or susceptibility data were provided. Responses on administration modalities and dosing might have been influenced by local patterns, case mix or use of extracorporeal circuits and RRT.
Secondly, the survey was sent by e-mail and responses were voluntary, with an unrecorded response rate; this might have caused an unknown responder bias. We recorded personal practices and opinions of the respondents and these may or may not represent the wider practice in that ICU or the local guidelines. Furthermore, although data were obtained from respondents from 53 different countries, most respondents were from either Europe or Australia and New Zealand as they were contacted either by the ESICM or the ANZICS, which may have caused the results to be more representative of these particular regions of the world. Likewise, there were 31 ICUs with two respondents, 5 with three respondents and 6 with four or more respondents. Having multiple respondents from some ICUS may have the capacity to skew the results, although these numbers represent a small number of the total number of respondents. Moreover, although obtaining responses from around the world increased the representativeness of this work, it might also have caused variability in the responses due to historic and cultural variations in treatment modalities across countries.
Despite the above limitations, we believe that these data are important for highlighting the significant variability in antimicrobial dosing and monitoring strategies in critically ill patients. Results point to an urgency for well conducted research that will allow the development of guidelines and more consistent prescribing behaviour on this topic.
In conclusion, in this multinational survey of a relatively simple clinical scenario, we found tremendous variability in dosing, administration and monitoring for five commonly used antimicrobials and antimicrobial classes. We believe this variability is due to an awareness of the complexity of accurate dosing in critically ill patients, but a lack of guidance of how one should prescribe and administer antimicrobials for these patients.
Funding
This work was performed by the authors without specific funding. J. A. R. is funded by a Career Development Fellowship from the National Health and Medical Research Council of Australia (APP1048652). J. D. W. is a Senior Clinical Investigator of the Research Foundation - Flanders (Belgium) (FWO). Online survey software (SurveyMonkey) access and formatting were provided at no cost by the ECCRN.
Transparency declarations
None to declare.
Acknowledgements
This survey was designed and conducted by the Working Group for Antimicrobial Use in the ICU within the Infection Section of the ESICM. It was endorsed by the ECCRN and benefited from mailings to its members.
References
- antibiotics
- gentamicin sulfate (usp)
- vancomycin
- amikacin
- carbapenem
- colistin
- anesthesiology
- gentamicins
- glycopeptides
- imipenem
- intensive care
- intensive care unit
- internal medicine
- pharmacists
- guidelines
- meropenem
- piperacillin/tazobactam
- therapeutic drug monitoring
- aminoglycosides
- antimicrobials
- trough concentration
- evidence-based practice
- prescribing behavior
- intravenous infusion, continuous
- infusion procedures



