-
PDF
- Split View
-
Views
-
Cite
Cite
James W Ryan, Aoife S Murray, Paddy J Gilligan, James M Bisset, Chris Nolan, Audrey Doyle, Barry Emerson, Joseph M Galvin, John G Murray, MRI safety management in patients with cardiac implantable electronic devices: Utilizing failure mode and effects analysis for risk optimization, International Journal for Quality in Health Care, Volume 32, Issue 7, August 2020, Pages 431–437, https://doi.org/10.1093/intqhc/mzaa067
Close - Share Icon Share
Abstract
Cardiac implantable electronic devices (CIEDs) are increasing in prevalence. Exposing patients with CIEDs to magnetic resonance imaging (MRI) can lead to adverse outcomes. This has led certain radiology departments to not accept MRI referrals related to patients with CIEDs. Patients with MR-conditional CIEDs can be safely scanned under specific conditions. Our institution has accepted such referrals since 2014. The aim of this study was to systematically identify and reduce risk in our CIED-MRI protocol using failure mode and effects analysis (FMEA).
A multidisciplinary FMEA team was assembled and included senior stakeholders from the CIED-MRI protocol. A process map was constructed followed by risk analysis and scoring. Targeted interventions were formulated and implemented; high-risk failure modes were prioritized. A new process map and protocol were drafted and repeat risk analysis was performed. Monitoring and re-evaluation of the CIED-MRI pathway were instigated at departmental quality assurance (QA) meetings.
Interventions included direct CIED characterization using wireless technology pre-MRI, CIED programming and reprogramming in the MRI suite before and immediately after MRI reducing device downtime and continuous patient monitoring during MRI by a cardiac physiologist. The cumulative risk priority number (RPN) decreased from 1190 pre-FMEA to 492 post-FMEA.
Despite the risk of exposing CIEDs to the MR environment, patients with MR-conditional CIEDs can be safely scanned with an appropriate multidisciplinary support. We found FMEA an indispensable tool in identifying and minimizing risk with no adverse events recorded since FMEA recommendations were implemented.
Introduction
Cardiac implantable electronic devices (CIEDs) is a term that refers to pacemakers, implantable loop recorders and implantable defibrillators. The increasing prevalence of CIEDs has been documented in the literature [1–5] and noted at our institution which has seen rising numbers of magnetic resonance imaging (MRI) referrals for patients with CIEDs. It is estimated that patients with CIEDs have a 75% chance of requiring an MRI in their lifetime [5]. There are numerous potential complications when exposing CIEDs to MRI including device failure, induction of ventricular fibrillation and heating of the adjacent soft tissue [6, 7]. Patients may also have hardware from previous devices that is therapeutically inactive but can pose a risk if exposed to MRI [6]. As a result, the presence of a CIED had until recently been considered a contraindication to MRI [8, 9].
The term MR-conditional CIED refers to a device ‘that has been shown to pose no known hazards in a specified MR environment with specific conditions of use’ [10]. The 2017 Heart Rhythm Society guidelines made a class I (strong) recommendation that MR-conditional CIED patients should only undergo MRI in institutions with a standardized workflow model [9]. It is thus essential that such institutions develop, implement and intermittently improve standardized CIED-MRI protocols to minimize risk.
Scanning of MR-nonconditional CIEDs is not currently supported by device manufacturers [11–14]. There is a reluctance to perform MRI in such patients [15, 16]. Recent large-scale, prospective studies have suggested that MR-nonconditional CIEDs can also safely undergo MRI using a standardized, protocol-driven approach [8, 17].
At our institution, we have processed over 600 CIED-MRI referrals since 2014 using a defined protocol and multidisciplinary support; 350/600 patients went on to MRI. In order to minimize the inherent risk of MRI in CIED patients, a failure mode and effects analysis (FMEA) was undertaken. An FMEA is a systematic methodology that allows for proactive identification and reduction of risk in complex clinical processes [18–20]. A recent review article described FMEA in the medical setting as an ‘efficient method’ in reducing risk and ‘improving service quality’ [21]; others have noted the benefits of FMEA in complex radiology care pathways [18].
Aims
We aimed to identify and reduce risk in our CIED-MRI protocol using FMEA and to share our findings.
Methods
Our private institution operates in an inner-city, tertiary referral environment. All our CIED-MRI patients are scanned in a 1.5-T magnet. We have onsite support from cardiology, cardiac physiology, medical physics and experienced radiographers and radiologists and access to 24-hour critical care facilities. We perform MRI on patients with MR-conditional CIEDs on an inpatient and outpatient basis.
FMEAs are composed of six sequential steps [18]. First, a topic was defined. We chose to critically appraise our local CIED-MRI protocol. Second, a committed team was assembled which included 10 individuals who were clinical contributors to the protocol. The team was made up of two members from the medical physics department, the chief cardiac physiologist and the MRI clinical specialist radiographer, three representatives from the quality department and three physicians including a radiology consultant, a radiology resident and an intern. Next, a process map of the current CIED-MRI protocol was constructed (Fig. 1) which allowed the team to gain a holistic understanding of the referral pathway.
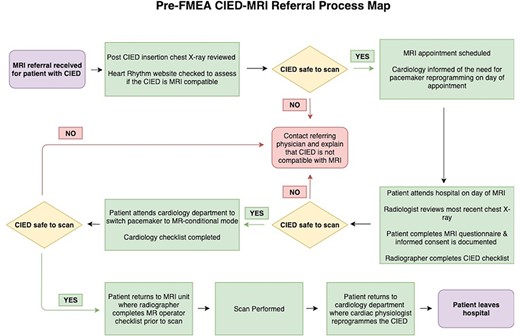
|
|
|
|
Failure mode: What could go wrong?
Failure causes: Why would failure happen?
Failure effects: What would be the consequences of failure?
Likelihood of occurrence: 1–10, 10 = very likely to occur
Likelihood of detection: 1–10, 10 = very unlikely to detect
Severity: 1–10, 10 = most severe effect
RPN: likelihood of occurrence x likelihood of detection x severity
|
|
|
|
Failure mode: What could go wrong?
Failure causes: Why would failure happen?
Failure effects: What would be the consequences of failure?
Likelihood of occurrence: 1–10, 10 = very likely to occur
Likelihood of detection: 1–10, 10 = very unlikely to detect
Severity: 1–10, 10 = most severe effect
RPN: likelihood of occurrence x likelihood of detection x severity
Fourth, a risk analysis was performed where each step on the process map was discussed and critically analysed by the FMEA team. Potential failure modes were identified; a failure mode is defined as anything that can go wrong during the completion of a step in a process [22]. An example of a failure mode would be the referring physician forgetting to mention that the patient has a CIED. ‘Likelihood of occurrence’, ‘likelihood of detection’ and ‘severity of impact’ scores were assigned to each failure mode and multiplied to yield a risk priority number (RPN) (see Table 1). RPNs provide a numeric assessment of risk for each failure mode; higher RPNs denote an increased risk. Team consensus was required when assigning RPNs.
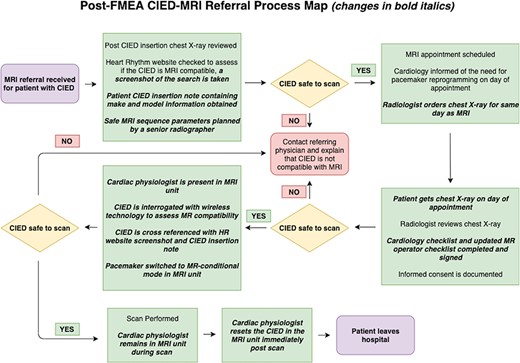
Fifth, an action plan was developed and implemented with targeted interventions aimed at addressing the highest-risk areas. A new process map was constructed including all interventions (Fig. 2). Finally, a repeat risk analysis was performed by the team post-intervention. Several methods were used to try and standardize RPN designation and limit subjectivity where possible. For example, the FMEA team utilized data from a recent departmental review of 125 CIED-MRIs when assigning ‘likelihood of occurrence’ scores [23], personal experience of team members was taken into account when assigning ‘likelihood of detection scores’, and the same team members were used for both risk analyses. Individual and cumulative RPNs were calculated to compare clinical risk in the new protocol to the original protocol. Continuous monitoring and re-evaluation of the CIED-MRI pathway was instigated at departmental quality assurance (QA) meetings.
Ethical considerations
This quality improvement project was deemed exempt from ethics review by the local institutional ethics committee.
Format
The reporting of this quality improvement project follows the proposed Standards for Quality Improvement Reporting Excellence guidelines [24].
Results
Main interventions and evolution of protocol
80% of the CIED-MRI referrals received at our institution were from external sources. The heterogeneity of information and quality in these referrals underlines the importance of having a standardized process for vetting and safety assessment. Incorrect CIED information in the referral was identified as a potential failure mode. As a result, pre-MRI CIED interrogation by a cardiac physiologist with wireless equipment was introduced to fully characterize the CIED components. In addition, a mandatory review of the patient’s CIED insertion note was implemented. This meant that staff were no longer solely reliant on the Heart Rhythm Ireland (HRI) website and chest X-ray for device characterization.
It was also noted that some referrers may not realize the risks of exposing CIEDs to MRI. To combat this, educational material has been shared locally with referrers and nationally with the Health Products Regulatory Authority (HPRA). These interventions were associated with a reduction in RPN for this failure mode from 180 pre-FMEA to 30 post-FMEA.
In some cases, CIED boxes or leads can be changed. As a result, a patient may have an MR-conditional box with an MR-nonconditional lead or vice versa. Failure to identify such cases has potential adverse consequences. The introduction of direct device interrogation and CIED insertion note review as described above was associated with an RPN reduction from 120 pre-FMEA to 60 post-FMEA for this failure mode.
The HRI website stores CIED make and model information and is a useful resource for patient-specific device characterization. However, this information is manually entered into the website and is thus open to human error. Instigation of device interrogation and CIED insertion note review decreased the RPN of this failure mode from 120 to 60. HRI is now informed of any errors or discrepancies noted on their website so that they can be corrected. In addition, we have contacted HRI and recommended the introduction of barcode scanning technology to replace manual data entry on the website.
In our pre-FMEA protocol, the patients attended the cardiology department on the day of their MRI where their CIED was set to MR-conditional mode by a cardiac physiologist. The patient was then sent to MRI. Once the MRI was complete, the patient returned to the cardiology department so that full CIED functionality could be restored before leaving the hospital. Whilst switching to MR-conditional mode CIEDs are not fully functional, this is a particularly vulnerable time for patients. The FMEA team noted that the patient may not return to the cardiology department post-MRI or could potentially have a cardiac event in an isolated area during CIED downtime, e.g. in the bathroom. To prevent such occurrences and minimize CIED downtime, the cardiac physiologist now attends the MRI suite for the duration of the patient visit, programming the CIED to MR-conditional mode, monitoring the patient continuously during the scan and reprogramming the CIED immediately post-MRI. This intervention reduced the RPN from 120 to 10 for this failure mode.
Secondary interventions
The HRI website is consulted for all CIED patient referrals to obtain make and model information. This search requires the user to select options from drop-down menus, a process that is prone to human error. The FMEA team noted this and introduced a policy whereby all HRI searches are compared to the CIED insertion note and to the findings from device interrogation on the day of the MRI, thus reducing the risk of incorrect device characterization. This new policy also facilitates the identification of errors on the HRI website. Competence-based training and CIED checklist sign-off for cardiac physiologists have been introduced to improve staff knowledge and accountability. Revision of the MR-operator checklist to include additional patient risk information has helped to ensure that patients are adequately informed pre-MRI. MRI parameter selection responsibilities have been designated to senior MR radiographers, thus decreasing the risk of inappropriate sequence selection.
Please refer to Table 1 for a step-by-step account of the risk analysis and interventions. The highest impact interventions are highlighted in grey on Table 1.
Discussion
We utilized the methodical approach afforded by FMEA to systematically identify risk within our CIED-MRI pathway, formulate interventions and develop a new protocol with an improved safety profile. The diverse nature of our team facilitated the identification of failure modes and interventions. Senior FMEA team members from radiology, medical physics, radiography, cardiology and the quality department helped ensure that interventions were implemented and adhered to locally. This project has given rise to a departmental mentality of iterative process improvement, facilitated by monthly QA meetings.
The cumulative RPN for our CIED-MRI protocol decreased from 1190 pre-FMEA to 492 post-FMEA indicating a decreased risk. To our knowledge, the benefits of FMEA in CIED-MRI protocols have not been documented in the literature. We believe that FMEA provides a defined path to risk reduction in the clinical setting and is generalizable to other MRI units and complex clinical processes outside of radiology as evidenced by prior studies [25–28].
Our MRI unit has accepted MR-conditional CIED referrals since 2014. An institutional review of 125 CIED patients who underwent MRI revealed temporary complications in three patients including diaphragmatic stimulation, transient dizziness and difficulty reprogramming the CIED post-MRI [23]. We have not experienced any long-term patient morbidity or mortality.
FMEA also allowed for the identification of several ancillary areas for improvement in the MRI suite including regular interval cardiac arrest simulation and the introduction of MRI suite safety signs to increase visitor awareness of the risks associated with MRI. Performing the FMEA in a multidisciplinary fashion led to stronger collegial links between various departments and specialities involved.
On a background of increasing demand, many hospitals still do not accept referrals from CIED patients despite evidence that such scans can be safely performed using standardized processes. Surveys performed in the UK and Ireland in 2017 and 2018, respectively, revealed that <50% of MRI units accepted referrals for patients with CIEDs [23, 29]. FMEA may provide a framework for MRI units that did not previously accept CIED-MRI referrals to plan and implement risk optimized protocols.
Limitations
There is an unavoidable element of subjectivity associated with failure mode RPN designation. The utilization of identical methodology and the same FMEA team members in both risk analyses provided consistency of interpretation and yielded pre- and post-intervention risk score that was directly comparable.
Staff members were afforded dedicated project time to participate in the FMEA, which was carried out in a cost-neutral fashion. Not all institutions may be able to provide staff with dedicated project time.
To date, we have not scanned MR-nonconditional CIEDs at our institution. However, there is evolving evidence to support this as a safe practice [8, 17].
Conclusion
We believe that lessons learnt from our FMEA study are widely applicable to other institutions given the multidisciplinary complex processes involved.
Acknowledgements
The authors wish to state that no funding was received for this study.





