-
PDF
- Split View
-
Views
-
Cite
Cite
Taiki Haga, Hiroshi Kurosawa, Junji Maruyama, Katsuko Sakamoto, Ryo Ikebe, Natsuko Tokuhira, Muneyuki Takeuchi, The prevalence and characteristics of rapid response systems in hospitals with pediatric intensive care units in Japan and barriers to their use, International Journal for Quality in Health Care, Volume 32, Issue 5, June 2020, Pages 325–331, https://doi.org/10.1093/intqhc/mzaa040
Close - Share Icon Share
Abstract
The use of pediatric rapid response systems (RRSs) to improve the safety of hospitalized children has spread in various western countries including the United States and the United Kingdom. We aimed to determine the prevalence and characteristics of pediatric RRSs and barriers to use in Japan, where epidemiological information is limited.
A cross-sectional online survey.
All 34 hospitals in Japan with pediatric intensive care units (PICUs) in 2019.
One PICU physician per hospital responded to the questionnaire as a delegate.
Prevalence of pediatric RRSs in Japan and barriers to their use.
The survey response rate was 100%. Pediatric RRSs had been introduced in 14 (41.2%) institutions, and response teams comprised a median of 6 core members. Most response teams employed no full-time members and largely comprised members from multiple disciplines and departments who served in addition to their main duties. Of 20 institutions without pediatric RRSs, 11 (55%) hoped to introduce them, 14 (70%) had insufficient knowledge concerning them and 11 (55%) considered that their introduction might be difficult. The main barrier to adopting RRSs was a perceived personnel and/or funding shortage. There was no significant difference in hospital beds (mean, 472 vs. 524, P = 0.86) and PICU beds (mean, 10 vs. 8, P = 0.34) between institutions with/without pediatric RRSs.
Fewer than half of Japanese institutions with PICUs had pediatric RRSs. Operating methods for and obstructions to RRSs were diverse. Our findings may help to popularize pediatric RRSs.
Introduction
Rapid response systems (RRSs) are medical safety systems designed to improve patient outcomes, using specialized teams of critical care-trained professionals, for early identification of hospitalized patients whose conditions are deteriorating. They provide appropriate management to prevent serious adverse events, such as cardiac arrest and unexpected death [1]. Although cardiac arrest in hospitalized children is rare, their outcomes are often poor despite appropriate resuscitation. Previously, two large studies reported that the rates of survival of children after hospital discharge following cardiac arrest were only 24% among 544 children [2] and 27% among 880 children [3] and of the survivors, 34% had severe neurological dysfunction [3]. Therefore, it is important to intervene and halt deterioration before cardiorespiratory arrest occurs. RRSs could effectively improve children’s outcomes. Single-center before-and-after studies have shown decreased rates of respiratory arrest outside the pediatric intensive care units (PICUs) [4], cardiopulmonary arrest [5–8] and mortality [6, 9] after RRS implementation in pediatric settings. A multicenter study of four pediatric hospitals demonstrated that pediatric RRSs reduced the rates of PICU mortality after readmission but not of actual cardiopulmonary arrests [10]. Similarly, systematic reviews and meta-analyses have shown that pediatric RRSs decrease hospital mortality and cardiopulmonary arrests outside of PICUs [11, 12].
There are regional differences in the number of reports and the prevalence of pediatric RRSs in the countries where RRSs have been reported frequently such as Australia, New Zealand, the United Kingdom, the United States, Canada, the Netherlands, Denmark, and Sweden [13]. However, in the other countries including Japan, there are few reports on pediatric RRSs, and their prevalence is unknown because epidemiological information is limited. Therefore, we conducted a cross-sectional survey of institutions with PICUs to determine the prevalence and characteristics of pediatric RRSs in Japan and the barriers to their implementation.
Methods
Study design
This study was a cross-sectional survey of PICU physicians in Japanese hospitals. It was performed through the Japanese Association of Pediatric Intensive and Critical Care Medicine (JAPIC), which has membership from all Japanese PICUs (34 institutions at the time of the survey). We created the questionnaire using online survey software (Survey Monkey®, www.surveymonkey.com) and sent it to each institution’s PICU medical director. To increase the response rate, we sent three reminders from April 20th through May 24th, 2019. One representative (the PICU medical director or another critical care physician knowledgeable of the hospital’s medical safety) per each institution responded. To prevent incomplete data, the survey was designed such that it could not be submitted unless the respondent fully completed the form. The Research Ethics Committee of our institution determined that an ethical review of this study was unnecessary because it used only anonymous data that could not be linked back to an individual participant.
Survey contents
We created two questionnaires: one for hospitals with pediatric RRSs (Supplementary Material 1) and one for hospitals without pediatric RRSs (Supplementary Material 2). Both questionnaires were sent to all institutions, and each responder chose the questionnaire that best applied to their hospital. Survey contents were as follows: (1) characteristics of responding hospitals (for both); (2) characteristics of pediatric RRSs (including the afferent, efferent and process improvement components, for institutions with pediatric RRSs) and (3) opinions regarding RRSs (for institutions without pediatric RRSs).
Definition of terms
An afferent component is a clear method of detecting ‘emergent unmet patient needs,’ defined as a disparity between what care a patient is receiving and what care he or she requires emergently, and triggering an RRS response. An efferent component is the response team that must (1) be able to provide an initial diagnosis, (2) be able to undertake initial therapeutic intervention and (3) have the authority to make transfer decisions and access other care providers to deliver definitive care. The process improvement component comprises feedback of event-related knowledge, evaluation of events, and application of process improvement strategies to prevent future occurrences (1). A medical emergency team (MET) includes at least one critical care physician or fellow with the following capabilities: (1) ability to prescribe therapy, (2) advanced airway management skills, (3) ability to establish central venous access and (4) ability to begin an intensive care unit (ICU) level of care at the bedside. A rapid response team (RRT) does not necessarily include a physician and can undertake the following: (1) rapidly assess patient needs, (2) begin basic care to stabilize the patient, (3) rapidly triage patients to a higher level of care if needed and (4) call in other resources to provide immediate ICU-level care on an expedited basis [14]. Critical care outreach, which is most commonly found in the United Kingdom, is generally staffed by ICU-trained nurses in close collaboration with ICU physicians and provides follow-up for patients discharged from the ICU [1]. The term ‘code blue’ is used in overhead announcements in hospitals to indicate an emergency in which a patient has entered cardiac and/or respiratory arrest. A general hospital is defined as a hospital that has major departments (including pediatrics) and deals with many types of sick patients; it does not specialize in the treatment of particular illnesses or patients. A single-calling criterion uses individual triggers or calling criteria to activate the RRS. A multiple-parameter calling criterion uses a combination of multiple parameters to generate scores intended to detect clinical deterioration, and the scores are compilations of points attributed to various physiological abnormalities (e.g. the Paediatric Early Warning System Score) [15]. A member-in-charge of an RRS is a daily staff member who responds to a patient’s deterioration as part of the response team.
Statistical analysis
Descriptive statistics were used to summarize pediatric RRS prevalence, hospital data, respondents’ opinions and pediatric RRS characteristics. Continuous variables were expressed as medians with interquartile ranges. Categorical variables were expressed as frequencies and percentages. Between-group differences were compared using the Wilcoxon rank-sum test for continuous data and Fisher’s exact test for categorical variables. For analyses involving survey opinions, Likert scale data were divided into three categories: disagree [1–2], neutral [3] and agree [4–5]. All P-values were two-sided, and P-values ≤ 0.05 were considered statistically significant. Statistical analyses were performed using R version 3.1.0 (R Foundation for Statistical Computing, Vienna, Austria, www.R-project.org).
Results
Surveys were sent to all 34 Japanese institutions with PICUs. The response rate was 100%. There were no missing data, and all data were included in the final analysis. Twenty-three of 34 (68%) surveys were completed by PICU medical directors, and the rest were completed by other critical care physicians.
Characteristics of responding institutions
Fourteen hospitals (41.2%) had pediatric RRSs, which had been implemented between 2009 and 2018. The number of RRSs had increased gradually over that period (Fig. 1). Hospital characteristics for institutions with and without pediatric RRSs are shown in Table 1; out of 18 general hospitals, six (33.3%) had a pediatric RRS and out of 16 children’s hospitals, eight (50%) had a pediatric RRS (P = 0.48). There were no significant differences in the number of hospital beds, PICU beds, percentages of children’s hospitals or traditional code team ownership between hospitals with and without pediatric RRSs. Institutions without pediatric RRSs were more likely to use an ‘Announcement of Code Blue’ compared with institutions with pediatric RRSs (100% vs. 50%, respectively; P = 0.001).
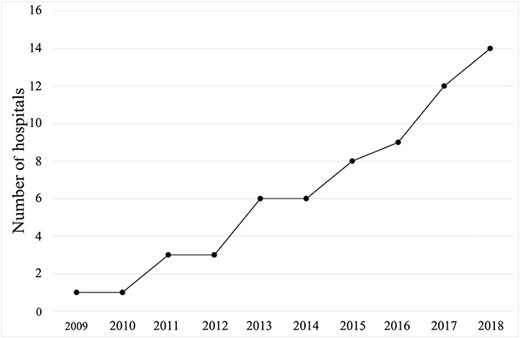
The number of hospitals with pediatric RRSs between 2009 and 2018.
| Characteristics . | All n = 34 . | Institutions with a pediatric RRSan = 14 . | Institutions without a pediatric RRS n = 20 . | P value . |
|---|---|---|---|---|
| Number of hospital beds, median (IQRb) | 495 (277, 795) | 472 (296, 969) | 524 (240, 747) | 0.86 |
| Number of PICUc beds, median (IQR) | 8 (8, 15.5) | 10 (8, 17) | 8 (7.5, 13) | 0.34 |
| Classification of hospital | ||||
| General hospital, n (%) | 18 (52.9) | 6 (42.9) | 12 (60) | 0.48 |
| Children’s hospital, n (%) | 16 (47.1) | 8 (57.1) | 8 (40) | |
| Ways to manage cardiorespiratory arrest | ||||
| Traditional code team, n (%) | 14 (41.2) | 7 (50) | 7 (35) | 0.48 |
| Announcement of a ‘code blue’ in the hospital, n (%) | 27 (79.4) | 7 (50) | 20 (100) | 0.001 |
| Characteristics . | All n = 34 . | Institutions with a pediatric RRSan = 14 . | Institutions without a pediatric RRS n = 20 . | P value . |
|---|---|---|---|---|
| Number of hospital beds, median (IQRb) | 495 (277, 795) | 472 (296, 969) | 524 (240, 747) | 0.86 |
| Number of PICUc beds, median (IQR) | 8 (8, 15.5) | 10 (8, 17) | 8 (7.5, 13) | 0.34 |
| Classification of hospital | ||||
| General hospital, n (%) | 18 (52.9) | 6 (42.9) | 12 (60) | 0.48 |
| Children’s hospital, n (%) | 16 (47.1) | 8 (57.1) | 8 (40) | |
| Ways to manage cardiorespiratory arrest | ||||
| Traditional code team, n (%) | 14 (41.2) | 7 (50) | 7 (35) | 0.48 |
| Announcement of a ‘code blue’ in the hospital, n (%) | 27 (79.4) | 7 (50) | 20 (100) | 0.001 |
RRS, rapid response system.
IQR, interquartile range.
PICU, pediatric intensive care unit.
| Characteristics . | All n = 34 . | Institutions with a pediatric RRSan = 14 . | Institutions without a pediatric RRS n = 20 . | P value . |
|---|---|---|---|---|
| Number of hospital beds, median (IQRb) | 495 (277, 795) | 472 (296, 969) | 524 (240, 747) | 0.86 |
| Number of PICUc beds, median (IQR) | 8 (8, 15.5) | 10 (8, 17) | 8 (7.5, 13) | 0.34 |
| Classification of hospital | ||||
| General hospital, n (%) | 18 (52.9) | 6 (42.9) | 12 (60) | 0.48 |
| Children’s hospital, n (%) | 16 (47.1) | 8 (57.1) | 8 (40) | |
| Ways to manage cardiorespiratory arrest | ||||
| Traditional code team, n (%) | 14 (41.2) | 7 (50) | 7 (35) | 0.48 |
| Announcement of a ‘code blue’ in the hospital, n (%) | 27 (79.4) | 7 (50) | 20 (100) | 0.001 |
| Characteristics . | All n = 34 . | Institutions with a pediatric RRSan = 14 . | Institutions without a pediatric RRS n = 20 . | P value . |
|---|---|---|---|---|
| Number of hospital beds, median (IQRb) | 495 (277, 795) | 472 (296, 969) | 524 (240, 747) | 0.86 |
| Number of PICUc beds, median (IQR) | 8 (8, 15.5) | 10 (8, 17) | 8 (7.5, 13) | 0.34 |
| Classification of hospital | ||||
| General hospital, n (%) | 18 (52.9) | 6 (42.9) | 12 (60) | 0.48 |
| Children’s hospital, n (%) | 16 (47.1) | 8 (57.1) | 8 (40) | |
| Ways to manage cardiorespiratory arrest | ||||
| Traditional code team, n (%) | 14 (41.2) | 7 (50) | 7 (35) | 0.48 |
| Announcement of a ‘code blue’ in the hospital, n (%) | 27 (79.4) | 7 (50) | 20 (100) | 0.001 |
RRS, rapid response system.
IQR, interquartile range.
PICU, pediatric intensive care unit.
RRS Characteristics
Afferent component
Most responding hospitals with pediatric RRSs (85.7%) used single-parameter calling criteria to activate the RRS; few hospitals (14.3%) used post-PICU follow-up and proactive rounding of general wards. Physicians and nurses could activate the RRS in all institutions with pediatric RRSs, and other healthcare providers could activate the RRS in approximately 50% of the institutions. No hospitals permitted patients or their family to activate the RRS (Table 2).
Characteristics of the afferent component in institutions with pediatric RRSs
| Characteristics . | n = 14 . |
|---|---|
| Ways to activate RRSsa | |
| Calling criteria, n (%) | 14 (100) |
| Single-parameter, n (%) | 12 (85.7) |
| Multiple-parameter, n (%) | 2 (14.3) |
| Post-PICUb follow-up, n (%) | 2 (14.3) |
| Proactive rounding of general wards, n (%) | 2 (14.3) |
| Members can activate RRSs | |
| Physician, n (%) | 14 (100) |
| Nurse, n (%) | 14 (100) |
| Other healthcare provider, n (%) | 8 (57.1) |
| Patient or family, n (%) | 0 (0) |
| Characteristics . | n = 14 . |
|---|---|
| Ways to activate RRSsa | |
| Calling criteria, n (%) | 14 (100) |
| Single-parameter, n (%) | 12 (85.7) |
| Multiple-parameter, n (%) | 2 (14.3) |
| Post-PICUb follow-up, n (%) | 2 (14.3) |
| Proactive rounding of general wards, n (%) | 2 (14.3) |
| Members can activate RRSs | |
| Physician, n (%) | 14 (100) |
| Nurse, n (%) | 14 (100) |
| Other healthcare provider, n (%) | 8 (57.1) |
| Patient or family, n (%) | 0 (0) |
RRS, rapid response system.
PICU, pediatric intensive care unit.
Characteristics of the afferent component in institutions with pediatric RRSs
| Characteristics . | n = 14 . |
|---|---|
| Ways to activate RRSsa | |
| Calling criteria, n (%) | 14 (100) |
| Single-parameter, n (%) | 12 (85.7) |
| Multiple-parameter, n (%) | 2 (14.3) |
| Post-PICUb follow-up, n (%) | 2 (14.3) |
| Proactive rounding of general wards, n (%) | 2 (14.3) |
| Members can activate RRSs | |
| Physician, n (%) | 14 (100) |
| Nurse, n (%) | 14 (100) |
| Other healthcare provider, n (%) | 8 (57.1) |
| Patient or family, n (%) | 0 (0) |
| Characteristics . | n = 14 . |
|---|---|
| Ways to activate RRSsa | |
| Calling criteria, n (%) | 14 (100) |
| Single-parameter, n (%) | 12 (85.7) |
| Multiple-parameter, n (%) | 2 (14.3) |
| Post-PICUb follow-up, n (%) | 2 (14.3) |
| Proactive rounding of general wards, n (%) | 2 (14.3) |
| Members can activate RRSs | |
| Physician, n (%) | 14 (100) |
| Nurse, n (%) | 14 (100) |
| Other healthcare provider, n (%) | 8 (57.1) |
| Patient or family, n (%) | 0 (0) |
RRS, rapid response system.
PICU, pediatric intensive care unit.
Efferent component
Two-thirds of response teams were METs, and the rest were RRTs. Teams primarily comprised physicians and nurses. The most common team member division/department was the ICU/intensive care medicine department (85.7%), followed in order by the emergency department (35.7%) and the operating room/anesthesiology department (28.6%). The median numbers of core members and members-in-charge of response teams were 6 and 2.5, respectively (Table 3). Moreover, of 14 response teams, three (21.4%) teams had full-time members. The members of 13 (92.9%) response teams worked in addition to their main duties, whereas the members of one (7.1%) response team worked exclusively for the RRS on the day in charge. In total, 11 (78.6%) response teams were available 7 days a week, 24 h a day, and three (21.4%) were available during the day on weekdays only. Six (42.9%) response teams had traditional code teams distinct from RRS teams and two (14.3%) had no traditional code teams; nine (64.3%) response teams covered the entire hospital, one (7.1%) covered the entire hospital except for the outpatient department and four (28.6%) covered only general wards.
| Characteristics . | n = 14 . |
|---|---|
| Types of response team | |
| Medical emergency team, n (%) | 10 (71.4) |
| Rapid response team, n (%) | 4 (28.6) |
| Critical care outreach team, n (%) | 0 (0) |
| Team composition | |
| Physician, n (%) | 14 (100) |
| Nurse, n (%) | 13 (92.9) |
| Other healthcare provider, n (%) | 1 (7.1) |
| Team member’s department | |
| Intensive care unit/intensive care medicine, n (%) | 12 (85.7) |
| Emergency department, n (%) | 5 (35.7) |
| Operation room/anesthesiology, n (%) | 4 (28.6) |
| Other, n (%) | 3 (21.4) |
| Number of core members in the response team, median (IQRa) | 6 (5, 9) |
| Number of members-in-charge (IQR) | 2.5 (2, 3) |
| Characteristics . | n = 14 . |
|---|---|
| Types of response team | |
| Medical emergency team, n (%) | 10 (71.4) |
| Rapid response team, n (%) | 4 (28.6) |
| Critical care outreach team, n (%) | 0 (0) |
| Team composition | |
| Physician, n (%) | 14 (100) |
| Nurse, n (%) | 13 (92.9) |
| Other healthcare provider, n (%) | 1 (7.1) |
| Team member’s department | |
| Intensive care unit/intensive care medicine, n (%) | 12 (85.7) |
| Emergency department, n (%) | 5 (35.7) |
| Operation room/anesthesiology, n (%) | 4 (28.6) |
| Other, n (%) | 3 (21.4) |
| Number of core members in the response team, median (IQRa) | 6 (5, 9) |
| Number of members-in-charge (IQR) | 2.5 (2, 3) |
IQR = interquartile range.
| Characteristics . | n = 14 . |
|---|---|
| Types of response team | |
| Medical emergency team, n (%) | 10 (71.4) |
| Rapid response team, n (%) | 4 (28.6) |
| Critical care outreach team, n (%) | 0 (0) |
| Team composition | |
| Physician, n (%) | 14 (100) |
| Nurse, n (%) | 13 (92.9) |
| Other healthcare provider, n (%) | 1 (7.1) |
| Team member’s department | |
| Intensive care unit/intensive care medicine, n (%) | 12 (85.7) |
| Emergency department, n (%) | 5 (35.7) |
| Operation room/anesthesiology, n (%) | 4 (28.6) |
| Other, n (%) | 3 (21.4) |
| Number of core members in the response team, median (IQRa) | 6 (5, 9) |
| Number of members-in-charge (IQR) | 2.5 (2, 3) |
| Characteristics . | n = 14 . |
|---|---|
| Types of response team | |
| Medical emergency team, n (%) | 10 (71.4) |
| Rapid response team, n (%) | 4 (28.6) |
| Critical care outreach team, n (%) | 0 (0) |
| Team composition | |
| Physician, n (%) | 14 (100) |
| Nurse, n (%) | 13 (92.9) |
| Other healthcare provider, n (%) | 1 (7.1) |
| Team member’s department | |
| Intensive care unit/intensive care medicine, n (%) | 12 (85.7) |
| Emergency department, n (%) | 5 (35.7) |
| Operation room/anesthesiology, n (%) | 4 (28.6) |
| Other, n (%) | 3 (21.4) |
| Number of core members in the response team, median (IQRa) | 6 (5, 9) |
| Number of members-in-charge (IQR) | 2.5 (2, 3) |
IQR = interquartile range.
Process improvement component
Of the 14 response teams, all used their own recording forms, and four (28.6%) used the online registry for Japanese multicenter RRSs (https://www.ihecj.jp) to record RRS activities. For quality improvement of the RRS, 12 (85.7%) conducted regular RRS meetings; 11 (78.6%) continuously evaluated RRS activities and outcomes, such as in-hospital mortality, unanticipated ICU admission, and incidence of cardiorespiratory arrest; 10 (71.4%) routinely reported RRS activities to the administration and nine (64.3%) gave feedback to RRS users.
Opinions of the institutions without RRSs
Table 4 shows the opinions of institutions without RRSs on RRSs. Of these, 11 (55%) hoped to introduce RRSs, and 14 (70%) had insufficient knowledge of RRSs. Regarding efforts to implement RRSs, one (5%) institution had launched a plan to install RRSs, and 11 (55%) considered that introducing RRSs would be difficult. The main barrier to the adoption of RRSs was an assumed lack of personnel and/or scarcity of funds (70%), followed by a lack of knowledge of RRSs: most hospital staff knows little about RRSs (40%).
| Question . | n, (% of Respondents) . | ||
|---|---|---|---|
| Disagree . | Neutral . | Agree . | |
| Your institution wants to introduce a pediatric RRSa | 2 (10) | 7 (35) | 11 (55) |
| Your PICUb staff has sufficient knowledge about pediatric RRSs | 6 (30) | 8 (40) | 6 (30) |
| The implementation of a pediatric RRS is difficult | 2 (10) | 7 (35) | 11 (55) |
| Question | n, (% of Respondents) | ||
| How does your institution feel about the implementation of pediatric RRSs? (select 1 item) | |||
| 1. Your institute has been advancing preparations toward the introduction of a pediatric RRS | 1 (5) | ||
| 2. Although your institute wants to introduce a pediatric RRS, it is not ready | 2 (10) | ||
| 3. The specific departments understand the need of a pediatric RRS, but it is not shared in the entire hospital | 10 (50) | ||
| 4. Your institute does not want to introduce a pediatric RRS | 1 (5) | ||
| 5. Does not apply | 6 (30) | ||
| What do you think are the barriers to implementing a pediatric RRS? (select all that apply) | |||
| 1. Lack of personnel and funds | 14 (70) | ||
| 2. Lack of knowledge of RRSs: most hospital staff know little about RRSs | 8 (40) | ||
| 3. Resistance from primary physicians | 3 (15) | ||
| 4. Acceptance by the finance department | 2 (10) | ||
| 5. Does not apply | 1 (5) | ||
| Question . | n, (% of Respondents) . | ||
|---|---|---|---|
| Disagree . | Neutral . | Agree . | |
| Your institution wants to introduce a pediatric RRSa | 2 (10) | 7 (35) | 11 (55) |
| Your PICUb staff has sufficient knowledge about pediatric RRSs | 6 (30) | 8 (40) | 6 (30) |
| The implementation of a pediatric RRS is difficult | 2 (10) | 7 (35) | 11 (55) |
| Question | n, (% of Respondents) | ||
| How does your institution feel about the implementation of pediatric RRSs? (select 1 item) | |||
| 1. Your institute has been advancing preparations toward the introduction of a pediatric RRS | 1 (5) | ||
| 2. Although your institute wants to introduce a pediatric RRS, it is not ready | 2 (10) | ||
| 3. The specific departments understand the need of a pediatric RRS, but it is not shared in the entire hospital | 10 (50) | ||
| 4. Your institute does not want to introduce a pediatric RRS | 1 (5) | ||
| 5. Does not apply | 6 (30) | ||
| What do you think are the barriers to implementing a pediatric RRS? (select all that apply) | |||
| 1. Lack of personnel and funds | 14 (70) | ||
| 2. Lack of knowledge of RRSs: most hospital staff know little about RRSs | 8 (40) | ||
| 3. Resistance from primary physicians | 3 (15) | ||
| 4. Acceptance by the finance department | 2 (10) | ||
| 5. Does not apply | 1 (5) | ||
RRS, rapid response system.
PICU, pediatric intensive care unit.
| Question . | n, (% of Respondents) . | ||
|---|---|---|---|
| Disagree . | Neutral . | Agree . | |
| Your institution wants to introduce a pediatric RRSa | 2 (10) | 7 (35) | 11 (55) |
| Your PICUb staff has sufficient knowledge about pediatric RRSs | 6 (30) | 8 (40) | 6 (30) |
| The implementation of a pediatric RRS is difficult | 2 (10) | 7 (35) | 11 (55) |
| Question | n, (% of Respondents) | ||
| How does your institution feel about the implementation of pediatric RRSs? (select 1 item) | |||
| 1. Your institute has been advancing preparations toward the introduction of a pediatric RRS | 1 (5) | ||
| 2. Although your institute wants to introduce a pediatric RRS, it is not ready | 2 (10) | ||
| 3. The specific departments understand the need of a pediatric RRS, but it is not shared in the entire hospital | 10 (50) | ||
| 4. Your institute does not want to introduce a pediatric RRS | 1 (5) | ||
| 5. Does not apply | 6 (30) | ||
| What do you think are the barriers to implementing a pediatric RRS? (select all that apply) | |||
| 1. Lack of personnel and funds | 14 (70) | ||
| 2. Lack of knowledge of RRSs: most hospital staff know little about RRSs | 8 (40) | ||
| 3. Resistance from primary physicians | 3 (15) | ||
| 4. Acceptance by the finance department | 2 (10) | ||
| 5. Does not apply | 1 (5) | ||
| Question . | n, (% of Respondents) . | ||
|---|---|---|---|
| Disagree . | Neutral . | Agree . | |
| Your institution wants to introduce a pediatric RRSa | 2 (10) | 7 (35) | 11 (55) |
| Your PICUb staff has sufficient knowledge about pediatric RRSs | 6 (30) | 8 (40) | 6 (30) |
| The implementation of a pediatric RRS is difficult | 2 (10) | 7 (35) | 11 (55) |
| Question | n, (% of Respondents) | ||
| How does your institution feel about the implementation of pediatric RRSs? (select 1 item) | |||
| 1. Your institute has been advancing preparations toward the introduction of a pediatric RRS | 1 (5) | ||
| 2. Although your institute wants to introduce a pediatric RRS, it is not ready | 2 (10) | ||
| 3. The specific departments understand the need of a pediatric RRS, but it is not shared in the entire hospital | 10 (50) | ||
| 4. Your institute does not want to introduce a pediatric RRS | 1 (5) | ||
| 5. Does not apply | 6 (30) | ||
| What do you think are the barriers to implementing a pediatric RRS? (select all that apply) | |||
| 1. Lack of personnel and funds | 14 (70) | ||
| 2. Lack of knowledge of RRSs: most hospital staff know little about RRSs | 8 (40) | ||
| 3. Resistance from primary physicians | 3 (15) | ||
| 4. Acceptance by the finance department | 2 (10) | ||
| 5. Does not apply | 1 (5) | ||
RRS, rapid response system.
PICU, pediatric intensive care unit.
Discussion
In this study, < 50% of Japanese institutions with PICUs were found to have RRSs. Of response teams in institutions with RRSs, two-thirds were METs, most used single-parameter calling criteria, only medical professionals (i.e. not the patient’s family) could activate RRSs, most comprised members from multiple disciplines and departments and employed no full-time members and less than one-third were registered in the online registry of Japanese multicenter RRSs. Of institutions without RRSs, approximately 50% wanted to introduce RRSs, only one-third had sufficient knowledge of RRSs, > 50% considered the implementation of RRSs would be difficult, and the main barrier to introducing RRSs was perceived to be insufficient personnel and/or funds.
Prevalence of pediatric RRSs in Japanese PICUs
The implementation of pediatric RRSs in Japan lags behind that of Australia, New Zealand, North America and other Western countries. A specific Japanese medical culture has been reported to be the main reason for this gap. One study [16] stated that the major feature of Japanese medical culture was ‘to place high importance on the primary care physician.’ Primary care physicians consider themselves best at understanding their patients and have very strong decision-making power concerning patient care. Furthermore, their patients and patients’ families tend to have complete confidence in their primary care physician and expect a physician to care comprehensively for them. As a result, both physicians and patients tend to refuse an intervention by third parties, including RRSs. This is supported by the fact that Japanese facilities had a very low rate of RRT/MET calls, reported by recent study using data from a Japanese multicenter database [17]. Although the primary care physician system has an advantage in maintaining continuity and inclusivity of patient care, there is a risk of delay in applying appropriate care when physicians work outside their specialist field, especially in high-urgency situations. Once RRSs are introduced into a medical culture such as that in Japan, RRSs could become effective medical safety systems to compensate for shortcomings of the primary care physician system through covering the fields outside of the primary care physician’s expertise and the times when the primary care physician cannot respond.
New policies are required to facilitate the spread of pediatric RRSs in Japan, because they are not popular in Japanese institutions with PICUs. The United States provides an example of successful adult and pediatric RRS implementation. The Institute for Healthcare Improvement has driven the implementation of RRSs, through organizing the 100 000 Lives Campaign (January 2005 through July 2006) and the 5 Million Lives Campaign (December 2006 through December 2008) [18, 19]. Additionally, the essential principles of RRSs were embraced by the United States Joint Commission, providing a mandate for United States hospitals in the form of a National Patient Safety Goal [20]. Subsequently, 79% of 130 hospitals with PICUs in the United States had RRTs as of 2010 [21]. In the United Kingdom, the National Institute of Clinical Excellence introduced RRSs. In 2005, 72.8% of National Health Service acute care hospitals in England had formal critical care outreach programs [22]. In Japan, the Japanese Coalition for Patient Safety has made an effort since 2008 to promote the adoption of RRSs as a goal in a Medical Safety Campaign (the so-called ‘Japanese version of the 100 000 Lives Campaign’) (http://kyodokodo.jp/). However, our study found that there was still a significant number of PICUs without pediatric RRSs. To resolve this issue, it might be helpful to reconsider the approach of the Japanese Medical Safety Campaign and to secure the cooperation of the Japanese Joint Commission.
Characteristics of pediatric RRSs in Japan
No Japanese hospitals with PICUs permitted family activation of RRSs. This may be a further feature of Japan’s medical culture. Japanese patients and patients’ families generally have strong confidence in their primary physicians; they tend to entrust medical care to their primary physicians and seldom address medical staff to request a change in therapeutic measures themselves, including family activation. This might be affected by a Japanese modest personality, too. However, in the United States, it was reported that 69% of pediatric RRSs permitted family activation in 2010 [21]. This readiness to initiate family activation was greatly influenced by a well-known case involving the death of a patient. This death was partly attributed to critical delays in escalation of medical care despite her family’s persistent verbal concerns [23].
Only 28.6% of Japanese hospitals with pediatric RRSs used the online registry for Japanese multicenter RRSs. This was likely to be because the registry remains largely unknown within children’s hospitals and because the registry was initially designed only for adult RRSs and then expanded to become applicable to pediatric RRSs. Most children’s hospitals already use their own recording forms and consider that it is not necessary to use the multicenter registry. For increasing registration of pediatric RRSs, it is important to enhance the usefulness of the registry (e.g. undertake a multicenter study). The first study to use this registry’s data has recently been published [17].
Barriers to implementing RRSs
Only 50% of responding institutions without pediatric RRSs hoped to introduce them. Insufficient RRS education could have contributed to this finding, because only 30% of these institutions had adequate information on RRSs. A Japanese investigation using a medical risk management seminar questionnaire reported the percentage of respondents knowledgeable about RRSs to be 38.5% in 2017 [24], which was similar to our results. These results suggest that implementing RRSs could be promoted through increasing information about them. Moreover, a previous study in the United States reported that the number of implemented RRSs increased sharply after several pediatric studies described favorable outcomes for them [21]. However, many Japanese remain unconvinced of the effectiveness of RRSs, because most previous reports showing positive outcomes for RRSs have been conducted in western countries where the medical culture differs from Japan. It is important to increase the number of reports of positive outcomes in Japanese hospitals after they adopt pediatric RRSs.
Our data suggest that the chief barrier to implementing pediatric RRSs is a belief in the scarcity of personnel and/or funding. However, there were no significant differences in size between institutions with and without RRSs, suggesting that hospitals without RRSs could work within their existing resources and funds to implement RRSs. In addition, of responding hospitals, most response teams comprised members of multiple disciplines and departments who worked in the RRS concurrently with their main operations, the size of the teams was relatively small (median number of core members and members-in-charge were six and 2.5, respectively), and some teams operated only during the day on weekdays. A recent study reported that RRTs included a median of three members and comprised physicians in 77% of institutions, nurses in 100% and respiratory therapists in 89% [21]. Therefore, the perceived problem of human resources might be overcome through monitoring the size of teams in these institutions. Moreover, RRSs might not necessarily need physicians. A systematic review and meta-analysis showed that the vast majority of rapid response interventions do not require a physician, and that the presence of a physician was not associated with improved outcomes [12]. Some studies have reported that RRTs result in cost reductions through avoiding unnecessary transfers to the ICU, cardiopulmonary arrests, complications that cause longer hospital stays [25] and critical deterioration events (such as unplanned transfers to the ICU with mechanical ventilation or vasopressors in the first 12 h after transfer) [26]. The annual cost savings of having an RRT available 24 h per day, 7 days a week have been reported to be $171 480 [25]. This positive cost-effectiveness offers further support for RRSs in terms of cost savings and efficiency.
Limitations
This study had some limitations. First, we could not exclude measurement bias. Only one representative per hospital responded to the survey, and differences in their positions, experience, knowledge and opinions might have influenced the quality of their responses. Moreover, the limited alternatives in the questionnaire might not have enabled respondents to answer accurately. Second, our data were restrictive. For example, to increase the response rate, we simplified the questionnaires and minimized the number of questions. Therefore, this survey did not include certain outcomes, such as unexpected cardiac arrest or death, and we could not evaluate whether the differences in variations of RRSs were associated with patient outcomes. Finally, we did not ask whether responding institution used automatic triggers or deep learning, concerning which there have been an increasing number of reports.
In conclusion, our findings indicated that < 50% of Japanese institutions with PICUs had pediatric RRSs. Possible approaches to increase the prevalence of Japanese RRSs include disseminating information on RRSs to encourage positive attitudes toward them and showing that the perceived issues concerning human resources and funding could be addressed with more careful planning. Future challenges relate to ensuring an increase in RRSs implementation and accessing RRS efficacy more thoroughly. Further epidemiological surveys to determine the actual status of Japanese pediatric RRSs over time are needed, as well as an increase in the registration of Japanese multicenter RRSs. Our results provide useful information that can be used to promote the spread of RRSs in Japan and other countries with medical cultures similar to that in Japan.
Acknowledgements
None.
Funding
No funding nor any specific grant was received from any funding agency in the public, commercial or not-for-profit sectors.
Conflicts of interest
The authors declare that there is no conflict of interest.



