-
PDF
- Split View
-
Views
-
Cite
Cite
L. L. Ross, E. Rouse, P. Gerondelis, E. DeJesus, C. Cohen, J. Horton, B. Ha, E. R. Lanier, R. Elion, on behalf of the COL40263 study, Low-abundance HIV species and their impact on mutational profiles in patients with virological failure on once-daily abacavir/lamivudine/zidovudine and tenofovir, Journal of Antimicrobial Chemotherapy, Volume 65, Issue 2, February 2010, Pages 307–315, https://doi.org/10.1093/jac/dkp419
Close - Share Icon Share
Abstract
HIV clonal genotypic analysis (CG) was used to investigate whether a more sensitive analysis method would detect additional low-abundance mutations compared with population genotyping (PG) in antiretroviral-naive patients who experienced virological failure (VF) during treatment with abacavir/lamivudine/zidovudine and tenofovir.
HIV was analysed by PG and CG (771 baseline and 657 VF clones) from subjects with VF (confirmed HIV RNA ≥ 400 copies/mL at 24–48 weeks).
Fourteen of 123 subjects (11%) met VF criteria; their median baseline HIV RNA was 5.4 log10 copies/mL, and 4.0 log10 copies/mL at VF. By baseline PG, 2/14 had HIV-1 with nucleoside reverse transcriptase inhibitor (NRTI) or non-NRTI mutations. By baseline CG, 9/14 had HIV-1 with NNRTI and/or NRTI mutations; 7/9 had study drug-associated mutations. By PG at VF, 10/14 had selected for resistance mutations [2, K65R; 1, M184V; and 7, thymidine analogue mutations (TAMs) ± M184V]. By CG at VF, for subjects with TAMs, T215F was more commonly detected (5/14 samples) than T215Y (2/14). For one subject who selected K65R at VF, both K65R-containing clones and TAM-containing clones (both T215A and T215F) were observed independently but not conjunctively in the same clone in a post-VF sample.
The majority of subjects with VF had major and minor mutations detected at VF; CG detected additional low-abundance variants at baseline and VF that could have influenced mutation selection pathways. Both PG and CG data suggest TAMs, not K65R selection, are the preferred resistance route, biased towards 215F selection. No HIV clone contained both K65R and T215F/Y mutations, suggesting in vivo antagonism between the two mutations. The once-daily zidovudine usage and high baseline viraemia may also have contributed to rapid selection of HIV with multiple mutations in VFs.
Introduction
When treating HIV-infected patients, HIV genotyping is commonly utilized as a diagnostic tool if the patient does not respond to therapy or if the patient experiences viral rebound. It has been previously observed that population genotyping (PG) can underestimate mutations, since minority quasi-species may not be detected.1,2 Certain drug resistance mutations in the reverse transcriptase have also been shown in vitro to be mutually antagonistic, e.g. the mutation K65R and the thymidine analogue mutations (TAMs; i.e. M41L, D67N, K70R, L210W, T215F/Y and K219Q/E), such that they are rarely detected in vivo in the same sample by population sequencing.3,4 K65R can be selected in vivo by the drugs tenofovir and abacavir but its frequency appears to be highly dependent on the other drugs used within the regimen.5,6In vitro, it reduces susceptibility to all marketed nucleoside reverse transcriptase inhibitors (NRTIs) except zidovudine.7,8 In studies where triple regimens of tenofovir, abacavir and lamivudine or tenofovir, didanosine and lamivudine were given to antiretroviral-naive subjects, the majority of subjects experiencing virological failure (VF) selected for M184V and K65R.5,9–11 In contrast, TAMs are typically selected in vivo by zidovudine and stavudine. TAMs confer reduced susceptibility to both drugs, and certain combinations of TAMs can also confer broad resistance to the NRTI class, including tenofovir.12–15
COL40263 was a pilot 48 week, open-label, multicentre study in antiretroviral-naive patients evaluating the efficacy and safety of a once-daily regimen including a fixed-dose combination of abacavir/lamivudine/zidovudine (300/150/300 mg/tablet, two tablets taken once daily) and tenofovir (300 mg, one tablet/day).16 Prior HIV analysis by PG and by population phenotypic analysis of samples from COL40263 subjects with VF had demonstrated that selection of resistance via the TAM pathway was preferred to selection via the K65R pathway, suggesting that inclusion of zidovudine in the tenofovir/abacavir/lamivudine regimen could modulate selection to favour selection of TAMs.17 Here, samples from the subjects who experienced VF in COL40263 were evaluated using clonal genotypic analysis (CG). Using this more sensitive methodology we hoped to determine whether there was additional resistance to study drugs undetected by PG at baseline or at the time of VF that could have impacted the selection pathway, and whether the mutational antagonism between K65R and TAMs, as suggested by the in vitro data, would result in detection of clonal variants in these VF samples that would contain either K65R or TAMs, but not both.
Methods
Study design
COL40263 was a 48 week non-randomized, single-arm, open-label observational pilot study conducted at 11 centres in the USA. All subjects received abacavir/lamivudine/zidovudine (300/150/300 mg/tablet, two tablets taken once daily) plus 300 mg of tenofovir once daily (one tablet), and the study was designed to assess the impact of this regimen in subjects with higher HIV RNA at entry; therefore, only subjects with HIV RNA ≥ 30 000 copies/mL at study screening were enrolled. All subjects were ≥18 and ≤65 years of age with documented HIV-1 infection and were antiretroviral therapy (ART) naive.
The multicentre COL40263 study was approved by either a central or a local Institutional Review Board. Subjects provided written informed consent to participate in the study and for sample analysis. VF was defined as confirmed HIV-1 RNA ≥ 400 copies/mL at ≥24 weeks. All plasma HIV-1 RNA concentrations were confirmed by a second measurement 2–4 weeks after the first measurement.
Genotypic analysis
HIV was isolated from plasma from the 14 patients experiencing VF. Samples were analysed by PG, and these results were compared with those obtained from samples collected at baseline (VIRCO, Mechelen, Belgium). CG was performed for the baseline and VF timepoints at GlaxoSmithKline using aliquots of the same plasma samples. A total of 771 baseline clonal sequences and 657 VF sequences were analysed. The clonal genotyping methodologies were as follows: viral RNA was extracted from 1 mL of patient plasma using a magnetic silica particle method (miniMag™, bioMérieux, Durham, NC, USA). RT–PCR amplification of the viral pol gene and confirmatory PG were performed using the TruGene™ HIV-1 Genotyping Kit and OpenGene™ System (Siemens Healthcare Diagnostics Inc., Deerfield, IL, USA). Amplicons were directly cloned from the same RT–PCR using the Zero-Blunt® TOPO PCR cloning system (Invitrogen, Carlsbad, CA, USA). Individual colonies grown on selective medium were separately isolated, followed by PCR amplification with HIV-1-specific primers. The colony PCR product was purified and sequenced with HIV-1-specific primers using an ABI 377 automated sequencer and Prism FS technology (PE Biosystems, Foster City, CA, USA). NRTI and non-NRTI (NNRTI) resistance mutations were analysed as per the current IAS-USA Drug Resistance Mutations group guidelines (http://www.iasusa.org),18 except that NNRTI mutations solely associated with etravirine resistance were not included, and the non-IAS-USA-defined mutations D67G and S68 (any) and T215 reversion mutations were included in the analysis. Unusual amino acid changes detected at resistance sites are also shown for the CG data.
Results
One hundred and twenty-three subjects enrolled in COL40263. The full safety and efficacy results from the COL40263 study have been published.16 Subjects who met the criteria for VF in COL40263 (n = 14) had a higher median baseline HIV RNA of 5.44 log10 copies/mL than the rest of the study population (n = 109, HIV RNA 5.04 log10 copies/mL). Twelve of the 14 subjects with VF had baseline HIV RNA ≥ 100 000 copies/mL compared with 60/109 of the non-failure population. The 14 subjects with VF also had a much lower median baseline CD4+ (124 cells/mm3) than the rest of the population (225 cells/mm3), were slightly younger (median 36.5 versus 38 years) and were more likely to be black (79% versus 36%) than white (21% versus 50%) or Hispanic (0% versus 15%).
Baseline HIV genotypic mutation profiles in the 14 subjects with VF
The mutations detected at baseline and at the time of VF by PG as compared with the mutations and mutational linkages detected by CG are shown in Table 1. By PG, 2/14 subjects had NNRTI or NRTI mutations at baseline. Of the 12 subjects with wild-type HIV at baseline by PG (without NRTI or NNRTI mutations), CG identified additional resistance mutations that were not detected by PG in 7/12 samples. Mutations detected by CG at baseline that could have impacted response to one or more study drugs included mutations at T215, K70E, D67G, M184V and the multi-NRTI Q151M-associated mutations A62V and F77L. The Y188L NNRTI mutation and unusual amino acid mutations at NNRTI resistance sites that might reflect reversion of NNRTI mutations (e.g. K103R, Y181H and G190E) from archived drug-resistant virus were also detected in some baseline samples by CG. In total, CG detected NNRTI or NRTI mutations, or both, at baseline in 9/14 VF subjects. Seven of these 14 subjects had baseline mutations with the potential to impact one or more study drugs.
Comparison of HIV resistance-associated mutations detected when analysed by conventional sequencing techniques (population genotype) and when using the more sensitive method of clonal analysis to analyse HIV-1 isolated at the baseline (pre-therapy) visit and virological failure (VF) for the 14 subjects who met VF criteria in COL40263
| . | Pre-therapy baseline sample . | VF sample . | |||||
|---|---|---|---|---|---|---|---|
| Subject . | HIV RNA log10 copies/mL . | population genotype . | clonal genotypea . | timepoint . | HIV RNA log10 copies/mL . | population genotype . | clonal genotypea . |
| 1 | 5.25 | WT | WT (17/17) | week 32 | 2.77 | K65K/R | K65R (16/61) |
| K65R + S68N (20/61) | |||||||
| K65R + Y115F (25/61) | |||||||
| 3.52 | Additional (post-VF) clonal analysis ∼at week 36 WT (6/23) | ||||||
| K65R + S68N (10/23) | |||||||
| K65R (2/23) | |||||||
| K65R + Y115F (3/23) | |||||||
| D67N + L210W + T215F (1/23) | |||||||
| T215A (1/23) | |||||||
| 2 | 5.3 | WT | WT (58/105) | week 48 | 2.87 | K65R + S68N/S + Y115F/Y + V118I | K65R + Y115F (6/48) |
| M184V (1/105) | K65R + S68N (16/48) | ||||||
| K70E (1/105) | K65R + S68N + Y115F (26/48) | ||||||
| V118I (45/105) | |||||||
| 3 | 5.85 | WT | WT (33/33) | week 32 | 3.36 | D67N + K70R + M184V | WT (7/46) |
| V118I (8/46) | |||||||
| D67N + K70R + M184V (18/46) | |||||||
| D67N + K70R + M184V + K219E (10/46) | |||||||
| D67N + K70R + M184V + K219Q (3/46) | |||||||
| 4 | 6.11 | WT | WT (77/77) | week 32 | 4.55 | T215Y | WT (7/74) |
| T215Y (63/74) | |||||||
| T215Y + M41L (4/74) | |||||||
| 5 | 5.34 | K103K/N + Y188F/H/L/Y | WT (8/30) | week 32 | 4.23 | M41L + D67N + K70R + M184V + T215F + K219E | WT (11/50) |
| Y188L (7/30) | M41L + D67N + K70R + Y188L + M184V + T215F + K219E (34/50) | ||||||
| K103N (13/30) | M41L + D67N + K70R + Y188L + M184V + T215L + K219E (1/50) | ||||||
| K103N + T215A (2/30) | Y188L (3/50) | ||||||
| M41L + D67N + K70R + Y188L (1/50) | |||||||
| 6 | 5.18 | WT | WT (127/128) | week 24 | 4.15 | WT | WT (31/31) |
| T215S (1/128) | |||||||
| 7 | 5.72 | WT | WT (53/53) | week 32 | 3.59 | M184V | M184V (36/62) |
| M184V + D67G (1/62) | |||||||
| D67N + T215F (21/62) | |||||||
| M184V + T215S (1/62) | |||||||
| D67N + K70R + T215Y (1/62) | |||||||
| D67N + S68G + T215Y (1/62) | |||||||
| K65R + S68N + Y115F + V118I (1/62) | |||||||
| 8 | 4.4 | WT | WT (44/46) | week 32 | 2.68 | WT | WT (35/36) |
| K219Q (2/46) | L210W (1/36) | ||||||
| 9 | 5.84 | T215T/A | WT (47/56) | week 28 | 5.08 | D67N + L210L/W + T215F | D67N + T215F (64/92) |
| D67G (1/56) | D67N + K70R + T215F (13/92) | ||||||
| T215A (8/56) | D67N + M184V + T215F (2/92) | ||||||
| D67N + L210W + T215F (13/92) | |||||||
| 10 | 5.30 | WT | WT (68/73) | week 24 | 3.54 | D67N + K70K/R + T215F | M184V + T215F (1/64) |
| M41I (1/73) | D67N + T215F (36/64) | ||||||
| K103R (1/73) | D67N + T215Y (1/64) | ||||||
| Y181H (1/73) | M41L + D67N + T215F (6/64) | ||||||
| M184V (1/73) | M41V + D67N + T215F (1/64) | ||||||
| G190E (1/73) | M41V + D67N + G190E + T215F (1/64) | ||||||
| A62T + D67N + T215F (1/64) | |||||||
| D67N + K70R + T215F (12/64) | |||||||
| D67N + K70R + M184V (1/64) | |||||||
| D67N + G190E + T215F (1/64) | |||||||
| D67N + K70T + T215F (1/64) | |||||||
| D67N + T215F + K219T (2/64) | |||||||
| 11 | 5.11 | WT | WT (13/13) | week 40 | 2.8 | D67N + K70R + M184V | WT (10/16) |
| D67N + K70R + 184V (1/16) | |||||||
| D67N + M184V (1/16) | |||||||
| D67N + K70R + M184V + K219E (4/16) | |||||||
| 12 | 4.72 | WT | WT (49/58) | week 24 | 4.2 | WT | WT (56/57) |
| Y188L (9/58) | Y188L (1/57) | ||||||
| 13 | 5.54 | WT | WT (66/67) | week 40 | 4.6 | WT | WT (30/31) |
| F77L (1/67) | M184I (1/31) | ||||||
| 14 | 5.56 | WT | WT (56/60) | week 24 | 5.66 | M41L/M + D67D/N + L210L/W + T215F | T215F (1/34) |
| A62V (2/60) | M41L + T215F (3/34) | ||||||
| D67G (1/60) | D67N + T215F (26/34) | ||||||
| M184V (1/60) | D67N + S68G + L210W + T215F (1/34) | ||||||
| D67N + K70R + T215F (2/34) | |||||||
| D67N + L210W + T215F (1/34) | |||||||
| . | Pre-therapy baseline sample . | VF sample . | |||||
|---|---|---|---|---|---|---|---|
| Subject . | HIV RNA log10 copies/mL . | population genotype . | clonal genotypea . | timepoint . | HIV RNA log10 copies/mL . | population genotype . | clonal genotypea . |
| 1 | 5.25 | WT | WT (17/17) | week 32 | 2.77 | K65K/R | K65R (16/61) |
| K65R + S68N (20/61) | |||||||
| K65R + Y115F (25/61) | |||||||
| 3.52 | Additional (post-VF) clonal analysis ∼at week 36 WT (6/23) | ||||||
| K65R + S68N (10/23) | |||||||
| K65R (2/23) | |||||||
| K65R + Y115F (3/23) | |||||||
| D67N + L210W + T215F (1/23) | |||||||
| T215A (1/23) | |||||||
| 2 | 5.3 | WT | WT (58/105) | week 48 | 2.87 | K65R + S68N/S + Y115F/Y + V118I | K65R + Y115F (6/48) |
| M184V (1/105) | K65R + S68N (16/48) | ||||||
| K70E (1/105) | K65R + S68N + Y115F (26/48) | ||||||
| V118I (45/105) | |||||||
| 3 | 5.85 | WT | WT (33/33) | week 32 | 3.36 | D67N + K70R + M184V | WT (7/46) |
| V118I (8/46) | |||||||
| D67N + K70R + M184V (18/46) | |||||||
| D67N + K70R + M184V + K219E (10/46) | |||||||
| D67N + K70R + M184V + K219Q (3/46) | |||||||
| 4 | 6.11 | WT | WT (77/77) | week 32 | 4.55 | T215Y | WT (7/74) |
| T215Y (63/74) | |||||||
| T215Y + M41L (4/74) | |||||||
| 5 | 5.34 | K103K/N + Y188F/H/L/Y | WT (8/30) | week 32 | 4.23 | M41L + D67N + K70R + M184V + T215F + K219E | WT (11/50) |
| Y188L (7/30) | M41L + D67N + K70R + Y188L + M184V + T215F + K219E (34/50) | ||||||
| K103N (13/30) | M41L + D67N + K70R + Y188L + M184V + T215L + K219E (1/50) | ||||||
| K103N + T215A (2/30) | Y188L (3/50) | ||||||
| M41L + D67N + K70R + Y188L (1/50) | |||||||
| 6 | 5.18 | WT | WT (127/128) | week 24 | 4.15 | WT | WT (31/31) |
| T215S (1/128) | |||||||
| 7 | 5.72 | WT | WT (53/53) | week 32 | 3.59 | M184V | M184V (36/62) |
| M184V + D67G (1/62) | |||||||
| D67N + T215F (21/62) | |||||||
| M184V + T215S (1/62) | |||||||
| D67N + K70R + T215Y (1/62) | |||||||
| D67N + S68G + T215Y (1/62) | |||||||
| K65R + S68N + Y115F + V118I (1/62) | |||||||
| 8 | 4.4 | WT | WT (44/46) | week 32 | 2.68 | WT | WT (35/36) |
| K219Q (2/46) | L210W (1/36) | ||||||
| 9 | 5.84 | T215T/A | WT (47/56) | week 28 | 5.08 | D67N + L210L/W + T215F | D67N + T215F (64/92) |
| D67G (1/56) | D67N + K70R + T215F (13/92) | ||||||
| T215A (8/56) | D67N + M184V + T215F (2/92) | ||||||
| D67N + L210W + T215F (13/92) | |||||||
| 10 | 5.30 | WT | WT (68/73) | week 24 | 3.54 | D67N + K70K/R + T215F | M184V + T215F (1/64) |
| M41I (1/73) | D67N + T215F (36/64) | ||||||
| K103R (1/73) | D67N + T215Y (1/64) | ||||||
| Y181H (1/73) | M41L + D67N + T215F (6/64) | ||||||
| M184V (1/73) | M41V + D67N + T215F (1/64) | ||||||
| G190E (1/73) | M41V + D67N + G190E + T215F (1/64) | ||||||
| A62T + D67N + T215F (1/64) | |||||||
| D67N + K70R + T215F (12/64) | |||||||
| D67N + K70R + M184V (1/64) | |||||||
| D67N + G190E + T215F (1/64) | |||||||
| D67N + K70T + T215F (1/64) | |||||||
| D67N + T215F + K219T (2/64) | |||||||
| 11 | 5.11 | WT | WT (13/13) | week 40 | 2.8 | D67N + K70R + M184V | WT (10/16) |
| D67N + K70R + 184V (1/16) | |||||||
| D67N + M184V (1/16) | |||||||
| D67N + K70R + M184V + K219E (4/16) | |||||||
| 12 | 4.72 | WT | WT (49/58) | week 24 | 4.2 | WT | WT (56/57) |
| Y188L (9/58) | Y188L (1/57) | ||||||
| 13 | 5.54 | WT | WT (66/67) | week 40 | 4.6 | WT | WT (30/31) |
| F77L (1/67) | M184I (1/31) | ||||||
| 14 | 5.56 | WT | WT (56/60) | week 24 | 5.66 | M41L/M + D67D/N + L210L/W + T215F | T215F (1/34) |
| A62V (2/60) | M41L + T215F (3/34) | ||||||
| D67G (1/60) | D67N + T215F (26/34) | ||||||
| M184V (1/60) | D67N + S68G + L210W + T215F (1/34) | ||||||
| D67N + K70R + T215F (2/34) | |||||||
| D67N + L210W + T215F (1/34) | |||||||
NRTI resistance mutations are shown in bold and WT indicates wild-type HIV. For Subject 1, clonal analysis results are also presented for an additional post-VF timepoint.
aClonal variant abundance is expressed as the number of clones with that genotype/total number of clones analysed from that sample.
Comparison of HIV resistance-associated mutations detected when analysed by conventional sequencing techniques (population genotype) and when using the more sensitive method of clonal analysis to analyse HIV-1 isolated at the baseline (pre-therapy) visit and virological failure (VF) for the 14 subjects who met VF criteria in COL40263
| . | Pre-therapy baseline sample . | VF sample . | |||||
|---|---|---|---|---|---|---|---|
| Subject . | HIV RNA log10 copies/mL . | population genotype . | clonal genotypea . | timepoint . | HIV RNA log10 copies/mL . | population genotype . | clonal genotypea . |
| 1 | 5.25 | WT | WT (17/17) | week 32 | 2.77 | K65K/R | K65R (16/61) |
| K65R + S68N (20/61) | |||||||
| K65R + Y115F (25/61) | |||||||
| 3.52 | Additional (post-VF) clonal analysis ∼at week 36 WT (6/23) | ||||||
| K65R + S68N (10/23) | |||||||
| K65R (2/23) | |||||||
| K65R + Y115F (3/23) | |||||||
| D67N + L210W + T215F (1/23) | |||||||
| T215A (1/23) | |||||||
| 2 | 5.3 | WT | WT (58/105) | week 48 | 2.87 | K65R + S68N/S + Y115F/Y + V118I | K65R + Y115F (6/48) |
| M184V (1/105) | K65R + S68N (16/48) | ||||||
| K70E (1/105) | K65R + S68N + Y115F (26/48) | ||||||
| V118I (45/105) | |||||||
| 3 | 5.85 | WT | WT (33/33) | week 32 | 3.36 | D67N + K70R + M184V | WT (7/46) |
| V118I (8/46) | |||||||
| D67N + K70R + M184V (18/46) | |||||||
| D67N + K70R + M184V + K219E (10/46) | |||||||
| D67N + K70R + M184V + K219Q (3/46) | |||||||
| 4 | 6.11 | WT | WT (77/77) | week 32 | 4.55 | T215Y | WT (7/74) |
| T215Y (63/74) | |||||||
| T215Y + M41L (4/74) | |||||||
| 5 | 5.34 | K103K/N + Y188F/H/L/Y | WT (8/30) | week 32 | 4.23 | M41L + D67N + K70R + M184V + T215F + K219E | WT (11/50) |
| Y188L (7/30) | M41L + D67N + K70R + Y188L + M184V + T215F + K219E (34/50) | ||||||
| K103N (13/30) | M41L + D67N + K70R + Y188L + M184V + T215L + K219E (1/50) | ||||||
| K103N + T215A (2/30) | Y188L (3/50) | ||||||
| M41L + D67N + K70R + Y188L (1/50) | |||||||
| 6 | 5.18 | WT | WT (127/128) | week 24 | 4.15 | WT | WT (31/31) |
| T215S (1/128) | |||||||
| 7 | 5.72 | WT | WT (53/53) | week 32 | 3.59 | M184V | M184V (36/62) |
| M184V + D67G (1/62) | |||||||
| D67N + T215F (21/62) | |||||||
| M184V + T215S (1/62) | |||||||
| D67N + K70R + T215Y (1/62) | |||||||
| D67N + S68G + T215Y (1/62) | |||||||
| K65R + S68N + Y115F + V118I (1/62) | |||||||
| 8 | 4.4 | WT | WT (44/46) | week 32 | 2.68 | WT | WT (35/36) |
| K219Q (2/46) | L210W (1/36) | ||||||
| 9 | 5.84 | T215T/A | WT (47/56) | week 28 | 5.08 | D67N + L210L/W + T215F | D67N + T215F (64/92) |
| D67G (1/56) | D67N + K70R + T215F (13/92) | ||||||
| T215A (8/56) | D67N + M184V + T215F (2/92) | ||||||
| D67N + L210W + T215F (13/92) | |||||||
| 10 | 5.30 | WT | WT (68/73) | week 24 | 3.54 | D67N + K70K/R + T215F | M184V + T215F (1/64) |
| M41I (1/73) | D67N + T215F (36/64) | ||||||
| K103R (1/73) | D67N + T215Y (1/64) | ||||||
| Y181H (1/73) | M41L + D67N + T215F (6/64) | ||||||
| M184V (1/73) | M41V + D67N + T215F (1/64) | ||||||
| G190E (1/73) | M41V + D67N + G190E + T215F (1/64) | ||||||
| A62T + D67N + T215F (1/64) | |||||||
| D67N + K70R + T215F (12/64) | |||||||
| D67N + K70R + M184V (1/64) | |||||||
| D67N + G190E + T215F (1/64) | |||||||
| D67N + K70T + T215F (1/64) | |||||||
| D67N + T215F + K219T (2/64) | |||||||
| 11 | 5.11 | WT | WT (13/13) | week 40 | 2.8 | D67N + K70R + M184V | WT (10/16) |
| D67N + K70R + 184V (1/16) | |||||||
| D67N + M184V (1/16) | |||||||
| D67N + K70R + M184V + K219E (4/16) | |||||||
| 12 | 4.72 | WT | WT (49/58) | week 24 | 4.2 | WT | WT (56/57) |
| Y188L (9/58) | Y188L (1/57) | ||||||
| 13 | 5.54 | WT | WT (66/67) | week 40 | 4.6 | WT | WT (30/31) |
| F77L (1/67) | M184I (1/31) | ||||||
| 14 | 5.56 | WT | WT (56/60) | week 24 | 5.66 | M41L/M + D67D/N + L210L/W + T215F | T215F (1/34) |
| A62V (2/60) | M41L + T215F (3/34) | ||||||
| D67G (1/60) | D67N + T215F (26/34) | ||||||
| M184V (1/60) | D67N + S68G + L210W + T215F (1/34) | ||||||
| D67N + K70R + T215F (2/34) | |||||||
| D67N + L210W + T215F (1/34) | |||||||
| . | Pre-therapy baseline sample . | VF sample . | |||||
|---|---|---|---|---|---|---|---|
| Subject . | HIV RNA log10 copies/mL . | population genotype . | clonal genotypea . | timepoint . | HIV RNA log10 copies/mL . | population genotype . | clonal genotypea . |
| 1 | 5.25 | WT | WT (17/17) | week 32 | 2.77 | K65K/R | K65R (16/61) |
| K65R + S68N (20/61) | |||||||
| K65R + Y115F (25/61) | |||||||
| 3.52 | Additional (post-VF) clonal analysis ∼at week 36 WT (6/23) | ||||||
| K65R + S68N (10/23) | |||||||
| K65R (2/23) | |||||||
| K65R + Y115F (3/23) | |||||||
| D67N + L210W + T215F (1/23) | |||||||
| T215A (1/23) | |||||||
| 2 | 5.3 | WT | WT (58/105) | week 48 | 2.87 | K65R + S68N/S + Y115F/Y + V118I | K65R + Y115F (6/48) |
| M184V (1/105) | K65R + S68N (16/48) | ||||||
| K70E (1/105) | K65R + S68N + Y115F (26/48) | ||||||
| V118I (45/105) | |||||||
| 3 | 5.85 | WT | WT (33/33) | week 32 | 3.36 | D67N + K70R + M184V | WT (7/46) |
| V118I (8/46) | |||||||
| D67N + K70R + M184V (18/46) | |||||||
| D67N + K70R + M184V + K219E (10/46) | |||||||
| D67N + K70R + M184V + K219Q (3/46) | |||||||
| 4 | 6.11 | WT | WT (77/77) | week 32 | 4.55 | T215Y | WT (7/74) |
| T215Y (63/74) | |||||||
| T215Y + M41L (4/74) | |||||||
| 5 | 5.34 | K103K/N + Y188F/H/L/Y | WT (8/30) | week 32 | 4.23 | M41L + D67N + K70R + M184V + T215F + K219E | WT (11/50) |
| Y188L (7/30) | M41L + D67N + K70R + Y188L + M184V + T215F + K219E (34/50) | ||||||
| K103N (13/30) | M41L + D67N + K70R + Y188L + M184V + T215L + K219E (1/50) | ||||||
| K103N + T215A (2/30) | Y188L (3/50) | ||||||
| M41L + D67N + K70R + Y188L (1/50) | |||||||
| 6 | 5.18 | WT | WT (127/128) | week 24 | 4.15 | WT | WT (31/31) |
| T215S (1/128) | |||||||
| 7 | 5.72 | WT | WT (53/53) | week 32 | 3.59 | M184V | M184V (36/62) |
| M184V + D67G (1/62) | |||||||
| D67N + T215F (21/62) | |||||||
| M184V + T215S (1/62) | |||||||
| D67N + K70R + T215Y (1/62) | |||||||
| D67N + S68G + T215Y (1/62) | |||||||
| K65R + S68N + Y115F + V118I (1/62) | |||||||
| 8 | 4.4 | WT | WT (44/46) | week 32 | 2.68 | WT | WT (35/36) |
| K219Q (2/46) | L210W (1/36) | ||||||
| 9 | 5.84 | T215T/A | WT (47/56) | week 28 | 5.08 | D67N + L210L/W + T215F | D67N + T215F (64/92) |
| D67G (1/56) | D67N + K70R + T215F (13/92) | ||||||
| T215A (8/56) | D67N + M184V + T215F (2/92) | ||||||
| D67N + L210W + T215F (13/92) | |||||||
| 10 | 5.30 | WT | WT (68/73) | week 24 | 3.54 | D67N + K70K/R + T215F | M184V + T215F (1/64) |
| M41I (1/73) | D67N + T215F (36/64) | ||||||
| K103R (1/73) | D67N + T215Y (1/64) | ||||||
| Y181H (1/73) | M41L + D67N + T215F (6/64) | ||||||
| M184V (1/73) | M41V + D67N + T215F (1/64) | ||||||
| G190E (1/73) | M41V + D67N + G190E + T215F (1/64) | ||||||
| A62T + D67N + T215F (1/64) | |||||||
| D67N + K70R + T215F (12/64) | |||||||
| D67N + K70R + M184V (1/64) | |||||||
| D67N + G190E + T215F (1/64) | |||||||
| D67N + K70T + T215F (1/64) | |||||||
| D67N + T215F + K219T (2/64) | |||||||
| 11 | 5.11 | WT | WT (13/13) | week 40 | 2.8 | D67N + K70R + M184V | WT (10/16) |
| D67N + K70R + 184V (1/16) | |||||||
| D67N + M184V (1/16) | |||||||
| D67N + K70R + M184V + K219E (4/16) | |||||||
| 12 | 4.72 | WT | WT (49/58) | week 24 | 4.2 | WT | WT (56/57) |
| Y188L (9/58) | Y188L (1/57) | ||||||
| 13 | 5.54 | WT | WT (66/67) | week 40 | 4.6 | WT | WT (30/31) |
| F77L (1/67) | M184I (1/31) | ||||||
| 14 | 5.56 | WT | WT (56/60) | week 24 | 5.66 | M41L/M + D67D/N + L210L/W + T215F | T215F (1/34) |
| A62V (2/60) | M41L + T215F (3/34) | ||||||
| D67G (1/60) | D67N + T215F (26/34) | ||||||
| M184V (1/60) | D67N + S68G + L210W + T215F (1/34) | ||||||
| D67N + K70R + T215F (2/34) | |||||||
| D67N + L210W + T215F (1/34) | |||||||
NRTI resistance mutations are shown in bold and WT indicates wild-type HIV. For Subject 1, clonal analysis results are also presented for an additional post-VF timepoint.
aClonal variant abundance is expressed as the number of clones with that genotype/total number of clones analysed from that sample.
HIV genotypic mutation profiles in the 14 VF subjects at VF
Seven of the VF subjects at baseline had resistance mutation-containing low-abundance viral species (including one of more of the following: A62V, F77L, K70E, T215A, T215S, D67G, M184V or K219Q) with the potential to impact response to one or more study drugs. At failure, virus from five of these seven subjects contained TAMs with or without M184V (Subjects 5, 8, 9, 10 and 14) or K65R (Subject 2). The only subject with TAMs detected at baseline who did not have detectable TAMs at failure was Subject 6, who had only a very low incidence of T215S (1/128 clones) at baseline. Certain TAM reversion mutations detected at baseline by CG were no longer detected at the time of VF and may have been outcompeted by other mutations. For example, Subjects 5 and 9 had HIV with T215A reversion mutations at baseline, but not at VF (where T215F was detected in virus from both subjects). Phylogenetic analysis of the clonal sequences from Subject 5 (Figure 1) illustrates the genetic diversity at baseline and the divergence into three distinct groups of variants at VF. At baseline, Subject 5 had viral clones containing K103N with or without T215A, or with Y188L resistance mutations, while at VF clones with one or more of the following mutations: M41L, D67N, K70R, M184V, Y188L, T215F or L, and K219E were detected, but K103N and T215A were not.
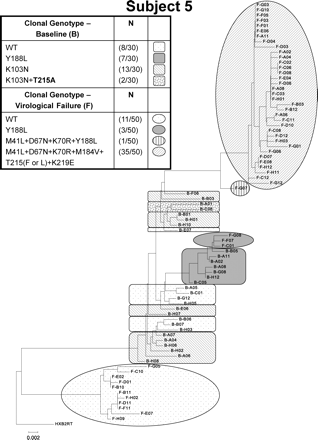
Phylogenetic analysis of plasma HIV variants isolated from a representative subject (Subject 5) whose baseline HIV-1 RNA was 5.34 log10 copies/mL and whose baseline (pre-therapy) population genotype resistance mutations included K103K/N + Y188F/H/L/Y and who at failure had an HIV-1 RNA of 4.23 log10 copies/mL and a population genotype of M41L + D67N + K70R + M184V + T215F + K219E. Clonal analysis detected distinct viral species, and some of these clonal variants contained different mutational patterns at baseline and at VF from those observed by population sequencing. Phylogenetic trees were constructed by using the neighbour-joining method and out-grouped with HXB2RT (HXB2 reverse transcriptase reference sequence). The scale indicates the relative phylogenetic distance. WT, wild-type.
Only four subjects (Subjects 6, 8, 12 and 13) had wild-type HIV as measured by PG at both baseline and VF. However, when analysed by CG, low-abundance viral species containing resistance mutations were detected in three of these four subjects at VF (L210W for Subject 8, Y188L for Subject 12 and M184I for Subject 13). The L210W and M184I mutations were treatment emergent for Subjects 8 and 13, respectively, while the NNRTI mutation Y188L that was observed by CG at VF for Subject 12 had also been detected at baseline by CG (but not by PG). Interestingly, although Subject 8 had low-abundance viral species containing the L210W mutation by VF, a different TAM (K219Q) had been detected at baseline by CG.
By PG at VF, virus from seven subjects had selected for TAMs or for TAMs plus an M184V mutation. In 6/7 of these subjects, CG revealed additional TAMs or M184V that were not detected by PG. The T215F variant was much more commonly detected than T215Y. In the remaining subject (Subject 5), 5/6 TAMs plus M184V were already detected by PG at VF. Although it is common for M184V mutations to be selected along with TAMs in subjects failing on regimens containing zidovudine and lamivudine, in this study a relatively small number of subjects had virus with M184V at VF (3/14 by PG, 7/14 by CG). Interestingly, when utilizing the more sensitive method of clonal analysis, at the last study visit examined by CG four of the VF subjects had virus with TAMs but no viral clones that also contained the M184V mutation (Subjects 1, 4, 8 and 14).
Two subjects with baseline wild-type virus by PG selected for the K65R mutation at VF. Viruses from both of these subjects were clade B. For Subject 1, both the VF and a timepoint 4 weeks after VF were analysed by CG. At baseline by both PG and clonal analysis, only wild-type HIV-1 was detected for Subject 1. At VF, Subject 1 population sequencing revealed only K65K/R, and only clones with the K65R mutation were observed at the first timepoint (with or without Y115F and S68 mutations), but at the later timepoint TAM-containing clones (one clone with D67N + L210W + T215F and another clone with T215A) were also detected in addition to K65R-containing clones. For Subject 2, K70E was detected by CG at baseline, followed by selection of K65R at failure. Interestingly, Subject 2 also had viral clones with M184V alone at baseline; however, M184V was not detected at failure. The phylogenetic profile from the clonal sequences for Subject 2 is shown in Figure 2. The tight clustering of the non-wild-type failure sequences suggests that they arose from a small segment of the baseline population, with replication occurring by only a few mutational pathways. This genetic bottlenecking could have resulted from the observed incomplete suppression of replication in the presence of drug for those viruses that had already selected for study drug resistance-associated mutations.

Phylogenetic analysis of plasma HIV variants isolated from a representative subject (Subject 2) whose baseline HIV-1 RNA was 5.30 log10 copies/mL and whose baseline (pre-therapy) population genotype was wild-type and who at failure had an HIV-1 RNA of 2.87 log10 copies/mL and a population genotype of K65R + S68N/S + Y115F/Y + V118I. Clonal analysis detected distinct viral species, and some of these clonal variants contained different mutational patterns at baseline and at VF from those observed by population sequencing. Phylogenetic trees were constructed by using the neighbour-joining method and out-grouped with HXB2RT (HXB2 reverse transcriptase reference sequence). The scale indicates the relative phylogenetic distance. WT, wild-type.
Discussion
The resistance pattern for the 14 subjects with VF in COL40263 differs significantly from that previously reported for tenofovir-containing triple nucleoside regimens.5,9–11 Virus from the majority of subjects with VF in COL40263 selected for TAMs at VF. The more sensitive technique of CG confirmed the PG findings that virus from the majority of VF selected for TAMs at failure, and detected the presence of one or more additional TAMs at low incidences.
The rapid selection of multiple HIV mutations in some of these subjects may be due in part to the high baseline viraemia observed for those subjects with VF in this study, as the opportunity for selection of mutations with a replication advantage under drug selection pressure would have been increased as compared with subjects with lower baseline viral loads. Although this was an ART-naive study population, for seven of the VF subjects low-abundance viral species possessing pre-existing NRTI mutations were detected at baseline. These included mutations considered to be multidrug resistance-conferring mutations (such as F77L and A62V) that could have provided a replication advantage under drug pressure from which more fit mutations might be selected. The presence of M184V has been previously associated with a lower incidence of treatment-emergent TAMs independent of time on therapy, suggesting a direct effect of M184V on reduced selection of TAMs.19 By both conventional (PG) and clonal sequencing methods, virus from four of the VF subjects (including the post-VF results of Subject 1) selected TAMs but had no detectable M184V by clonal analysis at their last study visit, and this lack of M184V selection may have resulted in a more rapid selection of multiple TAMs. It also cannot be ruled out that the use of zidovudine once daily rather than twice daily might have contributed to a lowered genetic barrier towards selection for TAMs.
Limited data from another clinical study by Moyle et al.20 support the hypothesis that TAMs is the preferred selection pathway in HIV-1-infected, ART-naive subjects treated with abacavir/lamivudine/zidovudine + tenofovir. In the as-treated analysis, 1/40 subjects treated with twice-daily abacavir/lamivudine/zidovudine + once-daily tenofovir experienced VF; virus from this subject also selected for M184V + TAMs (D67N, K70E, T215Y and K219E), and the authors noted that this subject had incomplete regimen adherence.20 For COL40263, anecdotal communication from the study sites suggested that incomplete adherence was also a factor in the majority of VFs, which may suggest that adherence is especially important in ART-naive subjects with high-level viraemia who initiate therapy, and may be more critical for subjects residing in geographic areas where the incidence of transmitted drug resistance mutations is known to be elevated.
Sturmer et al.21 have also extensively reviewed findings from a number of primarily single-arm studies in ART-experienced subjects who later received abacavir/lamivudine/zidovudine + tenofovir. In their cross-study comparison, they concluded that this regimen was an efficient treatment strategy in moderately pre-treated patients and that virological success was observed for this regimen in the presence of the M184V mutation and low numbers of TAMs, although the presence of at least two TAMs, especially when the T215Y/F mutation was present, or the L210W mutation alone was a predictor of VF. The role of pre-existing K65R as a predictor of VF for a later treatment regimen with quadruple abacavir/lamivudine/zidovudine + tenofovir therapy was unable to be clearly addressed in the review of Sturmer et al.21 due to low sample numbers. Virological response and mutational profile at failure results were also summarized by these authors for naive and pre-treated patients who received lamivudine/zidovudine + tenofovir therapy in several studies; the majority of these subjects achieved virological suppression on therapy. For those subjects who met VF criteria and had detectable resistance mutations at failure, TAMs + M184V was the predominant pattern, although a low incidence of K65R was detected.
K65R was also observed at low incidence (2/14 subjects) at VF in COL40263. Interestingly, one of the two subjects whose HIV did select for K65R may have been predisposed to selection of the K65R mutation by the presence of the K70E mutation, as low-abundance clones with this mutation were detected at baseline. K70E has been shown in macaques to precede selection for K65R when treated with tenofovir and also to precede selection for K65R in patients when tenofovir, abacavir and lamivudine are co-administered.22,23 While K70E and K65R are both selected by tenofovir, they appear to be structurally incompatible, and, when both are detected by PG after in vivo treatment with tenofovir, they occur on separate viral genomes. The K70E mutation also produces slightly less tenofovir resistance, suggesting that under tenofovir selection pressure virus with K65R will have a growth advantage.24 Since tenofovir was administered separately from the co-formulated abacavir/lamivudine/zidovudine, it is also possible that K65R could have emerged through partial adherence to the drug regimen, or that use of zidovudine once daily rather than twice daily was not sufficient in some patients to modulate selection in those patients experiencing VF towards a TAM pathway.
No clone from any of the subjects with VF contained both K65R and T215F or Y mutations. This agrees with the findings of Parikh et al.,3 who have suggested that there is no net evolutionary benefit for the virus to simultaneously select both TAMs and K65R. This antagonism may be a pan-HIV phenomenon, as a similar counterselection for K65R by mutations at T215 in subjects infected with HIV-2 was recently reported.25 Furthermore, the HIV-1 from Subject 1, which had selected K65R mutations at VF, continued to evolve under drug selection pressure and, at a post-VF timepoint, two distinct clonal populations emerged, those containing K65R and another that contained TAMs. Taken as a whole, the data from this study suggest that TAM selection is a preferred resistance route, rather than K65R, when zidovudine is combined with abacavir, lamivudine and tenofovir and is consistent with PG results from in vivo studies in HIV-1-infected antiviral-naive or -experienced subjects treated with this quadruple regimen in which TAMs were more likely to be detected than K65R.20,21 The clonal data from this study also suggest that for ART-naive subjects receiving this regimen, the pathway may also be biased towards selection via the 215F pathway, as this regimen was more common than T215Y. By utilizing the more sensitive technique of CG, it was possible to detect low-frequency drug-resistant variants in these ART-naive subjects at baseline, and the presence of these baseline variants in this cohort of ART-naive subjects with high baseline viraemia may have helped drive resistance down specific pathways. This suggests that for a minority of patients ultrasensitive techniques may be useful in detection of variants that can impact response on therapy and should alter choice of second-line regimens.
Funding
This work was supported by a collaborative research grant from GlaxoSmithKline (COL40263).
Transparency declarations
L. L. R., P. G., J. H. and B. H. are GlaxoSmithKline employees. E. R. and E. R. L. were employed by GlaxoSmithKline at the time of sample analysis. E. D.J., C. C. and R. E. have received consulting fees, lecture fees or research support from GlaxoSmithKline. L. L. R., E. R. L. and B. H. own GlaxoSmithKline stock or options.
Acknowledgements
We gratefully acknowledge the contributions of all of the patients, physicians and the study staff at all of the participating sites who made this study possible. The full COL40263 study team included the following physician investigators: Richard Elion, Edwin DeJesus, Cal Cohen, Thomas Jefferson, Gary Richmond, Julia Torres, Anne Morris, Larry Bush and Robert Schwartz, and the following members of the GlaxoSmithKline team: Lisa Ross, Belinda Ha, Elizabeth Rouse, Peter Gerondelis, Joseph Horton, Randall Lanier, Ilisse Minto, Denise Paulsen, Jaime Hernandez, Carol Humphries, Sue Pippin, Trevor Scott and Keith Pappa.
References
Author notes
Members of the study team are listed in the Acknowledgements section.



