-
PDF
- Split View
-
Views
-
Cite
Cite
Judith N. Steenbergen, Jeff Alder, Grace M. Thorne, Francis P. Tally, Daptomycin: a lipopeptide antibiotic for the treatment of serious Gram-positive infections, Journal of Antimicrobial Chemotherapy, Volume 55, Issue 3, March 2005, Pages 283–288, https://doi.org/10.1093/jac/dkh546
Close - Share Icon Share
Abstract
Infections caused by drug-resistant pathogens are on the rise. Daptomycin, a cyclic lipopeptide with activity against most Gram-positive pathogens, including vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus, is a newly US-FDA approved antimicrobial for complicated skin and skin structure infections (cSSSI). Daptomycin has a unique mechanism of action that results in destruction of the membrane potential. The rapid bactericidal activity of daptomycin makes it an attractive antibiotic for serious Gram-positive infections.
Introduction
The increase in infections caused by Gram-positive pathogens and the rise in antibiotic-resistant bacterial strains have prompted the need for novel antibiotics.1,2 Recent reports indicate that more than 25% of Staphylococcus aureus infections in Europe are caused by methicillin-resistant S. aureus (MRSA), and the majority of these isolates are resistant to additional antibiotics.3 The incidence of MRSA varies greatly by country. Over 50% of S. aureus isolates in Portugal and Italy are methicillin-resistant, isolates in England, Greece, and France have MRSA rates around 25%, whereas the Netherlands and Switzerland have the lowest incidence of MRSA.3 Vancomycin has been an effective antibiotic against MRSA; however, the increased use of vancomycin has led to the development of isolates with reduced susceptibility. The mechanism of reduced susceptibility to vancomycin in S. aureus has not been fully elucidated and appears to be heterogeneous. Reduced susceptibility to vancomycin is correlated with alterations in the bacterial cell wall leading to significantly thicker and more disorganized cell walls.4 These thicker cell walls may sequester the vancomycin from reaching the target nascent cell wall precursors.4 Additional in vitro studies have linked development of vancomycin reduced susceptibility with phenotypic changes such as loss of haemolysis and the mecA gene, and genotypic changes such as the presence of either the group I or group II polymorphism in the agr gene locus.5–7
To date, three vancomycin-resistant S. aureus strains (VRSA) have been isolated in the United States.8–11 Both the Pennsylvania and the New York strains were isolated from patients not on vancomycin therapy.9,12 Therefore, the need for new potent antimicrobial agents with MRSA activity is essential.
Mechanism of action
Daptomycin, a fermentation product produced by Streptomyces roseosporus, is a cyclic lipopeptide antibiotic with potent bactericidal activity against most Gram-positive organisms including multiple antibiotic-resistant and -susceptible strains.13–21 Daptomycin was recently approved in the United States for the treatment of complicated skin and skin structure infections (cSSSI) associated with S. aureus (methicillin-susceptible, MSSA, and methicillin-resistant, MRSA), Streptococcus pyogenes, Streptococcus agalactiae, Streptococcus dysgalactiae subsp. equisimilis and Enterococcus faecalis (vancomycin-susceptible only). Below, we discuss the in vitro potency of daptomycin against a range of other organisms including vancomycin-resistant E. faecalis and Enterococcus faecium.
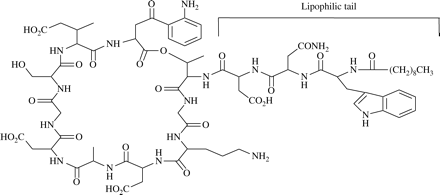
The unique structure of daptomycin consists of a 13-member amino acid cyclic lipopeptide with a decanoyl side-chain (Figure 1). This distinctive structure confers a novel mechanism of action.22 The proposed mechanism involves insertion of the lipophilic daptomycin tail into the bacterial cell membrane, causing rapid membrane depolarization and a potassium ion efflux. This is followed by arrest of DNA, RNA and protein synthesis resulting in bacterial cell death (Figure 2).22–24 The bactericidal effect of daptomycin is rapid with greater than 99.9% of both MRSA and MSSA bacteria dead in less than 1 h.25,26 This rapid cell death does not result in rapid bacterial cell lysis.24 Daptomycin also remains bactericidal (99.9% kill within 24 h) against stationary phase cultures of both MSSA and MRSA present at high density (109 cfu) in a simulated endocardial vegetation model.27
Microbiology
In vitro potency has been demonstrated for daptomycin against a range of aerobic and anaerobic Gram-positive bacteria including multidrug-resistant strains.13–21,28 MIC90 values along with MIC ranges for select pathogens can be found in Table 1. The table shows data from two recent studies illustrating the conserved MIC ranges and values for both European strains and isolates collected worldwide. Daptomycin's spectrum of activity encompasses the difficult to treat antibiotic-resistant organisms including methicillin-resistant and -susceptible Staphylococcus aureus (MRSA, MSSA), glycopeptide-intermediate S. aureus (GISA), methicillin-resistant coagulase-negative Staphylococcus spp. (CoNS), and vancomycin-resistant enterococci (VRE).13–21 Daptomycin demonstrated potency against the recently isolated vancomycin-resistant S. aureus as well as linezolid and quinupristin/dalfopristin-resistant S. aureus and E. faecium.14,17–20,24 Furthermore, daptomycin is also effective against a variety of streptococcal groups such as the β-haemolytic streptococci including S. pyogenes (Group A) and S. agalactiae (Group B) as well as other Streptococcus spp.13–15,20,21 Along with the commonly isolated Gram-positive organisms, daptomycin is also potent against Corynebacterium jeikeium, and a variety of anaerobic species including Peptostreptococcus spp., Clostridium perfringens, Clostridium difficile, and Propionibacterium acnes (Table 1).28 Drug synergy with daptomycin has been described in vitro with aminoglycosides and rifampicin antibiotics.29
Resistance
Daptomycin's efficacy is enhanced by the near absence of antibiotic resistance as verified by both in vitro and clinical studies.30 Resistance to daptomycin has been difficult to generate in the laboratory both in single passage and serial passage experi ments.30 The emergence of resistance was <0.2% across the entire set of Phase II and III clinical trials with over 1000 daptomycin-treated patients. The reason for this decrease in susceptibility is unknown and no transferable elements conferring daptomycin resistance have been isolated.
Pharmacology
Analysis of daptomycin pharmacodynamics determined that a once-daily dosing regimen increases the efficacy and safety of daptomycin.31In vitro and in vivo analysis established that daptomycin is effective in a concentration-dependent manner, has a long half-life (8 h), and demonstrates a prolonged post-antibiotic effect up to 6.8 h (Table 2).32 These findings resulted in a once a day dosing regimen recommendation of 4 mg/kg for complicated skin and skin structure infections (cSSSI) in the United States.
Once-daily dosing of daptomycin results in linear pharmacokinetics with minimal drug accumulation.31 Daptomycin distributes primarily in the plasma, with penetration to vascular tissues (Table 3). Daptomycin does not cross the blood–brain barrier and does not penetrate the cerebrospinal fluid of normal individuals. However, there was a 5% penetration (relative to serum) of daptomycin into the cerebrospinal fluid of rabbits with Streptococcus pneumoniae meningitis, resulting in clearance of the infection in this model.33 Daptomycin is primarily renally excreted, with the majority of the drug remaining intact in the urine.31 Since daptomycin is excreted through the kidneys, the dosing interval is increased to every 48 h in patients with severe renal impairment defined as a creatinine clearance of <30 mL/min. Because of daptomycin's unique mechanism of action and because it is not metabolized by cytochrome p450 or other hepatic enzymes, no antagonistic drug interactions have been observed.
Pre-clinical studies
In pre-clinical studies, daptomycin treatment has been linked to fully reversible skeletal muscle toxicity with no effect on smooth or cardiac muscle. Animal studies determined that both degenerative and regenerative changes are observed in skeletal muscle with no rhabdomyolysis.31 These effects, which can be associated with elevated creatine phosphokinase (CPK) levels, are fully reversible after cessation of daptomycin use and were not statistically significant when compared with comparator.31 The numbers of side effects for patients receiving daptomycin were comparable to standard therapy and less than 2% of patients receiving daptomycin discontinued therapy.34 The most common adverse events from the Phase III cSSSI clinical trials for daptomycin and comparator drugs are listed in Table 4.
The efficacy of daptomycin against a range of infections has been demonstrated in animal studies. Using a variety of antibiotic-resistant and -sensitive Gram-positive bacteria, daptomycin eradicated infections in the blood, muscle, kidney, heart and bone tissues of animals.35–41 These results show promise for daptomycin therapy for further clinical indications. An ongoing Phase III clinical trial is in progress to determine the efficacy of 6 mg/kg daptomycin once a day for endocarditis and bacteraemia caused by S. aureus.
Clinical studies
Daptomycin was evaluated in two large investigator-blinded, randomized, multicentre cSSSI studies in Europe, South Africa and the United States.34 Adults with cSSSI of known or suspected Gram-positive aetiology were enrolled. The predominant cSSSI infections studied included wound infections, major abscesses and ulcer infections. Daptomycin was compared with conventional therapy of a semi-synthetic penicillin (e.g. nafcillin, oxacillin, cloxacillin, or flucloxacillin) or vancomycin (for suspected MRSA). The clinical success rates for each treatment group (intent to treat, modified intent to treat, clinically evaluable, and microbiologically evaluable) are shown in Table 5.34 The study was designed to determine whether daptomycin was comparable to standard therapy and was not powered to show superiority. Therefore, statistical analysis determined that in the clinical trails, daptomycin was non-inferior to comparator therapy leading to daptomycin approval by the FDA in the United States.34 Results of the microbiologically evaluable population are detailed by pathogen in Table 6.34 Over 1000 patients were evaluated, and the following pathogens were the predominant organisms isolated; MSSA, MRSA, S. pyogenes, S. agalactiae, S. dysgalactiae subsp. equisimilis, and E. faecalis. The results from these Phase III trials confirmed the efficacy and safety of daptomycin.
Conclusions
In summary, daptomycin is a rapidly bactericidal antibiotic that is active against clinically relevant Gram-positive bacteria including antibiotic-resistant strains. Clinical data demonstrate that daptomycin is highly effective against cSSSI and ongoing clinical trials including infectious endocarditis caused by S. aureus, should expand treatment indications. The low occurrence of side effects, low resistance rates, and high potency demonstrate that daptomycin has significant clinical utility in the treatment of Gram-positive infections, including those caused by MRSA.

Daptomycin mechanism of action. Hypothetical steps: step 1, daptomycin binds to the cytoplasmic membrane in a calcium-dependent manner; step 2, daptomycin oligomerizes, disrupting the membrane; step 3, the release of intracellular ions and rapid cell death.
| Organism . | No. of strains . | MIC range (mg/L) . | MIC90 (mg/L) . | Reference . |
|---|---|---|---|---|
| Select aerobic pathogens | ||||
| Staphylococcus aureus | ||||
| oxacillin-resistant | ||||
| European isolates | 334 | 0.12–1 | 0.5 | 14 |
| worldwide isolates | 1247 | ≤ 0.12–1 | 0.5 | 21 |
| oxacillin-susceptible | ||||
| European isolates | 888 | ≤ 0.015–1 | 0.5 | 14 |
| worldwide isolates | 1955 | ≤ 0.12–2 | 0.5 | 21 |
| Coagulase-negative staphylococcia | ||||
| European isolates | 1040 | 0.03–1 | 0.5 | 14 |
| worldwide isolates | 838 | ≤ 0.12–2 | 0.5 | 21 |
| β-Haemolytic streptococci | ||||
| European isolatesb | 367 | 0.06–1 | 0.25 | 14 |
| worldwide isolates | 247 | ≤ 0.12–0.5 | 0.25 | 21 |
| Enterococcus faecalis | ||||
| vancomycin-susceptible | ||||
| European isolates | 1789 | ≤ 0.015–4 | 2 | 14 |
| worldwide isolates | 626 | ≤ 0.12–4 | 1 | 21 |
| vancomycin-resistant | ||||
| European isolates | 40 | ≤ 0.5–4 | 2 | 14 |
| worldwide isolates | 20 | 0.25–1 | 1 | 21 |
| Enterococcus faecium | ||||
| vancomycin-susceptible | ||||
| European isolates | 333 | 0.03–8 | 4 | 14 |
| worldwide isolates | 97 | ≤ 0.12–8 | 4 | 21 |
| vancomycin-resistant | ||||
| European isolates | 114 | 0.25–4 | 4 | 14 |
| worldwide isolates | 55 | 0.25–4 | 4 | 21 |
| Enterococcus spp.c | ||||
| European isolates | 160 | ≤ 0.015–4 | 4 | 14 |
| worldwide isolates | 21 | 0.5–4 | 2 | 21 |
| Corynebacterium jeikeium | 10 | 0.125–0.5 | 0.25 | 28 |
| Select anaerobic pathogens | ||||
| Actinomyces group | 22 | 0.06–16.0 | 4 | 28 |
| Bifidobacterium spp. | 13 | < 0.03–1.0 | 0.5 | 28 |
| Clostridium difficile | 18 | 0.125–1.0 | 1 | 28 |
| Clostridium perfringens | 11 | 0.06–0.5 | 0.5 | 28 |
| Lactobacillus spp.d | 37 | < 0.03–32.0 | 16 | 28 |
| Peptostreptococcus spp. | 14 | 0.125–1 | 1 | 28 |
| Propionibacterium spp. | 15 | 0.125–2 | 2 | 28 |
| Organism . | No. of strains . | MIC range (mg/L) . | MIC90 (mg/L) . | Reference . |
|---|---|---|---|---|
| Select aerobic pathogens | ||||
| Staphylococcus aureus | ||||
| oxacillin-resistant | ||||
| European isolates | 334 | 0.12–1 | 0.5 | 14 |
| worldwide isolates | 1247 | ≤ 0.12–1 | 0.5 | 21 |
| oxacillin-susceptible | ||||
| European isolates | 888 | ≤ 0.015–1 | 0.5 | 14 |
| worldwide isolates | 1955 | ≤ 0.12–2 | 0.5 | 21 |
| Coagulase-negative staphylococcia | ||||
| European isolates | 1040 | 0.03–1 | 0.5 | 14 |
| worldwide isolates | 838 | ≤ 0.12–2 | 0.5 | 21 |
| β-Haemolytic streptococci | ||||
| European isolatesb | 367 | 0.06–1 | 0.25 | 14 |
| worldwide isolates | 247 | ≤ 0.12–0.5 | 0.25 | 21 |
| Enterococcus faecalis | ||||
| vancomycin-susceptible | ||||
| European isolates | 1789 | ≤ 0.015–4 | 2 | 14 |
| worldwide isolates | 626 | ≤ 0.12–4 | 1 | 21 |
| vancomycin-resistant | ||||
| European isolates | 40 | ≤ 0.5–4 | 2 | 14 |
| worldwide isolates | 20 | 0.25–1 | 1 | 21 |
| Enterococcus faecium | ||||
| vancomycin-susceptible | ||||
| European isolates | 333 | 0.03–8 | 4 | 14 |
| worldwide isolates | 97 | ≤ 0.12–8 | 4 | 21 |
| vancomycin-resistant | ||||
| European isolates | 114 | 0.25–4 | 4 | 14 |
| worldwide isolates | 55 | 0.25–4 | 4 | 21 |
| Enterococcus spp.c | ||||
| European isolates | 160 | ≤ 0.015–4 | 4 | 14 |
| worldwide isolates | 21 | 0.5–4 | 2 | 21 |
| Corynebacterium jeikeium | 10 | 0.125–0.5 | 0.25 | 28 |
| Select anaerobic pathogens | ||||
| Actinomyces group | 22 | 0.06–16.0 | 4 | 28 |
| Bifidobacterium spp. | 13 | < 0.03–1.0 | 0.5 | 28 |
| Clostridium difficile | 18 | 0.125–1.0 | 1 | 28 |
| Clostridium perfringens | 11 | 0.06–0.5 | 0.5 | 28 |
| Lactobacillus spp.d | 37 | < 0.03–32.0 | 16 | 28 |
| Peptostreptococcus spp. | 14 | 0.125–1 | 1 | 28 |
| Propionibacterium spp. | 15 | 0.125–2 | 2 | 28 |
Includes methicillin-resistant isolates vancomycin-resistant isolates.
European isolates were S. agalactiae only.
Includes vancomycin-resistant isolates.
All Lactobacillus spp. were grown anaerobically.
| Organism . | No. of strains . | MIC range (mg/L) . | MIC90 (mg/L) . | Reference . |
|---|---|---|---|---|
| Select aerobic pathogens | ||||
| Staphylococcus aureus | ||||
| oxacillin-resistant | ||||
| European isolates | 334 | 0.12–1 | 0.5 | 14 |
| worldwide isolates | 1247 | ≤ 0.12–1 | 0.5 | 21 |
| oxacillin-susceptible | ||||
| European isolates | 888 | ≤ 0.015–1 | 0.5 | 14 |
| worldwide isolates | 1955 | ≤ 0.12–2 | 0.5 | 21 |
| Coagulase-negative staphylococcia | ||||
| European isolates | 1040 | 0.03–1 | 0.5 | 14 |
| worldwide isolates | 838 | ≤ 0.12–2 | 0.5 | 21 |
| β-Haemolytic streptococci | ||||
| European isolatesb | 367 | 0.06–1 | 0.25 | 14 |
| worldwide isolates | 247 | ≤ 0.12–0.5 | 0.25 | 21 |
| Enterococcus faecalis | ||||
| vancomycin-susceptible | ||||
| European isolates | 1789 | ≤ 0.015–4 | 2 | 14 |
| worldwide isolates | 626 | ≤ 0.12–4 | 1 | 21 |
| vancomycin-resistant | ||||
| European isolates | 40 | ≤ 0.5–4 | 2 | 14 |
| worldwide isolates | 20 | 0.25–1 | 1 | 21 |
| Enterococcus faecium | ||||
| vancomycin-susceptible | ||||
| European isolates | 333 | 0.03–8 | 4 | 14 |
| worldwide isolates | 97 | ≤ 0.12–8 | 4 | 21 |
| vancomycin-resistant | ||||
| European isolates | 114 | 0.25–4 | 4 | 14 |
| worldwide isolates | 55 | 0.25–4 | 4 | 21 |
| Enterococcus spp.c | ||||
| European isolates | 160 | ≤ 0.015–4 | 4 | 14 |
| worldwide isolates | 21 | 0.5–4 | 2 | 21 |
| Corynebacterium jeikeium | 10 | 0.125–0.5 | 0.25 | 28 |
| Select anaerobic pathogens | ||||
| Actinomyces group | 22 | 0.06–16.0 | 4 | 28 |
| Bifidobacterium spp. | 13 | < 0.03–1.0 | 0.5 | 28 |
| Clostridium difficile | 18 | 0.125–1.0 | 1 | 28 |
| Clostridium perfringens | 11 | 0.06–0.5 | 0.5 | 28 |
| Lactobacillus spp.d | 37 | < 0.03–32.0 | 16 | 28 |
| Peptostreptococcus spp. | 14 | 0.125–1 | 1 | 28 |
| Propionibacterium spp. | 15 | 0.125–2 | 2 | 28 |
| Organism . | No. of strains . | MIC range (mg/L) . | MIC90 (mg/L) . | Reference . |
|---|---|---|---|---|
| Select aerobic pathogens | ||||
| Staphylococcus aureus | ||||
| oxacillin-resistant | ||||
| European isolates | 334 | 0.12–1 | 0.5 | 14 |
| worldwide isolates | 1247 | ≤ 0.12–1 | 0.5 | 21 |
| oxacillin-susceptible | ||||
| European isolates | 888 | ≤ 0.015–1 | 0.5 | 14 |
| worldwide isolates | 1955 | ≤ 0.12–2 | 0.5 | 21 |
| Coagulase-negative staphylococcia | ||||
| European isolates | 1040 | 0.03–1 | 0.5 | 14 |
| worldwide isolates | 838 | ≤ 0.12–2 | 0.5 | 21 |
| β-Haemolytic streptococci | ||||
| European isolatesb | 367 | 0.06–1 | 0.25 | 14 |
| worldwide isolates | 247 | ≤ 0.12–0.5 | 0.25 | 21 |
| Enterococcus faecalis | ||||
| vancomycin-susceptible | ||||
| European isolates | 1789 | ≤ 0.015–4 | 2 | 14 |
| worldwide isolates | 626 | ≤ 0.12–4 | 1 | 21 |
| vancomycin-resistant | ||||
| European isolates | 40 | ≤ 0.5–4 | 2 | 14 |
| worldwide isolates | 20 | 0.25–1 | 1 | 21 |
| Enterococcus faecium | ||||
| vancomycin-susceptible | ||||
| European isolates | 333 | 0.03–8 | 4 | 14 |
| worldwide isolates | 97 | ≤ 0.12–8 | 4 | 21 |
| vancomycin-resistant | ||||
| European isolates | 114 | 0.25–4 | 4 | 14 |
| worldwide isolates | 55 | 0.25–4 | 4 | 21 |
| Enterococcus spp.c | ||||
| European isolates | 160 | ≤ 0.015–4 | 4 | 14 |
| worldwide isolates | 21 | 0.5–4 | 2 | 21 |
| Corynebacterium jeikeium | 10 | 0.125–0.5 | 0.25 | 28 |
| Select anaerobic pathogens | ||||
| Actinomyces group | 22 | 0.06–16.0 | 4 | 28 |
| Bifidobacterium spp. | 13 | < 0.03–1.0 | 0.5 | 28 |
| Clostridium difficile | 18 | 0.125–1.0 | 1 | 28 |
| Clostridium perfringens | 11 | 0.06–0.5 | 0.5 | 28 |
| Lactobacillus spp.d | 37 | < 0.03–32.0 | 16 | 28 |
| Peptostreptococcus spp. | 14 | 0.125–1 | 1 | 28 |
| Propionibacterium spp. | 15 | 0.125–2 | 2 | 28 |
Includes methicillin-resistant isolates vancomycin-resistant isolates.
European isolates were S. agalactiae only.
Includes vancomycin-resistant isolates.
All Lactobacillus spp. were grown anaerobically.
Mean (s.d.) daptomycin pharmacokinetic parameters in healthy volunteers on day 7
| Dose (mg/kg) . | Cmax (mg/L) . | Tmaxa (h) . | AUC0–24 (mg·h/L) . | t1/2 (h) . | V (L/kg) . | CLT (mL/h/kg) . | CLR (mL/h/kg) . | Ae24 (%) . |
|---|---|---|---|---|---|---|---|---|
| 4 (n=6) | 57.8 (3.0) | 0.8 (0.5, 1.0) | 494 (75) | 8.1 (1.0) | 0.096 (0.009) | 8.3 (1.3) | 4.8 (1.3) | 53.0 (10.8) |
| 6 (n=6) | 98.6 (12) | 0.5 (0.5,1.0) | 747 (91) | 8.9 (1.3) | 0.104 (0.013) | 8.1 (1.0) | 4.4 (0.3) | 47.4 (11.5) |
| 8 (n=6) | 133 (13.5) | 0.5 (0.5,1.0) | 1130 (117) | 9.0 (1.2) | 0.092 (0.012) | 7.2 (0.8) | 3.7 (0.5) | 52.1 (5.19) |
| Dose (mg/kg) . | Cmax (mg/L) . | Tmaxa (h) . | AUC0–24 (mg·h/L) . | t1/2 (h) . | V (L/kg) . | CLT (mL/h/kg) . | CLR (mL/h/kg) . | Ae24 (%) . |
|---|---|---|---|---|---|---|---|---|
| 4 (n=6) | 57.8 (3.0) | 0.8 (0.5, 1.0) | 494 (75) | 8.1 (1.0) | 0.096 (0.009) | 8.3 (1.3) | 4.8 (1.3) | 53.0 (10.8) |
| 6 (n=6) | 98.6 (12) | 0.5 (0.5,1.0) | 747 (91) | 8.9 (1.3) | 0.104 (0.013) | 8.1 (1.0) | 4.4 (0.3) | 47.4 (11.5) |
| 8 (n=6) | 133 (13.5) | 0.5 (0.5,1.0) | 1130 (117) | 9.0 (1.2) | 0.092 (0.012) | 7.2 (0.8) | 3.7 (0.5) | 52.1 (5.19) |
Cmax, maximum plasma concentration; Tmax, time to Cmax; AUC0–24, area under the concentration–time curve from 0 to 24 h; t1/2, terminal elimination half-life; V, apparent volume of distribution; CLT, systemic clearance; CLR, renal clearance; Ae24, percentage of dose recovered in urine over 24 h as unchanged daptomycin following the first dose.
Median (minimum, maximum).
Mean (s.d.) daptomycin pharmacokinetic parameters in healthy volunteers on day 7
| Dose (mg/kg) . | Cmax (mg/L) . | Tmaxa (h) . | AUC0–24 (mg·h/L) . | t1/2 (h) . | V (L/kg) . | CLT (mL/h/kg) . | CLR (mL/h/kg) . | Ae24 (%) . |
|---|---|---|---|---|---|---|---|---|
| 4 (n=6) | 57.8 (3.0) | 0.8 (0.5, 1.0) | 494 (75) | 8.1 (1.0) | 0.096 (0.009) | 8.3 (1.3) | 4.8 (1.3) | 53.0 (10.8) |
| 6 (n=6) | 98.6 (12) | 0.5 (0.5,1.0) | 747 (91) | 8.9 (1.3) | 0.104 (0.013) | 8.1 (1.0) | 4.4 (0.3) | 47.4 (11.5) |
| 8 (n=6) | 133 (13.5) | 0.5 (0.5,1.0) | 1130 (117) | 9.0 (1.2) | 0.092 (0.012) | 7.2 (0.8) | 3.7 (0.5) | 52.1 (5.19) |
| Dose (mg/kg) . | Cmax (mg/L) . | Tmaxa (h) . | AUC0–24 (mg·h/L) . | t1/2 (h) . | V (L/kg) . | CLT (mL/h/kg) . | CLR (mL/h/kg) . | Ae24 (%) . |
|---|---|---|---|---|---|---|---|---|
| 4 (n=6) | 57.8 (3.0) | 0.8 (0.5, 1.0) | 494 (75) | 8.1 (1.0) | 0.096 (0.009) | 8.3 (1.3) | 4.8 (1.3) | 53.0 (10.8) |
| 6 (n=6) | 98.6 (12) | 0.5 (0.5,1.0) | 747 (91) | 8.9 (1.3) | 0.104 (0.013) | 8.1 (1.0) | 4.4 (0.3) | 47.4 (11.5) |
| 8 (n=6) | 133 (13.5) | 0.5 (0.5,1.0) | 1130 (117) | 9.0 (1.2) | 0.092 (0.012) | 7.2 (0.8) | 3.7 (0.5) | 52.1 (5.19) |
Cmax, maximum plasma concentration; Tmax, time to Cmax; AUC0–24, area under the concentration–time curve from 0 to 24 h; t1/2, terminal elimination half-life; V, apparent volume of distribution; CLT, systemic clearance; CLR, renal clearance; Ae24, percentage of dose recovered in urine over 24 h as unchanged daptomycin following the first dose.
Median (minimum, maximum).
| Tissue . | Species . | Maximum concentration . | Percent relative to serum . | Reference . |
|---|---|---|---|---|
| Blister fluid | human | 27.6 mg/L | 68.4 | 42 |
| Blood clot–tissue | rat, rabbit | 3.5 μg/g | 72.7 | 43 |
| Peritoneal tissue chamber | rat | 11.8 mg/L | 35.1 | 44 |
| Lung | mouse, rat | 5 mg/L | 9.3 | 45 |
| BAL-ELF | mouse, rat, sheep | 1 mg/L | 2 | 45 |
| CSF | rabbit | 5.2 mg/L | 5.97 | 33 |
| Tissue . | Species . | Maximum concentration . | Percent relative to serum . | Reference . |
|---|---|---|---|---|
| Blister fluid | human | 27.6 mg/L | 68.4 | 42 |
| Blood clot–tissue | rat, rabbit | 3.5 μg/g | 72.7 | 43 |
| Peritoneal tissue chamber | rat | 11.8 mg/L | 35.1 | 44 |
| Lung | mouse, rat | 5 mg/L | 9.3 | 45 |
| BAL-ELF | mouse, rat, sheep | 1 mg/L | 2 | 45 |
| CSF | rabbit | 5.2 mg/L | 5.97 | 33 |
BAL-ELF, bronchoalveolar lavage epithelial lining fluid.
| Tissue . | Species . | Maximum concentration . | Percent relative to serum . | Reference . |
|---|---|---|---|---|
| Blister fluid | human | 27.6 mg/L | 68.4 | 42 |
| Blood clot–tissue | rat, rabbit | 3.5 μg/g | 72.7 | 43 |
| Peritoneal tissue chamber | rat | 11.8 mg/L | 35.1 | 44 |
| Lung | mouse, rat | 5 mg/L | 9.3 | 45 |
| BAL-ELF | mouse, rat, sheep | 1 mg/L | 2 | 45 |
| CSF | rabbit | 5.2 mg/L | 5.97 | 33 |
| Tissue . | Species . | Maximum concentration . | Percent relative to serum . | Reference . |
|---|---|---|---|---|
| Blister fluid | human | 27.6 mg/L | 68.4 | 42 |
| Blood clot–tissue | rat, rabbit | 3.5 μg/g | 72.7 | 43 |
| Peritoneal tissue chamber | rat | 11.8 mg/L | 35.1 | 44 |
| Lung | mouse, rat | 5 mg/L | 9.3 | 45 |
| BAL-ELF | mouse, rat, sheep | 1 mg/L | 2 | 45 |
| CSF | rabbit | 5.2 mg/L | 5.97 | 33 |
BAL-ELF, bronchoalveolar lavage epithelial lining fluid.
Incidence of adverse events that occurred in ≥ 2% of patients in either daptomycin or comparator treatment groups in Phase III cSSSI studies
| Adverse event . | Daptomycin %(n=534) . | Comparatora % (n=558) . | ||
|---|---|---|---|---|
| Gastrointestinal disorders | ||||
| constipation | 6.2 | 6.8 | ||
| nausea | 5.8 | 9.5 | ||
| diarrhoea | 5.2 | 4.3 | ||
| vomiting | 3.2 | 3.8 | ||
| dyspepsia | 0.9 | 2.5 | ||
| General disorders | ||||
| injection site reactions | 5.8 | 7.7 | ||
| fever | 1.9 | 2.5 | ||
| Nervous system disorders | ||||
| headache | 5.4 | 5.4 | ||
| insomnia | 4.5 | 5.4 | ||
| dizziness | 2.2 | 2.0 | ||
| Skin/subcutaneous disorders | ||||
| rash | 4.3 | 3.8 | ||
| pruritus | 2.8 | 3.8 | ||
| Diagnostic investigations | ||||
| abnormal liver function tests | 3.0 | 1.6 | ||
| elevated CPK | 2.8 | 1.8 | ||
| Infections | ||||
| fungal infections | 2.6 | 3.2 | ||
| urinary tract infections | 2.4 | 0.5 | ||
| Vascular disorders | ||||
| hypotension | 2.4 | 1.4 | ||
| hypertension | 1.1 | 2.0 | ||
| Renal/urinary disorders | ||||
| renal failure | 2.2 | 2.7 | ||
| Blood/lymphatic disorders | ||||
| anaemia | 2.1 | 2.3 | ||
| Respiratory disorders | ||||
| dyspnoea | 2.1 | 1.6 | ||
| Musculoskeletal disorders | ||||
| limb pain | 1.5 | 2.0 | ||
| arthralgia | 0.9 | 2.2 | ||
| Adverse event . | Daptomycin %(n=534) . | Comparatora % (n=558) . | ||
|---|---|---|---|---|
| Gastrointestinal disorders | ||||
| constipation | 6.2 | 6.8 | ||
| nausea | 5.8 | 9.5 | ||
| diarrhoea | 5.2 | 4.3 | ||
| vomiting | 3.2 | 3.8 | ||
| dyspepsia | 0.9 | 2.5 | ||
| General disorders | ||||
| injection site reactions | 5.8 | 7.7 | ||
| fever | 1.9 | 2.5 | ||
| Nervous system disorders | ||||
| headache | 5.4 | 5.4 | ||
| insomnia | 4.5 | 5.4 | ||
| dizziness | 2.2 | 2.0 | ||
| Skin/subcutaneous disorders | ||||
| rash | 4.3 | 3.8 | ||
| pruritus | 2.8 | 3.8 | ||
| Diagnostic investigations | ||||
| abnormal liver function tests | 3.0 | 1.6 | ||
| elevated CPK | 2.8 | 1.8 | ||
| Infections | ||||
| fungal infections | 2.6 | 3.2 | ||
| urinary tract infections | 2.4 | 0.5 | ||
| Vascular disorders | ||||
| hypotension | 2.4 | 1.4 | ||
| hypertension | 1.1 | 2.0 | ||
| Renal/urinary disorders | ||||
| renal failure | 2.2 | 2.7 | ||
| Blood/lymphatic disorders | ||||
| anaemia | 2.1 | 2.3 | ||
| Respiratory disorders | ||||
| dyspnoea | 2.1 | 1.6 | ||
| Musculoskeletal disorders | ||||
| limb pain | 1.5 | 2.0 | ||
| arthralgia | 0.9 | 2.2 | ||
Comparators included vancomycin (1 g iv every 12 h) and antistaphylococcal penicillins (i.e. nafcillin, oxacillin, cloxacillin, flucloxacillin; 4–12 g/day in divided doses).
Incidence of adverse events that occurred in ≥ 2% of patients in either daptomycin or comparator treatment groups in Phase III cSSSI studies
| Adverse event . | Daptomycin %(n=534) . | Comparatora % (n=558) . | ||
|---|---|---|---|---|
| Gastrointestinal disorders | ||||
| constipation | 6.2 | 6.8 | ||
| nausea | 5.8 | 9.5 | ||
| diarrhoea | 5.2 | 4.3 | ||
| vomiting | 3.2 | 3.8 | ||
| dyspepsia | 0.9 | 2.5 | ||
| General disorders | ||||
| injection site reactions | 5.8 | 7.7 | ||
| fever | 1.9 | 2.5 | ||
| Nervous system disorders | ||||
| headache | 5.4 | 5.4 | ||
| insomnia | 4.5 | 5.4 | ||
| dizziness | 2.2 | 2.0 | ||
| Skin/subcutaneous disorders | ||||
| rash | 4.3 | 3.8 | ||
| pruritus | 2.8 | 3.8 | ||
| Diagnostic investigations | ||||
| abnormal liver function tests | 3.0 | 1.6 | ||
| elevated CPK | 2.8 | 1.8 | ||
| Infections | ||||
| fungal infections | 2.6 | 3.2 | ||
| urinary tract infections | 2.4 | 0.5 | ||
| Vascular disorders | ||||
| hypotension | 2.4 | 1.4 | ||
| hypertension | 1.1 | 2.0 | ||
| Renal/urinary disorders | ||||
| renal failure | 2.2 | 2.7 | ||
| Blood/lymphatic disorders | ||||
| anaemia | 2.1 | 2.3 | ||
| Respiratory disorders | ||||
| dyspnoea | 2.1 | 1.6 | ||
| Musculoskeletal disorders | ||||
| limb pain | 1.5 | 2.0 | ||
| arthralgia | 0.9 | 2.2 | ||
| Adverse event . | Daptomycin %(n=534) . | Comparatora % (n=558) . | ||
|---|---|---|---|---|
| Gastrointestinal disorders | ||||
| constipation | 6.2 | 6.8 | ||
| nausea | 5.8 | 9.5 | ||
| diarrhoea | 5.2 | 4.3 | ||
| vomiting | 3.2 | 3.8 | ||
| dyspepsia | 0.9 | 2.5 | ||
| General disorders | ||||
| injection site reactions | 5.8 | 7.7 | ||
| fever | 1.9 | 2.5 | ||
| Nervous system disorders | ||||
| headache | 5.4 | 5.4 | ||
| insomnia | 4.5 | 5.4 | ||
| dizziness | 2.2 | 2.0 | ||
| Skin/subcutaneous disorders | ||||
| rash | 4.3 | 3.8 | ||
| pruritus | 2.8 | 3.8 | ||
| Diagnostic investigations | ||||
| abnormal liver function tests | 3.0 | 1.6 | ||
| elevated CPK | 2.8 | 1.8 | ||
| Infections | ||||
| fungal infections | 2.6 | 3.2 | ||
| urinary tract infections | 2.4 | 0.5 | ||
| Vascular disorders | ||||
| hypotension | 2.4 | 1.4 | ||
| hypertension | 1.1 | 2.0 | ||
| Renal/urinary disorders | ||||
| renal failure | 2.2 | 2.7 | ||
| Blood/lymphatic disorders | ||||
| anaemia | 2.1 | 2.3 | ||
| Respiratory disorders | ||||
| dyspnoea | 2.1 | 1.6 | ||
| Musculoskeletal disorders | ||||
| limb pain | 1.5 | 2.0 | ||
| arthralgia | 0.9 | 2.2 | ||
Comparators included vancomycin (1 g iv every 12 h) and antistaphylococcal penicillins (i.e. nafcillin, oxacillin, cloxacillin, flucloxacillin; 4–12 g/day in divided doses).
| . | Daptomycin . | . | Comparatora . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| Population . | n . | % success . | n . | % success . | 95% CIb . | |||
| Intent-to-treat | 534 | 71.5 | 558 | 71.1 | (–5.8, 5.0) | |||
| Modified intent-to-treat | 428 | 74.5 | 471 | 74.7 | (–5.5, 5.9) | |||
| Clinically evaluable | 446 | 83.4 | 456 | 84.2 | (–4.0, 5.6) | |||
| Microbiologically evaluable | 365 | 84.7 | 396 | 85.9 | (–3.8, 6.3) | |||
| . | Daptomycin . | . | Comparatora . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| Population . | n . | % success . | n . | % success . | 95% CIb . | |||
| Intent-to-treat | 534 | 71.5 | 558 | 71.1 | (–5.8, 5.0) | |||
| Modified intent-to-treat | 428 | 74.5 | 471 | 74.7 | (–5.5, 5.9) | |||
| Clinically evaluable | 446 | 83.4 | 456 | 84.2 | (–4.0, 5.6) | |||
| Microbiologically evaluable | 365 | 84.7 | 396 | 85.9 | (–3.8, 6.3) | |||
Cloxacillin, flucloxacillin, nafcillin, oxacillin or vancomycin.
95% confidence interval around the difference in success rate (comparator—daptomycin).
| . | Daptomycin . | . | Comparatora . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| Population . | n . | % success . | n . | % success . | 95% CIb . | |||
| Intent-to-treat | 534 | 71.5 | 558 | 71.1 | (–5.8, 5.0) | |||
| Modified intent-to-treat | 428 | 74.5 | 471 | 74.7 | (–5.5, 5.9) | |||
| Clinically evaluable | 446 | 83.4 | 456 | 84.2 | (–4.0, 5.6) | |||
| Microbiologically evaluable | 365 | 84.7 | 396 | 85.9 | (–3.8, 6.3) | |||
| . | Daptomycin . | . | Comparatora . | . | . | |||
|---|---|---|---|---|---|---|---|---|
| Population . | n . | % success . | n . | % success . | 95% CIb . | |||
| Intent-to-treat | 534 | 71.5 | 558 | 71.1 | (–5.8, 5.0) | |||
| Modified intent-to-treat | 428 | 74.5 | 471 | 74.7 | (–5.5, 5.9) | |||
| Clinically evaluable | 446 | 83.4 | 456 | 84.2 | (–4.0, 5.6) | |||
| Microbiologically evaluable | 365 | 84.7 | 396 | 85.9 | (–3.8, 6.3) | |||
Cloxacillin, flucloxacillin, nafcillin, oxacillin or vancomycin.
95% confidence interval around the difference in success rate (comparator—daptomycin).
| . | Success rate, n/N (%) . | . | |
|---|---|---|---|
| Pathogen . | Daptomycin . | comparatora . | |
| Methicillin-susceptible Staphylococcus aureus (MSSA)b | 170/198 (85.9) | 180/207 (87.0) | |
| Methicillin-resistant Staphylococcus aureus (MRSA)b | 21/28 (75.0) | 25/36 (69.4) | |
| Streptococcus pyogenes | 79/84 (94.0) | 80/88 (90.9) | |
| Streptococcus agalactiae | 23/27 (85.2) | 22/29 (75.9) | |
| Streptococcus dysgalactiae subsp. equisimilis | 8/8 (100) | 9/11 (81.8) | |
| Enterococcus faecalis (vancomycin-susceptible only)b | 27/37 (73.0) | 40/53 (75.5) | |
| . | Success rate, n/N (%) . | . | |
|---|---|---|---|
| Pathogen . | Daptomycin . | comparatora . | |
| Methicillin-susceptible Staphylococcus aureus (MSSA)b | 170/198 (85.9) | 180/207 (87.0) | |
| Methicillin-resistant Staphylococcus aureus (MRSA)b | 21/28 (75.0) | 25/36 (69.4) | |
| Streptococcus pyogenes | 79/84 (94.0) | 80/88 (90.9) | |
| Streptococcus agalactiae | 23/27 (85.2) | 22/29 (75.9) | |
| Streptococcus dysgalactiae subsp. equisimilis | 8/8 (100) | 9/11 (81.8) | |
| Enterococcus faecalis (vancomycin-susceptible only)b | 27/37 (73.0) | 40/53 (75.5) | |
Vancomycin or semi-synthetic penicillins (e.g. nafcillin, oxacillin, cloxacillin or flucloxacillin).
As determined by the central laboratory.
| . | Success rate, n/N (%) . | . | |
|---|---|---|---|
| Pathogen . | Daptomycin . | comparatora . | |
| Methicillin-susceptible Staphylococcus aureus (MSSA)b | 170/198 (85.9) | 180/207 (87.0) | |
| Methicillin-resistant Staphylococcus aureus (MRSA)b | 21/28 (75.0) | 25/36 (69.4) | |
| Streptococcus pyogenes | 79/84 (94.0) | 80/88 (90.9) | |
| Streptococcus agalactiae | 23/27 (85.2) | 22/29 (75.9) | |
| Streptococcus dysgalactiae subsp. equisimilis | 8/8 (100) | 9/11 (81.8) | |
| Enterococcus faecalis (vancomycin-susceptible only)b | 27/37 (73.0) | 40/53 (75.5) | |
| . | Success rate, n/N (%) . | . | |
|---|---|---|---|
| Pathogen . | Daptomycin . | comparatora . | |
| Methicillin-susceptible Staphylococcus aureus (MSSA)b | 170/198 (85.9) | 180/207 (87.0) | |
| Methicillin-resistant Staphylococcus aureus (MRSA)b | 21/28 (75.0) | 25/36 (69.4) | |
| Streptococcus pyogenes | 79/84 (94.0) | 80/88 (90.9) | |
| Streptococcus agalactiae | 23/27 (85.2) | 22/29 (75.9) | |
| Streptococcus dysgalactiae subsp. equisimilis | 8/8 (100) | 9/11 (81.8) | |
| Enterococcus faecalis (vancomycin-susceptible only)b | 27/37 (73.0) | 40/53 (75.5) | |
Vancomycin or semi-synthetic penicillins (e.g. nafcillin, oxacillin, cloxacillin or flucloxacillin).
As determined by the central laboratory.
References
Bell, J. M. & Turnidge, J. D. (
Stefani, S. & Varaldo, P. E. (
Fluit, A. C., Wielders, C. L., Verhoef, J. et al. (
Cui, L., Ma, X., Sato, K. et al. (
Verdier, I., Reverdy, M. E., Etienne, J. et al. (
Sakoulas, G., Eliopoulos, G. M., Moellering, R. C. et al. (
Adhikari, R. P., Scales, G. C., Kobayashi, K. et al. (
Centers for Disease Control and Prevention. (
Centers for Disease Control and Prevention. (
Centers for Disease Control and Prevention. (
Mutnick, A. H., Biedenbach, D. J. & Jones, R. N. (
Kacica, M. A., Scott, C., Johnson, G. et al. (
Barry, A. L., Fuchs, P. C. & Brown, S. D. (
Critchley, I. A., Draghi, D. C., Sahm, D. F. et al. (
Fluit, A. C., Schmitz, F. J., Verhoef, J. et al. (
Fluit, A. C., Schmitz, F. J., Verhoef, J. et al. (
Petersen, P. J., Bradford, P. A., Weiss, W. J. et al. (
Richter, S. S., Kealey, D. E., Murray, C. T. et al. (
Rybak, M. J., Hershberger, E., Moldovan, T. et al. (
Snydman, D. R., Jacobus, N. V., McDermott, L. A. et al. (
Streit, J. M., Jones, R. N. & Sader, H. S. (
Silverman, J. A., Perlmutter, N. G. & Shapiro, H. M. (
Canepari, P., Boaretti, M., del Mar Lleo, M. et al. (
Silverman, J., Harris, B., Cotroneo, N., et al. (
Cha, R. & Rybak, M. J. (
Fuchs, P. C., Barry, A. L. & Brown, S. D. (
Tedesco, K. L. & Rybak, M. J. (
Goldstein, E. J., Citron, D. M., Merriam, C. V. et al. (
Rand, K. H. & Houck, H. (
Silverman, J. A., Oliver, N., Andrew, T. et al. (
Oleson, F. B., Jr, Berman, C. L., Kirkpatrick, J. B. et al. (
Safdar, N., Andes, D. & Craig, W. A. (
Cottagnoud, P., Pfister, M., Acosta, F. et al. (
Arbeit, R. D., Maki, D., Tally, F. P. et al. (
Alder, J., Li, T., Yu, D. et al. (
Cha, R., Grucz, R. G., Jr & Rybak, M. J. (
Dandekar, P. K., Tessier, P. R., Williams, P. et al. (
Kaatz, G. W., Seo, S. M., Reddy, V. N. et al. (
Louie, A., Kaw, P., Liu, W. et al. (
Mader, J. T. & Adams, K. (
Smith, K., Cobbs, G., Dill, R. et al. (
Wise, R., Gee, T., Andrews, J. M. et al. (
Michiels, M. J. & Bergeron, M. G. (
Vaudaux, P., Francois, P., Bisognano, C. et al. (
Alder, J., Arbeit, R., Eisenstein, B., et al. (



