-
PDF
- Split View
-
Views
-
Cite
Cite
Dong Sik Jung, Frank P. Tverdek, Ying Jiang, Dimitrios P. Kontoyiannis, Switching to anidulafungin from caspofungin in cancer patients in the setting of liver dysfunction is associated with improvement of liver function tests, Journal of Antimicrobial Chemotherapy, Volume 70, Issue 11, November 2015, Pages 3100–3106, https://doi.org/10.1093/jac/dkv235
Close - Share Icon Share
Abstract
Anidulafungin does not undergo hepatic metabolism like the other echinocandins. Therefore, there is a perception that anidulafungin may be less hepatotoxic or less likely to exacerbate existing liver damage. This has not been substantiated in the literature.
We retrospectively reviewed all cancer patients in whom anidulafungin treatment was immediately preceded by treatment with caspofungin and there existed clinical or laboratory evidence of hepatic damage or dysfunction at M. D. Anderson Cancer Center from January 2010 to December 2013.
Sixty-one patients were included in the study. Most patients had haematological malignancies (58, 95%), and the patients were administered hepatotoxic agents such as chemotherapeutic agents (47, 77%) and other medications (38, 62%) simultaneously. There were significant decreases in AST and ALT (P < 0.029 and P < 0.0017, respectively) between two timepoints (switch from caspofungin to anidulafungin and end of anidulafungin therapy). The median changes in AST, ALT and total bilirubin during anidulafungin therapy were −43 IU/L, −25 IU/L and −0.15 mg/dL, respectively. Over 70% of patients had favourable changes in hepatic enzymes or function, and values were stable and decreased at the end of anidulafungin therapy. On average, the percentage of patients with laboratory results meeting common terminology criteria for adverse events (CTCAE) grade ≥2 at the time of switching to anidulafungin was decreased at the end of treatment.
Median serum values and trajectory of hepatic enzymes and hepatotoxicity usually decreased after switching to anidulafungin treatment in patients with abnormal liver function tests. Anidulafungin could be useful in the management of cancer patients with hepatotoxicity occurring during caspofungin therapy.
Introduction
The echinocandins (caspofungin, micafungin and anidulafungin) are considered first-line treatment for invasive candidiasis, and are useful agents for the treatment of Aspergillus moulds.1,2 While the risk of elevated liver enzymes or dysfunction is relatively low with the echinocandin antifungals, there is concern about administering them to patients with acute hepatotoxicity or hepatic dysfunction.3 Anidulafungin is unique, compared with micafungin and caspofungin, in that unlike them it does not undergo hepatic metabolism.4 Therefore, there is a perception among clinicians that anidulafungin may be less hepatotoxic and less likely to exacerbate pre-existing liver dysfunction, but this has not been substantiated in the literature. Prior audits of internal medication use aiming at quality improvement have identified this as one of the main indications for anidulafungin in our institution. Given this practice, there was the opportunity to evaluate the role this strategy plays in the management of patients with hepatotoxicity occurring during treatment with caspofungin.
Patients and methods
Data collection
In this single-centre, retrospective study, pharmacy databases were queried for adult cancer patients (age ≥18 years) who received at least three consecutive doses of anidulafungin (200 mg loading dose and then 100 mg once daily), which had been immediately preceded by treatment with caspofungin (50 mg once daily) at the University of Texas M. D. Anderson Cancer Center from January 2010 to December 2013, with the indication of invasive fungal infections (IFIs), as defined by established criteria5 or antifungal prophylaxis. We included all patients that exhibited clinical or laboratory evidence of hepatic injury or dysfunction [elevation of ALT, AST or serum total bilirubin (TB) levels] within 48 h prior to initiation of anidulafungin. We excluded patients with baseline abnormal liver enzymes at the start of caspofungin treatment. The patients' medical records were reviewed for demographic, clinical and laboratory characteristics. We calculated a Child–Pugh Score6,7 for all patients who had hepatic injury or dysfunction while switching from caspofungin to anidulafungin and identified sequential liver enzymes at three time periods: (i) start of caspofungin therapy (SC); (ii) switch from caspofungin to anidulafungin therapy (STA); and (iii) end of anidulafungin therapy (EA). Pharmacy databases were also queried for all medications among patients included in our study, to search for concomitant hepatotoxic medications8 and chemotherapeutic agents.9 The study was approved by the institutional review board of the M. D. Anderson Cancer Center, with a waiver of informed consent for anonymous data collection.
Definitions
Invasive fungal disease was defined according to the consensus criteria of the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycosis Study Group (EORTC/MSG)5 and as previously described in the literature. Neutropenia was defined as <500 neutrophils/mm3. Septic shock was defined as the requirement for administration of vasoactive agents (pressors, adrenaline) and/or transfer to the ICU. Nephrotoxicity was defined as an increase in creatinine of 2 × baseline, a decrease in urine output to <0.5 mL/kg/h, when that information was available, or a decrease in the glomerular filtration rate by 50%, in accordance with the definition of acute kidney injury by the RIFLE criteria.10 Liver dysfunction at the time of switching from caspofungin to anidulafungin was defined according to the Child–Pugh scoring system (score 1–6, class A; 7–9, class B; ≥10, class C).6,7 The clinical patterns of liver injury were characterized as follows: hepatocellular, predominant initial elevation of ALT; cholestatic, predominant initial elevation of alkaline phosphatase (ALP); mixed, not fulfilling the hepatocellular or cholestatic patterns.11 Liver injury/hepatotoxicity was defined according to the common terminology criteria for adverse events (CTCAE, Version 4.0).12 CTCAE grade refers to the severity of the adverse event. We used grade 2 as a definition of clinically relevant hepatotoxicity, which means moderate toxicity; minimal, local or non-invasive interventions only; ALT >3-fold the upper limit of the normal range (ULN), AST >3-fold the ULN and TB >1.5-fold the ULN. In this study, we did not evaluate hepatotoxicity with respect to ALP, which is commonly increased by cancer progression or other factors in patients with cancer.13 With regard to the trajectory of hepatic enzymes, stable change was defined as a similar value in ALT and AST (±10 IU/L) and TB (±0.3 mg/dL) between STA and EA. Hepatotoxic chemotherapeutic agents were defined as drugs that were included in lists of hepatotoxic medications in the literature.9 Hepatotoxic medications were defined as drugs that showed frequent elevation of liver enzymes and could cause moderate severity,8 or were included in lists of hepatotoxic drugs in the literature11,14 We excluded medications that have been reported to be associated with liver function test abnormalities in <10% of patients during therapy and for which the liver enzyme abnormalities were mild, asymptomatic and transient, typically reversing with continuation of medication.
Statistical analysis
Descriptive statistics were used to summarize patients' demographic, clinical and treatment variables. Continuous variables are presented with median and IQR and categorical variables are presented with frequency and percentage. The Wilcoxon signed-rank test was used to compare patients' values of AST, ALT and TB between two timepoints (STA and EA). The χ2 or Fisher's exact test was used to compare the proportions of patients with laboratory results of CTCAE grade ≥2 between STA and EA, as appropriate. All tests were two-sided, with a significance level of 0.05. All analyses were performed using SPSS statistical software version 21 (SPSS 21.0, IBM Corporation, Armonk, NY, USA).
Results
Baseline characteristics
We found 192 adult cancer patients (age ≥18 years) who received at least three doses of anidulafungin after switching from caspofungin due to increased values in liver enzyme or function tests at our cancer centre during the study periods. Among these 192 patients, we excluded those with abnormal baseline liver function or enzymes prior to starting caspofungin treatment (111 patients), those with normal liver function or enzymes at the start of anidulafungin treatment (9) and those who had a lapse in echinocandin treatment between caspofungin and anidulafungin treatment of >48 h (11). We ended up with 61 patients that met inclusion and exclusion criteria on the day of switching from caspofungin to anidulafungin treatment.
Thirty-one (51%) patients were male. The median age of patients was 58 years, with a range of 19–81 years. Most of the patients had haematological malignancies (58, 95%). Fourteen (23%) patients had received an allogeneic stem cell transplant. Five patients (8%) had graft-versus-host disease with hepatic involvement. Forty-two patients (69%) had Child–Pugh class B. Types of hepatotoxicity and concomitant disorders are listed in Table 1. Most patients (44, 72%) received anidulafungin as treatment for an IFI (Table 1). A patient with invasive mucormycosis had a mixed infection with invasive aspergillosis, and was treated with a combination of posaconazole and anidulafungin. The mean and median durations of anidulafungin treatment were 14 days (SD 16.4) and 9 days (IQR 6–14), respectively. The numbers of patients receiving concomitant hepatotoxic drugs and combination antifungal therapy are shown in Table 1.
Baseline characteristics of 61 patients who switched from caspofungin to anidulafungin
| Parameter . | Results . |
|---|---|
| Male sex | 31 (51) |
| Age, years, median (range) | 58.0 (19–81) |
| Body weight, mean, kg (SD) | 82.3 (18.2) |
| Underlying malignancies | |
| leukaemia | 50 (82) |
| myelodysplastic syndrome | 3 (5) |
| lymphoma/multiple myeloma | 5 (8) |
| solid tumour | 3 (5) |
| Allogeneic haematopoietic stem cell transplant | 14 (23) |
| Graft-versus-host diseases with hepatic involvement | 5 (8) |
| Neutropeniaa | 30 (49) |
| Child–Pugh class | |
| A | 11 (18) |
| B | 42 (69) |
| C | 8 (13) |
| Type of hepatotoxicity | |
| hepatocellular | 16 (26) |
| cholestatic | 26 (43) |
| mixed | 19 (31) |
| Concomitant disorders | |
| renal failure | 31 (51) |
| septic shock | 17 (28) |
| chronic hepatitis Cb | 1 (2) |
| Indication for anidulafungin therapy | |
| prophylaxis | 17 (28) |
| treatment for presumed or documented fungal infection (species identified)c | 44 (72) |
| Hepatotoxic chemotherapyd | 47 (77) |
| Immunosuppressive agentse | 15 (25) |
| Hepatotoxic medicationsf | 38 (62) |
| Combination antifungal therapyg | 18 (30) |
| liposomal amphotericin B | 11 (18) |
| voriconazole | 5 (8) |
| posaconazole | 2 (3) |
| Duration of anidulafungin treatment (days) | |
| mean (SD) | 14 (16.4) |
| median (IQR) | 9 (6–14) |
| Parameter . | Results . |
|---|---|
| Male sex | 31 (51) |
| Age, years, median (range) | 58.0 (19–81) |
| Body weight, mean, kg (SD) | 82.3 (18.2) |
| Underlying malignancies | |
| leukaemia | 50 (82) |
| myelodysplastic syndrome | 3 (5) |
| lymphoma/multiple myeloma | 5 (8) |
| solid tumour | 3 (5) |
| Allogeneic haematopoietic stem cell transplant | 14 (23) |
| Graft-versus-host diseases with hepatic involvement | 5 (8) |
| Neutropeniaa | 30 (49) |
| Child–Pugh class | |
| A | 11 (18) |
| B | 42 (69) |
| C | 8 (13) |
| Type of hepatotoxicity | |
| hepatocellular | 16 (26) |
| cholestatic | 26 (43) |
| mixed | 19 (31) |
| Concomitant disorders | |
| renal failure | 31 (51) |
| septic shock | 17 (28) |
| chronic hepatitis Cb | 1 (2) |
| Indication for anidulafungin therapy | |
| prophylaxis | 17 (28) |
| treatment for presumed or documented fungal infection (species identified)c | 44 (72) |
| Hepatotoxic chemotherapyd | 47 (77) |
| Immunosuppressive agentse | 15 (25) |
| Hepatotoxic medicationsf | 38 (62) |
| Combination antifungal therapyg | 18 (30) |
| liposomal amphotericin B | 11 (18) |
| voriconazole | 5 (8) |
| posaconazole | 2 (3) |
| Duration of anidulafungin treatment (days) | |
| mean (SD) | 14 (16.4) |
| median (IQR) | 9 (6–14) |
Data are number (%) of patients on the day of switching from caspofungin to anidulafungin unless otherwise indicated.
aAbsolute neutrophil count <500 cells/μL.
bOther viral hepatitis and cirrhosis.
cCandida spp., 2 isolated in blood; Aspergillus spp., 5 probable aspergillosis; Zygomycetes, 1 found in tissue; Saccharomyces cerevisiae, 1 found in tissue.
dVincristine, cyclophosphamide, doxorubicin, methotrexate, cytarabine and melphalan, etc.; during anidulafungin treatment or within 1 month before starting anidulafungin.
eTacrolimus, mycophenolate mofetil and cyclosporin.
fAntibiotics: trimethoprim/sulfamethoxazole, clindamycin, ciprofloxacin, doxycycline, minocycline and amoxicillin/clavulanate; anticonvulsants: valproic acid, phenytoin; anti-psychotics, etc.; during anidulafungin treatment, all concomitant potentially hepatotoxic drugs were continued except in one patient.
gAnidulafungin switched from caspofungin and other antifungal agents.
Baseline characteristics of 61 patients who switched from caspofungin to anidulafungin
| Parameter . | Results . |
|---|---|
| Male sex | 31 (51) |
| Age, years, median (range) | 58.0 (19–81) |
| Body weight, mean, kg (SD) | 82.3 (18.2) |
| Underlying malignancies | |
| leukaemia | 50 (82) |
| myelodysplastic syndrome | 3 (5) |
| lymphoma/multiple myeloma | 5 (8) |
| solid tumour | 3 (5) |
| Allogeneic haematopoietic stem cell transplant | 14 (23) |
| Graft-versus-host diseases with hepatic involvement | 5 (8) |
| Neutropeniaa | 30 (49) |
| Child–Pugh class | |
| A | 11 (18) |
| B | 42 (69) |
| C | 8 (13) |
| Type of hepatotoxicity | |
| hepatocellular | 16 (26) |
| cholestatic | 26 (43) |
| mixed | 19 (31) |
| Concomitant disorders | |
| renal failure | 31 (51) |
| septic shock | 17 (28) |
| chronic hepatitis Cb | 1 (2) |
| Indication for anidulafungin therapy | |
| prophylaxis | 17 (28) |
| treatment for presumed or documented fungal infection (species identified)c | 44 (72) |
| Hepatotoxic chemotherapyd | 47 (77) |
| Immunosuppressive agentse | 15 (25) |
| Hepatotoxic medicationsf | 38 (62) |
| Combination antifungal therapyg | 18 (30) |
| liposomal amphotericin B | 11 (18) |
| voriconazole | 5 (8) |
| posaconazole | 2 (3) |
| Duration of anidulafungin treatment (days) | |
| mean (SD) | 14 (16.4) |
| median (IQR) | 9 (6–14) |
| Parameter . | Results . |
|---|---|
| Male sex | 31 (51) |
| Age, years, median (range) | 58.0 (19–81) |
| Body weight, mean, kg (SD) | 82.3 (18.2) |
| Underlying malignancies | |
| leukaemia | 50 (82) |
| myelodysplastic syndrome | 3 (5) |
| lymphoma/multiple myeloma | 5 (8) |
| solid tumour | 3 (5) |
| Allogeneic haematopoietic stem cell transplant | 14 (23) |
| Graft-versus-host diseases with hepatic involvement | 5 (8) |
| Neutropeniaa | 30 (49) |
| Child–Pugh class | |
| A | 11 (18) |
| B | 42 (69) |
| C | 8 (13) |
| Type of hepatotoxicity | |
| hepatocellular | 16 (26) |
| cholestatic | 26 (43) |
| mixed | 19 (31) |
| Concomitant disorders | |
| renal failure | 31 (51) |
| septic shock | 17 (28) |
| chronic hepatitis Cb | 1 (2) |
| Indication for anidulafungin therapy | |
| prophylaxis | 17 (28) |
| treatment for presumed or documented fungal infection (species identified)c | 44 (72) |
| Hepatotoxic chemotherapyd | 47 (77) |
| Immunosuppressive agentse | 15 (25) |
| Hepatotoxic medicationsf | 38 (62) |
| Combination antifungal therapyg | 18 (30) |
| liposomal amphotericin B | 11 (18) |
| voriconazole | 5 (8) |
| posaconazole | 2 (3) |
| Duration of anidulafungin treatment (days) | |
| mean (SD) | 14 (16.4) |
| median (IQR) | 9 (6–14) |
Data are number (%) of patients on the day of switching from caspofungin to anidulafungin unless otherwise indicated.
aAbsolute neutrophil count <500 cells/μL.
bOther viral hepatitis and cirrhosis.
cCandida spp., 2 isolated in blood; Aspergillus spp., 5 probable aspergillosis; Zygomycetes, 1 found in tissue; Saccharomyces cerevisiae, 1 found in tissue.
dVincristine, cyclophosphamide, doxorubicin, methotrexate, cytarabine and melphalan, etc.; during anidulafungin treatment or within 1 month before starting anidulafungin.
eTacrolimus, mycophenolate mofetil and cyclosporin.
fAntibiotics: trimethoprim/sulfamethoxazole, clindamycin, ciprofloxacin, doxycycline, minocycline and amoxicillin/clavulanate; anticonvulsants: valproic acid, phenytoin; anti-psychotics, etc.; during anidulafungin treatment, all concomitant potentially hepatotoxic drugs were continued except in one patient.
gAnidulafungin switched from caspofungin and other antifungal agents.
Median laboratory values and trajectory of hepatic enzymes and function at three timepoints
Generally, median laboratory values for the hepatic enzymes AST and ALT and for TB (hepatic function) increased during caspofungin therapy and decreased after switching to anidulafungin (Table 2 and Figure 1). There were significant decreases in AST and ALT between the timepoints STA and EA (P = 0.029 and P = 0.0017, respectively). The median decreases during anidulafungin therapy were −43 IU/L (IQR −196 to 26), −25 IU/L (−145 to 14) and −0.15 mg/dL (−1.2 to 2.6) for AST, ALT and TB, respectively (Table 2).
Median (IQR) laboratory values at three timepoints during therapy with caspofungin or anidulafungin
| Parameter . | 1. Start of caspofungin therapy (SC) . | 2. Switch from caspofungin to anidulafungin therapy (STA) . | 3. End of anidulafungin therapy (EA) . | Change (IQR) between STA and EA . | P valuea . |
|---|---|---|---|---|---|
| AST (IU/L) (n = 33) | 31 (22–39) | 132 (49–404) | 71 (50–159) | −43 (−196 to 26) | 0.029 |
| ALT (IU/L) (n = 54) | 25 (16–41) | 122 (34–258) | 85 (41–160) | −25 (−145 to 14) | 0.0017 |
| TB (mg/dL) (n = 54) | 0.6 (0.4–0.8) | 2.5 (1.3–5.5) | 2.2 (1.1–5.4) | −0.15 (−1.2 to 2.6) | 0.98 |
| Parameter . | 1. Start of caspofungin therapy (SC) . | 2. Switch from caspofungin to anidulafungin therapy (STA) . | 3. End of anidulafungin therapy (EA) . | Change (IQR) between STA and EA . | P valuea . |
|---|---|---|---|---|---|
| AST (IU/L) (n = 33) | 31 (22–39) | 132 (49–404) | 71 (50–159) | −43 (−196 to 26) | 0.029 |
| ALT (IU/L) (n = 54) | 25 (16–41) | 122 (34–258) | 85 (41–160) | −25 (−145 to 14) | 0.0017 |
| TB (mg/dL) (n = 54) | 0.6 (0.4–0.8) | 2.5 (1.3–5.5) | 2.2 (1.1–5.4) | −0.15 (−1.2 to 2.6) | 0.98 |
Serum ULN: AST, 46 IU/L; ALT, 56 IU/L; TB, 1 mg/dL. Only data for patients with evaluable data at all three timepoints (SC, STA and EA) are included.
aWilcoxon signed-rank test between STA and EA.
Median (IQR) laboratory values at three timepoints during therapy with caspofungin or anidulafungin
| Parameter . | 1. Start of caspofungin therapy (SC) . | 2. Switch from caspofungin to anidulafungin therapy (STA) . | 3. End of anidulafungin therapy (EA) . | Change (IQR) between STA and EA . | P valuea . |
|---|---|---|---|---|---|
| AST (IU/L) (n = 33) | 31 (22–39) | 132 (49–404) | 71 (50–159) | −43 (−196 to 26) | 0.029 |
| ALT (IU/L) (n = 54) | 25 (16–41) | 122 (34–258) | 85 (41–160) | −25 (−145 to 14) | 0.0017 |
| TB (mg/dL) (n = 54) | 0.6 (0.4–0.8) | 2.5 (1.3–5.5) | 2.2 (1.1–5.4) | −0.15 (−1.2 to 2.6) | 0.98 |
| Parameter . | 1. Start of caspofungin therapy (SC) . | 2. Switch from caspofungin to anidulafungin therapy (STA) . | 3. End of anidulafungin therapy (EA) . | Change (IQR) between STA and EA . | P valuea . |
|---|---|---|---|---|---|
| AST (IU/L) (n = 33) | 31 (22–39) | 132 (49–404) | 71 (50–159) | −43 (−196 to 26) | 0.029 |
| ALT (IU/L) (n = 54) | 25 (16–41) | 122 (34–258) | 85 (41–160) | −25 (−145 to 14) | 0.0017 |
| TB (mg/dL) (n = 54) | 0.6 (0.4–0.8) | 2.5 (1.3–5.5) | 2.2 (1.1–5.4) | −0.15 (−1.2 to 2.6) | 0.98 |
Serum ULN: AST, 46 IU/L; ALT, 56 IU/L; TB, 1 mg/dL. Only data for patients with evaluable data at all three timepoints (SC, STA and EA) are included.
aWilcoxon signed-rank test between STA and EA.
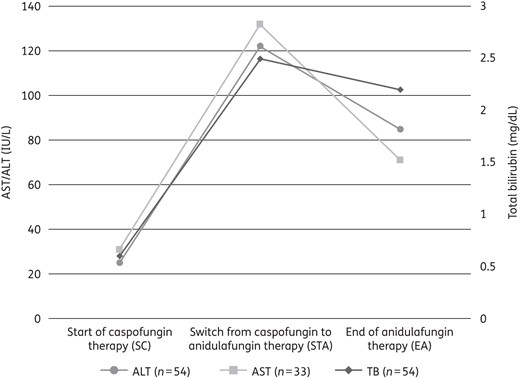
Median laboratory values of AST, ALT, and TB at three timepoints during therapy with caspofungin or anidulafungin. There were significant decreases (P < 0.05) in AST and ALT between STA and EA. Serum ULN: AST, 46 IU/L; ALT, 56 IU/L; TB, 1 mg/dL. Only data of patients with evaluable data at all three timepoints (SC, STA and EA) are shown.
With regard to the trajectory of hepatic enzymes or function between STA and EA, >70% of patients had favourable changes; values were stable and lower at the end of anidulafungin therapy compared with values at the switch to anidulafungin (Figure 2). Among 54 patients who had ALT with CTCAE grade ≥2 at the time of switching to anidulafungin therapy, 34 (63%) had decreased values and 12 (22%) had stable values at the end of anidulafungin therapy. Among 33 patients who had AST with CTCAE grade ≥2 at the time of switching, 18 (55%) who had decreased values and 8 (24%) had stable values at the end of anidulafungin therapy. Among 54 patients who had TB of CTCAE grade ≥2 at the time of switching, 28 (52%) had decreased values at the end of anidulafungin therapy (Figure 2).
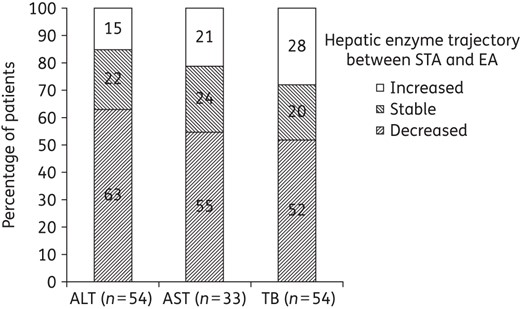
Proportion of patients with change in hepatic enzyme trajectory between STA and EA. Over 70% of patients had favourable changes (stable or decreased values) of hepatic enzymes; only data for patients with evaluable data at both timepoints are included.
Change in hepatotoxicity after switching to anidulafungin
On average, after switching to anidulafungin treatment, the percentage of patients with laboratory results more than CTCAE grade 2 decreased in total and in patients according to Child–Pugh classification B and C at STA (AST and TB) (Figure 3). There were significant decreases in ALT more than CTCAE grade 2 between STA and EA in total (P = 0.004) and in patients with Child–Pugh class A (P = 0.05) (Table 3). Among nine patients with Child–Pugh class A, three (33%) had TB above CTCAE grade 2 level at the end of anidulafungin therapy. The elevated values of TB persisted similarly in two patients (4.5 and 1.6 mg/dL, respectively) and in one patient the value decreased from 6.1 mg/dL (peak value) to 4.7 mg/dL during anidulafungin therapy.
| . | P value . | |||
|---|---|---|---|---|
| . | Total . | Child–Pugh A . | Child–Pugh B . | Child–Pugh C . |
| ALT | 0.004 | 0.05 | 0.136 | 0.282 |
| AST | 0.125 | 0.09 | 0.210 | 0.524 |
| TB | 0.227 | 0.303 | 0.127 | 0.467 |
| . | P value . | |||
|---|---|---|---|---|
| . | Total . | Child–Pugh A . | Child–Pugh B . | Child–Pugh C . |
| ALT | 0.004 | 0.05 | 0.136 | 0.282 |
| AST | 0.125 | 0.09 | 0.210 | 0.524 |
| TB | 0.227 | 0.303 | 0.127 | 0.467 |
| . | P value . | |||
|---|---|---|---|---|
| . | Total . | Child–Pugh A . | Child–Pugh B . | Child–Pugh C . |
| ALT | 0.004 | 0.05 | 0.136 | 0.282 |
| AST | 0.125 | 0.09 | 0.210 | 0.524 |
| TB | 0.227 | 0.303 | 0.127 | 0.467 |
| . | P value . | |||
|---|---|---|---|---|
| . | Total . | Child–Pugh A . | Child–Pugh B . | Child–Pugh C . |
| ALT | 0.004 | 0.05 | 0.136 | 0.282 |
| AST | 0.125 | 0.09 | 0.210 | 0.524 |
| TB | 0.227 | 0.303 | 0.127 | 0.467 |
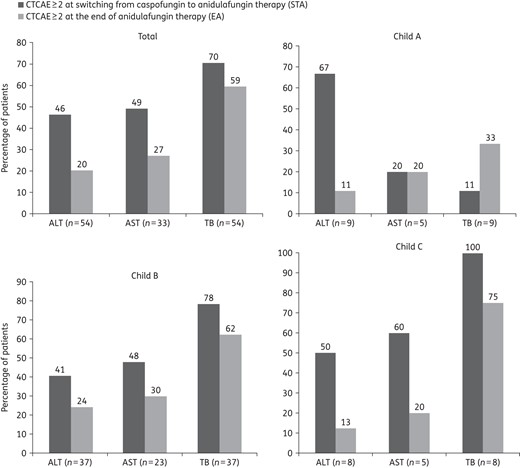
Proportion of patients with laboratory results of CTCAE grade ≥2. There was a tendency to decline between two time periods in the total population and in patients according to Child–Pugh classification at STA; only data for patients with evaluable data at both timepoints are included. CTCAE grade ≥2: ALT >3 × ULN, AST >3 × ULN, TB >1.5 × ULN.
Discussion
In studying a population with a high risk of hepatotoxicity, we found that the serum median values and the trajectory of hepatic enzymes declined at the end of anidulafungin therapy after switching from caspofungin to anidulafungin in cancer patients in the setting of liver injury. The percentage of patients with moderate or severe hepatotoxicity (CTCAE grade ≥2) also decreased at the end of anidulafungin therapy. It appears that anidulafungin may be a safe alternative drug in the management of cancer patients with hepatotoxicity or dysfunction occurring during treatment or prophylaxis with caspofungin.
Drug-induced hepatotoxicity, especially severe hepatocellular injury with jaundice, is associated with high mortality or the requirement for liver transplantation.15,16 A previous prospective study in the USA reported that antimicrobials were most commonly responsible for hepatotoxicity.17,18 Therefore, caution should be used when administering antifungal agents capable of causing hepatic injury in patients at risk of hepatotoxicity.19 Previous reports have shown that fluconazole and posaconazole are less hepatotoxic than others in the azole class.20–22 However, within the echinocandin class, the use of anidulafungin in patients with hepatotoxicity occurring while on caspofungin has not been reported in the literature. Since anidulafungin is inactivated by gradual spontaneous degradation and is not metabolized hepatically,23 dosage adjustment or avoidance in subjects with varying degrees of hepatic dysfunction is not needed, unlike the situation with the other echinocandins, caspofungin and micafungin.4,24,25 Even though anidulafungin has been available on the market since 2006, real-life experience of the switch from caspofungin to anidulafungin due to the presence of liver injury has not been described. Our study population included cancer patients, mostly patients with haematological malignancies (95%), with multiple factors that can damage the liver and lead to hepatic dysfunction (Child–Pugh classes A–C were represented in our study population). Nevertheless, elevated hepatic enzymes tended to decline after switching to anidulafungin (Figure 2) and in all Child–Pugh classes most patients with CTCAE grade ≥2 had lower values at the end of anidulafungin therapy (Figure 3). Interestingly, this change was only statistically significant for ALT reductions in patients with Child–Pugh class A. The reason for this is unclear, but it may be representative of self-limited enzyme elevations in the setting of minimal functional impact. In contrast, patients with more severe hepatic dysfunction (classes B and C) might have had a much slower improvement in the kinetics of transaminase recovery due to multiple factors, more frequent use of other hepatotoxins or more limited hepatic reserves due to underlying liver pathology. However, our data do not allow testing of this hypothesis.
Our results support the recent Prospective Spanish Survey, which reached a consensus using the Delphi technique,26 which showed that clinicians or specialists usually preferred to use anidulafungin (100%) or caspofungin (82.6%) in patients with moderate hepatic dysfunction (Child–Pugh class B) for invasive candidiasis.
With respect to other risk factors for hepatotoxicity, co-administration of hepatotoxic chemotherapy or medications can also cause hepatotoxicity in cancer patients, either independently or through drug–drug interactions.9,11,24 Indeed, 47 patients (77%) received hepatotoxic chemotherapy such as vincristine, cyclophosphamide, doxorubicin, methotrexate or cytarabine and 38 (62%) received hepatotoxic medications such as trimethoprim/sulfamethoxazole, amoxicillin/clavulanate or valproic acid during anidulafungin therapy. Moreover, with regard to host susceptibility to hepatotoxicity, patients with neutropenia and septic shock were 50% and 28%, respectively (Table 1). Among the patients with little or no improvement in hepatotoxicity subsequent to the change to anidulafungin therapy, it is possible that the above potentially confounding factors could be driving the toxicity rather than the caspofungin.
Our study has limitations that should be taken into consideration. First, it was a retrospective, single-centre study with a relatively small number of cancer patients who had multifactorial liver injury or dysfunction at the time of switching from caspofungin to anidulafungin. Second, our study includes the potential for selection bias and the lack of international standards for diagnosis of hepatotoxicity. To this end, we used the CTCAE (Version 4.0) published by the National Cancer Institute as a measure of hepatotoxicity, and included ALT, AST and TB as parameters of hepatotoxicity. For a variety of reasons, ALP is frequently elevated in patients with cancer, making it potentially less sensitive as a marker of hepatotoxicity as we do not routinely check specific isoenzymes in order to confirm the hepatic origin of ALP. Third, during caspofungin treatment, hepatotoxicity could potentially not be caused by caspofungin, but by chemotherapeutic agents, other concomitant medications or other confounding factors. Furthermore, it is possible that continuation or dose reduction (35 mg/day) of caspofungin for patients with hepatic dysfunction (Child–Pugh classes B and C) would also yield similar downtrending liver enzyme values. Fourth, we did not compare clinical efficacy, though many would assume equivalent efficacy. Fifth, while receiving anidulafungin, the patients commonly received another antifungal agent in combination therapy, which could also cause hepatic injury in patients with cancer. The difficulties of ascribing drug-specific hepatotoxicity in this patient population have been encountered in prior studies.27–29
Despite these limitations, this study stands as a real-life experience of the clinical practice of switching among echinocandins in the setting of biochemical liver damage. The bedside clinician is often unable to delineate the actual cause of liver enzyme elevation, especially in this patient population with multiple risk factors. Furthermore, there is often concern about further worsening of hepatic damage and deterioration of liver function in the absence of an intervention. The strategy of switching from caspofungin to anidulafungin is an attempt by the clinician to mitigate hepatic toxicity while maintaining clinical efficacy. Resolution of liver enzyme elevation is important as it may prevent delays in chemotherapy, prevent delays or modification of stem cell transplantation conditioning regimens and reduce diagnostic and treatment expenditures, and allow antifungal therapy to continue for difficult-to-treat IFIs.
Funding
Supported in part by an academic grant from Pfizer, Inc. to D. P. K. who is also a recipient of the Frances King Black Endowment.
Transparency declarations
D. P. K. has received research support and honoraria from: Pfizer; Astellas Pharma US; Gilead Sciences, Inc.; Merck & Co., Inc.; and T2 Biosystems. All other authors report no potential conflicts.
Acknowledgements
We thank Cai Wu (Pharmacy Medication Management and Analytics) for pharmacy data and Parag Mahale for technical assistance.



