-
PDF
- Split View
-
Views
-
Cite
Cite
Abdurrahman Sagir, Mark Oette, Rolf Kaiser, Martin Däumer, Gerd Fätkenheuer, Jürgen Kurt Rockstroh, Heribert Knechten, Günther Schmutz, Martin Hower, Jetske Emmelkamp, Herbert Pfister, Dieter Häussinger, on behalf of the RESINA Study Team, Trends of prevalence of primary HIV drug resistance in Germany, Journal of Antimicrobial Chemotherapy, Volume 60, Issue 4, October 2007, Pages 843–848, https://doi.org/10.1093/jac/dkm274
Close - Share Icon Share
Abstract
Primary HIV drug resistance (PDR) is associated with poor treatment outcome of first-line highly active antiretroviral therapy (HAART). The aim of the study was to observe the trend of prevalence of PDR between 2001 and 2005.
In a prospective multicentre study in the state of Nordrhein-Westfalen, Germany, 831 treatment-naive chronically HIV-infected patients underwent genotypic resistance testing.
Six hundred and forty (77%) of them were male. Two-thirds of the patients (558, 67%) were infected with HIV subtype B. PDR was found in 75 of 831 [9%; 95% confidence interval (CI) 7.1–10.9] cases entering the study between January 2001 and December 2005. An increasing trend of PDR was found from 2001 (4.8%; CI 2.1–9.4) to 2005 (9.0%; CI 5.4–12.6; P = 0.08). A significant tendency to higher PDR was observed for ethnicity other than Caucasian (P = 0.04), HIV subtypes other than B (P = 0.02) and transmission routes other than homosexual (P = 0.03).
A non-significant increase in prevalence of PDR was observed from 2001 to 2005. A significant trend to higher PDR rate was detected in non-Caucasian patients, patients infected with non-B subtypes, and in patients with risk factors for acquisition of HIV other than homosexual transmission. Based on the fact that there is a trend to higher PDR rate, resistance testing in untreated HIV-infected patients starting HAART becomes more important in clinical routine. The identification of patient subgroups with a remarkable risk of PDR makes continuous monitoring of PDR mandatory.
Introduction
In countries with wide access to antiretroviral therapy, various drugs are available to target HIV replication. Despite documented efficacy of highly active antiretroviral therapy (HAART) of HIV infection, viral replication cannot be suppressed sufficiently in a substantial proportion of patients.1 One major reason for this is the emergence of drug-resistant variants. Unfortunately, the frequent development of drug resistance during combination therapy limits the sustained response to antiretroviral therapy in many HIV-infected patients.2–4 Mutations indicating resistance were found in about half of a series of resistance tests and in almost 80% of the population with detectable viral replication while treated with HAART.5 Resistance against antiretroviral drugs in previously untreated HIV-positive patients, defined as primary HIV drug resistance (PDR), is of growing relevance. It has been hypothesized that a wide use of antiretroviral drugs could result in an increase in drug-resistant virus transmission and more generally in the prevalence of resistant variants. In the years 1995–98, the prevalence of drug-resistant variants in patients with recent HIV infection ranged from 10% to 20% in western Europe and in the United States.6,7 The increase in resistance-associated mutations in newly infected patients between the early 1990s and recent years has been debated in different studies.8–10 The reason for this phenomenon is infection with resistant virus strains, as described for sexual, vertical and parenteral path of transmission.11,12 HAART guided by resistance testing showed a similar efficacy in patients with PDR as compared with patients with wild-type virus.13 PDR is associated with poor treatment outcome of first-line HAART when the physicians do not take PDR into account before starting therapy.8,14,15
The objective of this prospective study was to evaluate the prevalence of PDR among treatment-naive patients in the years 2001–05 and to identify risk factors associated with an increase in PDR.
Methods
The project on ‘primary drug RESIstance in treatment-NAive HIV-infected patients’ (RESINA) is a prospective multicentre study in the state of Nordrhein-Westfalen, Germany. With ∼18 million inhabitants, it is the largest state of the country and accounts for ∼21% of documented HIV cases in Germany. Genotypic resistance testing was performed in HAART-naive HIV-1-infected patients before first application of HAART. The study received approval by local and collaborating institutional review boards. Across the state, 42 centres specialized in the treatment of HIV and AIDS patients were identified and initially asked for participation. Thirty-seven centres consisting of 5 hospitals, 4 outpatient units of university clinics, 2 outpatient units of regional hospitals and 26 private practices collaborated in the project. A substantial part of the HIV-infected subgroup as defined by the study protocol has been reached by covering the majority of treatment centres of the state. Thus, the study population may be regarded as representative for the state of Nordrhein-Westfalen, Germany.
Inclusion criteria were documented HIV-1 infection, eligibility for application of HAART, and informed consent. Exclusion criteria were previous intake of antiretroviral drugs (as determined by self-report) and unwillingness to participate. The following baseline parameters were analysed: sex, CDC stage of disease, HIV subtype (subdivision of group M of HIV-1), ethnicity and route of HIV transmission. Subtyping was performed from sequencing of the pol gene region and interpretation was carried out with both geno2pheno (www.genafor.org) and the National Center for Biotechnology Information (NCBI) rules (www.ncbi.nlm.nih.gov/projects/genotyping/genotype.cgi). Recently subtyping is performed with the Rega Subtyping Tool, (http://dbpartners.stanford.edu/RegaSubtyping/) instead of the NCBI-Tool.
Genotypic resistance testing was performed as described previously.13 Mutations were considered as significant in accordance with the International AIDS Society (IAS) rules with the exclusion of V188I, interpretation of genotyping results was done by the geno2pheno drug resistance interpretation tool and additional expert advice was included into the communication of results.16,17 Multiclass resistance was defined as resistance in cases with involvement of at least two substance groups. All treatment-naive patients who gave their informed consent between January 2001 and December 2005 were included in this study. PDR rates were analysed for each year.
The statistical analysis was performed with SAS, release 12.0. Trend analysis was performed by using Cochran–Armitage trend-test. Univariate comparisons were applied using Wilcoxons rank sum test or two-sided Fishers exact test, where appropriate. P values of < 0.05 were considered significant; no adjustment for multiple testing was applied.
Results
Between January 2001 and December 2005, a total of 831 patients were enrolled into the RESINA study prospectively. Baseline characteristics of the patients for each year are shown in Table 1. Most patients were male (640, 77%), the dominant HIV subtype was B (558, 67%) and 80% (661) of the patients were Caucasian. The cumulative baseline characteristics after division of HIV subtypes, ethnicity, CDC stage and route of transmission are shown in Table 2. PDR was identified in 75 of the 831 patients [9%; 95% confidence interval (CI) 7.1–10.9]. Multiclass resistance was detected in 11 cases (1.3%; CI 0.5–2.1). The PDR rate was 4.8% (4/83; CI 2.1–9.4) in 2001 and reached the highest rate in 2004 with 11.6% (28/242; CI 7.5–15.6). The PDR rate decreased from 2004 to 2005 from 11.6% to 9.0% (22/245; CI 5.4–12.6), but was higher than in the years 2001, 2002 and 2003. The trend analysis of PDR rates from 2001 to 2005 showed a trend to an increase in PDR rates during this period, but did not reach significance (P = 0.08, Figure 1a). In the next step, we studied trends within the different drug classes. The highest overall PDR rate was observed for nucleoside reverse transcriptase inhibitors (NRTIs; 5.4%; CI 3.9–6.9), followed by non-nucleoside reverse transcriptase inhibitors (NNRTIs; 3.0%; CI 1.8–4.2) and protease inhibitors (PIs; 2.4%; CI 1.4–3.5). None of the substance groups reached the significance level in the trend analysis (P = 0.5 for NRTIs, P = 0.07 for NNRTIs and P = 0.1 for PIs). Trends for drug class and multiclass resistance are shown in Figure 1(b). The cumulative prevalence, 95% CI and the detected mutations for each substance group are shown in Table 3.
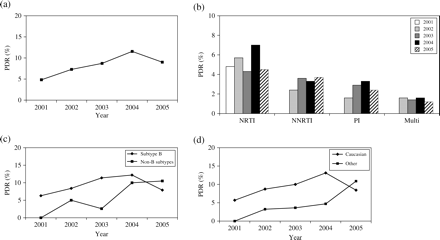
(a) Trend of PDR rates from 2001 to 2005. (b) PDR trends within the drug classes. (c) PDR trends in patients infected with HIV subtype B and non-B subtypes. (d) PDR trends in Caucasian patients and patients with other ethnicity.
Characteristics of patients included in the study divided concerning the time of resistance testing
| . | 2001 n = 83 . | 2002 n = 123 . | 2003 n = 138 . | 2004 n = 242 . | 2005 n = 245 . | Cumulative n = 831 . |
|---|---|---|---|---|---|---|
| Male, n (%) | 61 (73%) | 90 (73%) | 113 (82%) | 183 (76%) | 193 (79%) | 640 (77%) |
| CDC C, n (%) | 36 (43%) | 42 (34%) | 45 (33%) | 60 (25%) | 68 (28%) | 251 (30%) |
| HIV subtype, n (%) | ||||||
| B | 64 (77%) | 83 (67%) | 99 (72%) | 172 (71%) | 140 (57%) | 558 (67%) |
| other | 19 (23%) | 40 (33%) | 39 (28%) | 70 (29%) | 105 (43%) | 273 (33%) |
| Ethnicity, n (%) | ||||||
| Caucasian | 70 (84%) | 92 (75%) | 110 (80%) | 199 (82%) | 190 (78%) | 661 (80%) |
| other | 13 (16%) | 31 (25%) | 28 (20%) | 43 (18%) | 55 (22%) | 170 (20%) |
| HIV transmission, n (%) | ||||||
| homosexual | 46 (55%) | 58 (47%) | 72 (52%) | 135 (56%) | 114 (47%) | 425 (51%) |
| other | 37 (45%) | 65 (53%) | 66 (48%) | 107 (44%) | 131 (54%) | 406 (49%) |
| . | 2001 n = 83 . | 2002 n = 123 . | 2003 n = 138 . | 2004 n = 242 . | 2005 n = 245 . | Cumulative n = 831 . |
|---|---|---|---|---|---|---|
| Male, n (%) | 61 (73%) | 90 (73%) | 113 (82%) | 183 (76%) | 193 (79%) | 640 (77%) |
| CDC C, n (%) | 36 (43%) | 42 (34%) | 45 (33%) | 60 (25%) | 68 (28%) | 251 (30%) |
| HIV subtype, n (%) | ||||||
| B | 64 (77%) | 83 (67%) | 99 (72%) | 172 (71%) | 140 (57%) | 558 (67%) |
| other | 19 (23%) | 40 (33%) | 39 (28%) | 70 (29%) | 105 (43%) | 273 (33%) |
| Ethnicity, n (%) | ||||||
| Caucasian | 70 (84%) | 92 (75%) | 110 (80%) | 199 (82%) | 190 (78%) | 661 (80%) |
| other | 13 (16%) | 31 (25%) | 28 (20%) | 43 (18%) | 55 (22%) | 170 (20%) |
| HIV transmission, n (%) | ||||||
| homosexual | 46 (55%) | 58 (47%) | 72 (52%) | 135 (56%) | 114 (47%) | 425 (51%) |
| other | 37 (45%) | 65 (53%) | 66 (48%) | 107 (44%) | 131 (54%) | 406 (49%) |
Characteristics of patients included in the study divided concerning the time of resistance testing
| . | 2001 n = 83 . | 2002 n = 123 . | 2003 n = 138 . | 2004 n = 242 . | 2005 n = 245 . | Cumulative n = 831 . |
|---|---|---|---|---|---|---|
| Male, n (%) | 61 (73%) | 90 (73%) | 113 (82%) | 183 (76%) | 193 (79%) | 640 (77%) |
| CDC C, n (%) | 36 (43%) | 42 (34%) | 45 (33%) | 60 (25%) | 68 (28%) | 251 (30%) |
| HIV subtype, n (%) | ||||||
| B | 64 (77%) | 83 (67%) | 99 (72%) | 172 (71%) | 140 (57%) | 558 (67%) |
| other | 19 (23%) | 40 (33%) | 39 (28%) | 70 (29%) | 105 (43%) | 273 (33%) |
| Ethnicity, n (%) | ||||||
| Caucasian | 70 (84%) | 92 (75%) | 110 (80%) | 199 (82%) | 190 (78%) | 661 (80%) |
| other | 13 (16%) | 31 (25%) | 28 (20%) | 43 (18%) | 55 (22%) | 170 (20%) |
| HIV transmission, n (%) | ||||||
| homosexual | 46 (55%) | 58 (47%) | 72 (52%) | 135 (56%) | 114 (47%) | 425 (51%) |
| other | 37 (45%) | 65 (53%) | 66 (48%) | 107 (44%) | 131 (54%) | 406 (49%) |
| . | 2001 n = 83 . | 2002 n = 123 . | 2003 n = 138 . | 2004 n = 242 . | 2005 n = 245 . | Cumulative n = 831 . |
|---|---|---|---|---|---|---|
| Male, n (%) | 61 (73%) | 90 (73%) | 113 (82%) | 183 (76%) | 193 (79%) | 640 (77%) |
| CDC C, n (%) | 36 (43%) | 42 (34%) | 45 (33%) | 60 (25%) | 68 (28%) | 251 (30%) |
| HIV subtype, n (%) | ||||||
| B | 64 (77%) | 83 (67%) | 99 (72%) | 172 (71%) | 140 (57%) | 558 (67%) |
| other | 19 (23%) | 40 (33%) | 39 (28%) | 70 (29%) | 105 (43%) | 273 (33%) |
| Ethnicity, n (%) | ||||||
| Caucasian | 70 (84%) | 92 (75%) | 110 (80%) | 199 (82%) | 190 (78%) | 661 (80%) |
| other | 13 (16%) | 31 (25%) | 28 (20%) | 43 (18%) | 55 (22%) | 170 (20%) |
| HIV transmission, n (%) | ||||||
| homosexual | 46 (55%) | 58 (47%) | 72 (52%) | 135 (56%) | 114 (47%) | 425 (51%) |
| other | 37 (45%) | 65 (53%) | 66 (48%) | 107 (44%) | 131 (54%) | 406 (49%) |
Baseline characteristics of all patients after division concerning their route of transmission, CDC stage, HIV subtype and ethnicity
| Baseline characteristica . | n . | % . |
|---|---|---|
| Route of transmission | ||
| homosexual/bisexual | 425 | 51.5 |
| heterosexual | 153 | 18.5 |
| endemic area | 123 | 14.9 |
| intravenous drug abuse | 41 | 5.0 |
| blood products | 4 | 0.5 |
| vertical | 2 | 0.2 |
| unknown | 78 | 9.4 |
| CDC stage | ||
| A | 324 | 41.8 |
| B | 200 | 25.8 |
| C | 251 | 32.4 |
| HIV subtype | ||
| B | 558 | 69.4 |
| CRF 02_AG | 60 | 7.5 |
| CRF 01_AE | 47 | 5.8 |
| D | 29 | 3.6 |
| C | 28 | 3.5 |
| no PCR product obtained | 82 | 10.2 |
| Ethnicity | ||
| Caucasian | 661 | 80.0 |
| African | 121 | 14.6 |
| Asian | 31 | 3.8 |
| other | 13 | 1.6 |
| Baseline characteristica . | n . | % . |
|---|---|---|
| Route of transmission | ||
| homosexual/bisexual | 425 | 51.5 |
| heterosexual | 153 | 18.5 |
| endemic area | 123 | 14.9 |
| intravenous drug abuse | 41 | 5.0 |
| blood products | 4 | 0.5 |
| vertical | 2 | 0.2 |
| unknown | 78 | 9.4 |
| CDC stage | ||
| A | 324 | 41.8 |
| B | 200 | 25.8 |
| C | 251 | 32.4 |
| HIV subtype | ||
| B | 558 | 69.4 |
| CRF 02_AG | 60 | 7.5 |
| CRF 01_AE | 47 | 5.8 |
| D | 29 | 3.6 |
| C | 28 | 3.5 |
| no PCR product obtained | 82 | 10.2 |
| Ethnicity | ||
| Caucasian | 661 | 80.0 |
| African | 121 | 14.6 |
| Asian | 31 | 3.8 |
| other | 13 | 1.6 |
aWhere subtotals do not equal 831 (the number of study participants) this indicates that data were not provided for some patients.
Baseline characteristics of all patients after division concerning their route of transmission, CDC stage, HIV subtype and ethnicity
| Baseline characteristica . | n . | % . |
|---|---|---|
| Route of transmission | ||
| homosexual/bisexual | 425 | 51.5 |
| heterosexual | 153 | 18.5 |
| endemic area | 123 | 14.9 |
| intravenous drug abuse | 41 | 5.0 |
| blood products | 4 | 0.5 |
| vertical | 2 | 0.2 |
| unknown | 78 | 9.4 |
| CDC stage | ||
| A | 324 | 41.8 |
| B | 200 | 25.8 |
| C | 251 | 32.4 |
| HIV subtype | ||
| B | 558 | 69.4 |
| CRF 02_AG | 60 | 7.5 |
| CRF 01_AE | 47 | 5.8 |
| D | 29 | 3.6 |
| C | 28 | 3.5 |
| no PCR product obtained | 82 | 10.2 |
| Ethnicity | ||
| Caucasian | 661 | 80.0 |
| African | 121 | 14.6 |
| Asian | 31 | 3.8 |
| other | 13 | 1.6 |
| Baseline characteristica . | n . | % . |
|---|---|---|
| Route of transmission | ||
| homosexual/bisexual | 425 | 51.5 |
| heterosexual | 153 | 18.5 |
| endemic area | 123 | 14.9 |
| intravenous drug abuse | 41 | 5.0 |
| blood products | 4 | 0.5 |
| vertical | 2 | 0.2 |
| unknown | 78 | 9.4 |
| CDC stage | ||
| A | 324 | 41.8 |
| B | 200 | 25.8 |
| C | 251 | 32.4 |
| HIV subtype | ||
| B | 558 | 69.4 |
| CRF 02_AG | 60 | 7.5 |
| CRF 01_AE | 47 | 5.8 |
| D | 29 | 3.6 |
| C | 28 | 3.5 |
| no PCR product obtained | 82 | 10.2 |
| Ethnicity | ||
| Caucasian | 661 | 80.0 |
| African | 121 | 14.6 |
| Asian | 31 | 3.8 |
| other | 13 | 1.6 |
aWhere subtotals do not equal 831 (the number of study participants) this indicates that data were not provided for some patients.
| . | n . | % . | 95% CI . | Mutations . |
|---|---|---|---|---|
| Altogether | 75 | 9.0 | 7.1–11.0 | see below |
| NRTI-associated mutations (including revertants) | 45 | 5.4 | 3.9–7.0 | M41L, A62V, D67N, T69D/N, L74V, V75I, M184V, L210W, K219Q |
| NNRTI-associated mutations | 25 | 3.0 | 1.8–4.2 | K103N, V106A, V108I, Y181C/E, G190A |
| PI-associated mutations | 20 | 2.4 | 1.4–3.4 | D30N, M46I/L, V82A/F/T, I84V, L90M |
| Revertants | 32 | 3.9 | 2.5–5.2 | T215A/C/D/E/L/N/S/V |
| Two-class resistance | 7 | 0.8 | 0.2–1.5 | combinations of the above mentioned |
| Three-class resistance | 4 | 0.5 | 0.01–1.0 | mutations |
| . | n . | % . | 95% CI . | Mutations . |
|---|---|---|---|---|
| Altogether | 75 | 9.0 | 7.1–11.0 | see below |
| NRTI-associated mutations (including revertants) | 45 | 5.4 | 3.9–7.0 | M41L, A62V, D67N, T69D/N, L74V, V75I, M184V, L210W, K219Q |
| NNRTI-associated mutations | 25 | 3.0 | 1.8–4.2 | K103N, V106A, V108I, Y181C/E, G190A |
| PI-associated mutations | 20 | 2.4 | 1.4–3.4 | D30N, M46I/L, V82A/F/T, I84V, L90M |
| Revertants | 32 | 3.9 | 2.5–5.2 | T215A/C/D/E/L/N/S/V |
| Two-class resistance | 7 | 0.8 | 0.2–1.5 | combinations of the above mentioned |
| Three-class resistance | 4 | 0.5 | 0.01–1.0 | mutations |
| . | n . | % . | 95% CI . | Mutations . |
|---|---|---|---|---|
| Altogether | 75 | 9.0 | 7.1–11.0 | see below |
| NRTI-associated mutations (including revertants) | 45 | 5.4 | 3.9–7.0 | M41L, A62V, D67N, T69D/N, L74V, V75I, M184V, L210W, K219Q |
| NNRTI-associated mutations | 25 | 3.0 | 1.8–4.2 | K103N, V106A, V108I, Y181C/E, G190A |
| PI-associated mutations | 20 | 2.4 | 1.4–3.4 | D30N, M46I/L, V82A/F/T, I84V, L90M |
| Revertants | 32 | 3.9 | 2.5–5.2 | T215A/C/D/E/L/N/S/V |
| Two-class resistance | 7 | 0.8 | 0.2–1.5 | combinations of the above mentioned |
| Three-class resistance | 4 | 0.5 | 0.01–1.0 | mutations |
| . | n . | % . | 95% CI . | Mutations . |
|---|---|---|---|---|
| Altogether | 75 | 9.0 | 7.1–11.0 | see below |
| NRTI-associated mutations (including revertants) | 45 | 5.4 | 3.9–7.0 | M41L, A62V, D67N, T69D/N, L74V, V75I, M184V, L210W, K219Q |
| NNRTI-associated mutations | 25 | 3.0 | 1.8–4.2 | K103N, V106A, V108I, Y181C/E, G190A |
| PI-associated mutations | 20 | 2.4 | 1.4–3.4 | D30N, M46I/L, V82A/F/T, I84V, L90M |
| Revertants | 32 | 3.9 | 2.5–5.2 | T215A/C/D/E/L/N/S/V |
| Two-class resistance | 7 | 0.8 | 0.2–1.5 | combinations of the above mentioned |
| Three-class resistance | 4 | 0.5 | 0.01–1.0 | mutations |
To identify risk factors that were associated with a trend to higher PRD rate, we divided the patients with regard to the following characteristics: (i) CDC stage (A and B versus C); (ii) sex (male versus female); (iii) ethnicity (Caucasian versus other); (iv) route of transmission (homosexual contact versus other); and (v) HIV subtype (subtype B versus subtypes other than B).
PDR was detected in 20 (7.9%; CI 4.6–11.3) of the 251 patients with CDC C compared with 55 (9.5%; CI 7.1–11.7) of the 580 patients with stage CDC A or B (P = 0.52). PDR rate in patients classified in CDC stage C increased from 2.8% (1/36) in 2001 to 11.7% (7/60) in 2004, but decreased to 7.4% (5/68) in 2005. The trend from 2001 to 2005 was not significant (P = 0.14). PDR rate in patients in CDC stage A or B increased from 6.4% (3/47) in 2001 to 9.6% (17/177) in 2005 with the highest PDR rate in 2004 (11.5%; 21/182), but the trend was also not significant (P = 0.19).
In 62 (9.7%; CI 7.4–11.9) of the 640 men and 13 (6.8%; CI 3.2–10.4) of the 191 women, PDR was detected over the period. This difference of PDR between the genders was not significant (P = 0.28). In the trend analysis, PDR rate increased in women from 1/22 (4.5%; CI 0–13.2) in 2001 to 5/49 (10.2%; CI 1.7–18.7) in 2005 but was not significant (P = 0.08). In men, PDR rate increased from 3/61(4.9%; CI 0–10.3) to 17/193 (8.8%; CI 4.8–12.8) and was also not significant (P = 0.19). In the next step, we studied HIV subtypes. Differentiation was made between patients infected with HIV subtype B and non-B HIV subtypes. Of the 831 patients, 558 (67%) were infected with HIV subtype B and 273 (33%) with other subtypes. Over the study period, PDR was observed in 54/558 (9.7%; CI 7.2–12.1) patients infected with HIV subtype B and 21/273 (7.7%; CI 4.5–10.8) infected with other subtypes (P = 0.4). The trend analysis showed a significant increase in the PDR rate in patients infected with non-B subtypes. Although none (0/19) of the patients infected with other subtypes than B showed PDR in 2001, PDR rate increased during the period [2002 2/40 (5.0%; CI 0–11.7), 2003 1/39 (2.6%; CI 0–7.5), 2004 7/70 (10%; CI 2.9–17.1)] to 11/105 (10.5%; CI 4.6–16.3) in 2005. This trend was significant (P = 0.02). In patients infected with HIV subtype B, PDR rate increased from 4/64 (6.3%; CI 0.3–12.2) in 2001 to 11/140 (7.9%; CI 3.4–12.2) in 2005. Trend analysis for these patients did not reach the significance level (P = 0.31). Trends of both patient groups are shown in Figure 1(c).
HIV is transmitted via different ways. We divided all patients according to their way of transmission. The first group included all patients whose risk factor was homosexual contact (n = 425), all other patients we included in the second group (n = 406). PDR rate was 10.4% (44/424; CI 7.5–13.3) in the first group compared with 7.6% (31/407; CI 5.1–10.2) in the second group (P = 0.2). While the trend to higher PDR rate was not significant in the first group [2001 3/46 (6.5%; CI 0–13.6), 2002 6/58 (10.3%; CI 2.5–18.2), 2003 7/72 (9.7%; CI 2.9–16.6), 2004 20/135 (14.8%; CI 8.8–20.8) and 2005 8/114 (7.0%; CI 2.3–11.7), P = 0.41], the second group showed a significant trend to higher PDR rate during the study period [2001 1/37 (2.7%; CI 0–7.9), 2002 3/65 (4.6%; CI 0–9.7), 2003 5/66 (7.6%; CI 1.2–13.9), 2004 8/107 (7.5%; CI 2.5–12.6) and 2005 14/131 (10.7%; CI 5.4–15.9), P = 0.03].
To clarify if PDR rates are different between Caucasian patients and other patients, all patients were divided according to their ethnicity. The first group included Caucasian patients, the second group included all other patients. Overall, PDR rate was 9.8% (65/661; CI 7.6–12.1) in Caucasian patients compared with 5.9% (10/170; CI 2.3–19.1) in the other patients (P = 0.13). While no significant trend to higher PDR rate was observed for Caucasian patients [2001 4/70 (5.7%; CI 2.8–11.2), 2002 8/92 (8.7%; CI 2.9–14.4), 2003 11/110 (10%; CI 4.4–15.6), 2004 26/199 (13.1%; CI 8.4–17.7) and 2005 16/190 (8.4%; CI 4.5–12.4), P = 0.22], the trend was significant in the second group [2001 0/13 (0%), 2002 1/31 (3.2%; CI 0–9.4), 2003 1/28 (3.6%; CI 0–10.4), 2004 2/43 (4.7%; CI 0–10.9) and 2005 6/55 (10.9%; CI 2.7–10.9), P = 0.04]. The trends are shown in Figure 1(d).
A significant association was found for these three factors: HIV subtype with ethnicity, P < 0.001; HIV subtype with HIV transmission route, P < 0.001; ethnicity with HIV transmission route, P < 0.001.
Discussion
The RESINA project is a prospective multicentre study on the epidemiology of PDR and treatment outcome in Nordrhein-Westfalen, Germany. PDR was determined in chronically infected patients at the time point of initiation of HAART. The reason for this approach was to give an estimation of prevalence in the largest subgroup of cases in clinical routine, in contrast to the rare patients with seroconversion. Here, we analysed PDR rates in different groups from 2001 to 2005. A total of 831 treatment-naive chronically HIV-infected cases was studied; compared with other studies more patients were included over a longer observation period.6,8,18–22 The result of this study showed that 9.0% of patients who had never been exposed to antiretroviral therapy carried mutations conferring HIV drug resistance. This finding is consistent with other reports.18,23–26
A trend to higher PDR rates was observed in our study (from 4.8% in 2001 to 9.0% in 2005) but did not reach the level of significance (P = 0.08). The highest PDR was observed in 2004 with 11.6% and decreased in 2005 (to 9%). This may indicate a stabilization of prevalence at a rate of 9–10%, but further studies are needed to support this assumption. Still, we found an overall increase in prevalence. This result is consistent with several but not all studies. While a stable or declining prevalence of PDR was detected in some studies, others showed an increase in PDR prevalence.8,18,21,24,27,28 A remarkable increase in PDR in treatment-naive patients was observed in the UK. During the study period from 1996 to 2003, PDR rate increased from 11.0% to 19.2%.24 Our result reflects also the discrepancy between these studies. While the prevalence of PDR doubled in value during the study period, the difference did not reach the level of significance. A reason for not reaching the level of significance could be the different numbers of patients who were included in the different years. While in the first year (2001) only 83 patients were included into the study, the number of patients increased every year, so that in 2005 nearly thrice as many patients (245) were included compared with 2001.
After dividing the PDR concerning their drug classes, the highest PDR rate was found for NRTIs (5.4%) followed by NNRTIs (3.0%) and PIs (2.4%). None of the drug classes reached the level of significance in the trend analysis, but the PDR rates for PIs (0% in 2001; 2.4% in 2005; P = 0.1) and for NNRTIs (0% in 2001, 3.7% in 2005; P = 0.07) increased during the observation period.
In the next step, we studied PDR rates in subgroups. PDR did not depend on CDC stage or gender overall. The trend analysis also showed that CDC stage and gender are not risk factors that are associated with a trend of increased PDR rate during the study period.
Factors that were associated with a significant trend to higher PDR rate were ethnicity other than Caucasian, infection with HIV subtypes other than B and way of transmission other than homosexual contact. These findings are in contrast to published data. In several studies the following factors were associated with an increased risk for PDR: Caucasian, infection with HIV subtype B, homosexual contact, and antiretroviral medication intake of the index person.23,29 In 2001, PDR was detected only in Caucasian patients (5.7%). During the observation period PDR rate in these individuals increased up to 8.4% (P = 0.22). In patients with another ethnicity PDR rate developed faster. While PDR was not detected in 2001, the PDR rate reached 10.9% (6/55) in 2005. The proportion of patients who were not Caucasian increased from 16% (13/83) to 22% (55/245) during the study period. In case these developments continue, the PDR rate in treatment-naive patients would increase further in the near future.
While in none of the patients infected with non-B subtype was PDR detectable in 2001, the PDR rate increased to 10.5% (11/105) in 2005. The proportion of patients who were infected with HIV non-B subtype increased from 23% (19/83) in 2001 to 43% (105/245) in 2005. Taking a look at these both developments, PDR rates may rise in the next years faster than observed in the past. PDR rates were similar in patients with different risk factors for acquisition of HIV overall. But an important result was noticed in the trend analysis. While the PDR rate was stable in homosexual patients, the PDR rate increased in non-homosexual patients from 2.7% (1/37) in 2001 to 10.7% (14/131) in 2005 (P = 0.03). These results indicate a change in risk factors that are associated with higher PDR.
Taking our results together, an increase in PDR rate could be possible in the future if the observed developments continue. We showed that the proportion of patients who are infected with HIV subtype other than B increased and a significant trend to higher PDR rate was detected in this group. These tendencies were found also for patients who were not Caucasian and for patients who had a risk factor for acquisition HIV other than homosexual contact. These three factors were significantly associated, so that these subgroups are likely to contain similar patient groups. Drug resistance testing is recommended for patients failing antiretroviral therapy and for HIV-infected pregnant women.30 Recently updated guidelines recommend drug-susceptibility testing for patients presenting with recent infection (<1 year) and for all newly diagnosed cases when the regional prevalence of drug resistance increases to > 5% to 10% in an area.31 Moreover, the results of modelling studies have suggested that offering genotypic resistance testing before the initiation of therapy was cost-effective in a US healthcare setting at a 8.3% prevalence of baseline resistance.32 Here, we detected PDR in 9.0% of the patients. Furthermore, HAART guided by resistance testing had similar efficacy in patients with PDR as compared with patients with wild-type virus.13 These facts support the demand of resistance testing before initiation of antiretroviral therapy.
Finally, most HIV specialists of the largest state in Germany worked together for this prospective study. By covering a defined geographical region, selection bias is likely to be low in our investigation. Therefore, the PDR rates and trends in the present study reflect the development in treatment-naive patients in Germany. Here, we see a change in risk factors that are associated with PDR.
In conclusion, PDR rates are increasing in subgroups. A trend to significantly higher PDR rates was infection with HIV subtype non-B, ethnicity other than Caucasian and other risk factors for acquisition of HIV than by homosexual contact. The positive effect of resistance testing in the therapy of treatment-naive patients is known. Our results support the demand of resistance testing before therapy initiation in regard to the increasing PDR rates in subgroups.
Funding
The RESINA study was supported by a grant of the German Ministry of Health and Social Security (grant no. AZ 319-4476-02/3). A preparatory study was supported by the Heinz Ansmann Foundation for AIDS research.
Acknowledgements
We wish to thank Claudia Müller for valuable help in data acquisition.
Study centres: P. Arbter, Krefeld; R. Baumann, Neuss; I. Becker-Boost, Duisburg; A. Busch, S. Christensen, Münster; H. Carls, Düsseldorf; G. Fätkenheuer, Köln; B. Gantke, Düsseldorf; R. Gippert, P. Hartmann, H. Quaing, Münster; M. Grüneberg, Münster; M. Oette, S. Koch, A. Sagir, D. Häussinger, Düsseldorf; I. Herrmann, Köln; M. Hower, Dortmund; K. Isernhagen, K. Römer, Köln; H. Knechten, P. Braun, R. Ehret, Aachen; W. Köthemann, A. Neuwirth, Köln; F. Kwirant, Duisburg; U. Marder, D. Petry, A. Strehlow, Düsseldorf; S. Mauruschat, Wuppertal; S. Mauss, G. Schmutz, Düsseldorf; V. Miasnikov, Düsseldorf; T. Schumacher, D. Mitrenga, Köln; A. Mutz, Osnabrück; M. Paffenholz, Köln; M. Reith, Düsseldorf; K. Remberg, Krefeld; A. Rieke, Koblenz; J. K. Rockstroh, Bonn; M. Sandmann, J. Velardi, Wuppertal; M. Schäfer, Bielefeld; S. Schoelzel, Troisdorf; S. Scholten, Köln; T. Scholten, Hagen; S. Schons, Düsseldorf; D. Schuster, K. Schuster, Wuppertal; J. Stechel, Köln; M. Wichmann, Köln; W. Wiesel, A. Theisen, Köln.
Transparency declarations
None to declare.



