-
PDF
- Split View
-
Views
-
Cite
Cite
Nicole E. Stoller, Teshome Gebre, Berhan Ayele, Mulat Zerihun, Yared Assefa, Dereje Habte, Zhaoxia Zhou, Travis C. Porco, Jeremy D. Keenan, Jenafir I. House, Bruce D. Gaynor, Thomas M. Lietman, Paul M. Emerson, Efficacy of latrine promotion on emergence of infection with ocular Chlamydia trachomatis after mass antibiotic treatment: a cluster-randomized trial, International Health, Volume 3, Issue 2, June 2011, Pages 75–84, https://doi.org/10.1016/j.inhe.2011.03.004
Close - Share Icon Share
Summary
The World Health Organization (WHO) recommends environmental improvements such as latrine construction in the integrated trachoma control strategy, SAFE. We report a cluster-randomized trial assessing the effect of intensive latrine promotion on emergence of infection with ocular Chlamydia trachomatis after mass treatment with antibiotics.
Twenty-four communities in Goncha Seso Enesie woreda, Amhara Regional State, Ethiopia, were enumerated, and a random selection of 60 children aged 0–9 years in each was monitored for clinical signs of trachoma and ocular chlamydial infection at baseline, 12 and 24 months. All community members were offered treatment with a single dose of oral azithromycin or topical tetracycline. After treatment, 12 subkebeles were randomized to receive intensive latrine promotion. Mean cluster ocular infection in the latrine and the non-latrine arms were reduced from 45.5% (95% CI 34.1–56.8%) and 43.0% (95% CI 31.1–54.8%), respectively, at baseline to 14.6% (95% CI 7.4–21.8%) and 14.8% (95% CI 8.9–20.8%), respectively, at 24 months (P = 0.93). Clinical signs fell from 72.0% (95% CI 58.2–85.5%) and 61.3% (95% CI 44.0–78.5%) at baseline to 45.8% (36.0–55.6%) and 48.5% (34.0–62.9%), respectively, at 24 months (P = 0.69). At 24 months, estimated household latrine coverage and use were 80.8% and 61.7%, respectively, where there had been intensive latrine promotion and 30.0% and 25.0% respectively in the single treatment only arm. We were unable to detect a difference in the prevalence of ocular chlamydial infection in children due to latrine construction.
1 Introduction
Behaviour change and environmental improvements are cornerstones of the World Health Organization’s (WHO) control efforts for trachoma, the world’s leading infectious cause of blindness. Along with the other components of the SAFE strategy, surgery and mass antibiotic distributions, these form the foundation for eliminating blinding trachoma.1 Some national programs have included efforts on building household latrines as a means of vector control since Musca sorbens, the eye-seeking fly that plays a role in spreading the infection, breeds preferentially on human faeces on the soil surface.2
Risk factor analysis from several countries has shown ownership of a latrine to be associated with a lower risk of having clinical signs of trachoma (reviewed in Kuper et al., 2003).3 Multivariate analyses of program evaluation data from Sudan and Ethiopia have shown that household latrine ownership is independently associated with a lower risk of having signs of active clinical trachoma when controlling for participation in azithromycin distribution and health education.4,5 Despite the evidence from risk factor analyses and program evaluations, there is an absence of direct evidence from a prospective trial that latrine promotion alone can reduce trachoma transmission. A cluster-randomized controlled trial of the effects of latrines on trachoma transmission (measured as the prevalence of clinical signs of trachoma) showed that the village population of Musca sorbens flies was significantly reduced by the latrine intervention, and the frequency of flies contacting the eyes of children was lower in the latrine communities.6 However, although the reduction in Musca sorbens was accompanied by a decline in the prevalence of trachoma in the communities randomized to the latrine intervention compared to the controls, the difference did not achieve statistical significance.
Trachoma is caused by ocular infection with the bacterium Chlamydia trachomatis, and several clinical trials have shown that treatments with antibiotic results in a deep reduction in the prevalence of ocular C. trachomatis infection—where infection is defined as the identification of C. trachomatis DNA from an ocular swab.7–9 A follow-up study of children from a hyper-endemic area showed that after four treatments six months apart there was a rapid re-emergence of ocular C. trachomatis infection. The proportion of positive children fell from an average of 63.5% at baseline to 2.6% six months after the fourth treatment; by 24 months after the last treatment, infection had risen to 25.2%.10
We hypothesized that in an area hyper-endemic for trachoma, ocular infection with C. trachomatis would return rapidly after a single round of treatment with antibiotic, as it had in the Lakew study,10 and that the rate of emergence of infection would be affected if the treatment were followed by a program of intensive latrine promotion similar to programming in other parts of Amhara, where the proportion of the population with access to improved sanitation increased by more than 50% in less than three years.11 We designed a cluster-randomized controlled trial to determine whether the rate of return of ocular C. trachomatis infection was affected by a program of intensive latrine promotion.
2 Methods
2.1 Study site and randomization
The study was conducted in the Goncha Seso Enesie woreda (district) of the Amhara Regional State in northern Ethiopia from May 2006 to July 2008. We selected and censused 72 contiguous subkebeles (analogous to a village, a subkebele is an administrative unit with a population of around 1500 people sub divided into 4 or 5 ‘state teams’ of around 50 households each). The 72 subkebeles constituted our study clusters and were randomized to one of six arms of 12 subkebeles each, forming three separate clinical trial comparisons (Figure 1 ). Arm generation and subkebele assignment was done using RANDOM() and SORT() in Microsoft Excel® (Microsoft Corp., Redmond, WA, USA). The assignment of subkebeles to the trial arms was concealed from the field teams until the start of the different interventions. Twenty-four of the subkebeles were randomly assigned to the two arms of this trial, assessing the added benefit of intensive latrine promotion to the rate of emergence of ocular C. trachomatis infection after a single round of mass treatment with antibiotic. The remaining 48 subkebeles were randomly assigned to two other comparisons: a trial comparing annual versus biannual mass treatment of communities over 42 months and a trial assessing the effect of quarterly antibiotic treatment of children on untreated adults.12 Using a simple random sample, we selected a single sentinel state team from each subkebele to monitor the prevalence of ocular C. trachomatis infection. Other state teams in the subkebele were treated in the same manner as the sentinel state team to reduce contamination between arms. For all 72 randomization units in the study, the mean distance between a sentinel state team and its nearest neighbouring sentinel state team was 12.7 km (range 0.06–30.3 km), 3/276 pairwise distances were less than 1.0 km.
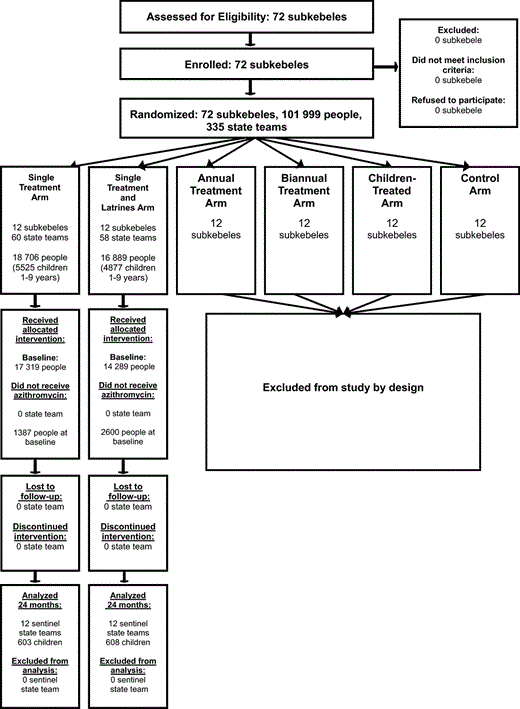
At baseline (May 2006), 12 months, and 24 months, 60 children aged 0–9 years were randomly selected from the census of each sentinel state team of both treatment arms for clinical examination and conjunctival swabbing using a Microsoft Excel® macro on the current census list. For baseline, this was the original census and at months 12 and 24 it was an updated census list. Children born after baseline were included through census updates at 12 and 24 months. Children who aged out of the study cohort were removed from the list once they turned 10. The simple random sample of 60 children was re-drawn at each visit, so the same individuals may or may not have been chosen.
2.2 Sample size and power calculation
The pre-specified primary outcome was the prevalence of ocular C. trachomatis infection in children aged 0–9 years at 24 months after baseline and the secondary outcome measure was clinical signs of trachoma using the WHO simplified grading system,13 adjusting for baseline prevalence. We estimated that the inclusion of 12 subkebeles per arm would provide 80% power to detect a 6% difference in the prevalence of infection assuming a standard deviation of 5.0% in the 24-month prevalence, a correlation between baseline and 24 months of 0.5, and a two-tailed alpha of 0.05.
2.3 Trachoma grading and detection of infection with ocular Chlamydia trachomatis infection
Examination for trachoma signs was conducted by independent integrated eye care workers using the WHO simplified grading system.13 The examiners were recruited from a non-study district and were not informed of the intervention status of the villages or the purpose of the study. Examiners had to achieve at least 80% inter-observer agreement in identifying trachoma signs compared to the senior examiner to participate in the survey. The right upper tarsal conjunctiva of each participant was considered as ‘clinically active’ if a grade of TF (trachomatous inflammation follicular) and/or TI (trachomatous inflammation intense) was present. Validation photographs were not taken. To collect PCR samples, a Dacron swab was passed firmly across the tarsal conjunctiva three times, rotating approximately 120˚ between each pass. Examiners changed gloves between study participants. All samples were kept at 4 ˚C in the field and frozen at -20 ˚C within 6 hours. The swabs were shipped at 4 ˚C to San Francisco where they were stored at −80 ˚C until processed.14 Two types of control swabs were taken. At baseline, a duplicate swab was collected from five randomly selected participants to assess concordance between samples. At baseline, 12 months and 24 months, ‘air swabs’ were taken for five randomly selected participants per subkebele in which the swab was passed within 2.5 cm of the conjunctiva but did not touch it. Air controls were used to assess contamination between sequential samples.
The Amplicor PCR test (Roche Diagnostics, Branchburg, NJ, USA) was used to detect C. trachomatis DNA on pooled samples using the method described by House et al.12 Baseline samples were tested in pools of two and post-treatment samples in pools of five. Pooled samples resulting in an equivocal signal from the test were retested individually. The prevalence of ocular C. trachomatis infection was assessed by maximum likelihood estimation.15
2.4 Interventions
2.4.1 Antibiotic treatment
At baseline, all individuals aged one year and older in all subkebeles of both arms were offered a single dose of directly observed oral azithromycin (Zithromax® Pfizer Inc, NY, USA; 1 gm in adults or height-based dosing to approximately 20 mg/kg in children). Children less than one year of age and participants self-reporting as pregnant were offered a 6-week course of topical 1% tetracycline ointment (Shanghai General Pharmaceuticals, Shanghai, China) applied twice daily to both eyes and not directly observed. Twenty four months after baseline all individuals in both arms were again offered oral azithromycin or topical tetracycline, following the same guidelines.
2.4.2 Latrine provision
A latrine promotion program is currently under way in Amhara which promotes simple pit latrine construction by participating households using all locally available materials. In the subkebeles randomized to the intensive latrine promotion arm, female health extension workers and additional sanitation volunteers intensified the existing program and added a free ferro-reinforced cement latrine slab. The health workers and volunteers trained all the heads of households in groups of 1–8 people on latrine construction and offered them a cement slab when the pit had been dug. There was no limit on the number of slabs available. Guidelines for construction specified a pit approximately 2.5–3.5m deep with slightly tapering sides and an opening of 1 × 1.5m. Stout eucalyptus logs were laid over the pit and the cement slab (dimensions 60 × 60 cm, 5 cm thick, with a drop hole of 18 × 38 cm) was laid on a bed of mud plaster on the logs. Guidelines for the superstructure construction included a roof to protect the latrine from rain, and a door and walls which provided privacy, but since homeowners were responsible for the superstructure construction, the appearance of the finished latrines differed between households. After provision of training, the health workers and volunteers made multiple visits to the households during the first three months of the study to encourage latrine construction and use. No payments were made to heads of households for latrine construction and participation in the intensified latrine promotion program was entirely voluntary.
2.5 Assessment of uptake of interventions
2.5.1 Antibiotic treatment
Antibiotic treatments were recorded in pre-printed record books and coverage was assessed from the books. No attempt was made to validate use of tetracycline eye ointment. Antibiotic coverage was calculated relative to the previous household-based census of the area.
2.5.2 Latrine coverage and usage
Intensive latrine promotion and construction took place after the baseline census, collection, and treatment were completed. For the subkebeles randomized to receive intensive latrine promotion, latrine coverage was calculated relative to the baseline household-based census of the area.
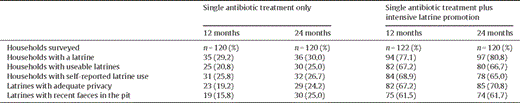
Latrine use surveys were conducted at 12 months after baseline (June 2007) to assess the ability of the intensive latrine promotion team to achieve good coverage and usage, and at 24 months to assess whether exposure to latrines in the single antibiotic treatment plus intensive latrine promotion arm was maintained to the end of the study. In both surveys, 10 households were selected at random from each of the 12 sentinel state teams in both arms for a total of 240 households per survey. The survey team conducted a visual inspection of the latrine and determined usability (defined as whether the interviewer would use it), privacy (defined as the presence of sufficient walls surrounding the latrine), and recent use (defined as the presence of recent faeces in the pit). The household head was interviewed about who used the latrine.
2.6 Masking
The field team was masked to the subkebele assignment until after the baseline survey and antibiotic treatment had taken place. Health extension workers residing in the study area performed antibiotic distributions and a separate team of integrated eye care workers from outside the study area performed clinical assessments and took ocular swabs. Lab personnel were masked to individual, community, and treatment-arm identifications.
2.7 Statistical methods
Baseline characteristics of the participants in the communities of the two study arms and latrine coverage and usage were compared using the Student’s t test or paired sample t test. The pre-specified primary outcome was an analysis of covariance in the prevalence of ocular C. trachomatis in children aged 0–9 years after 24 months, adjusting for baseline prevalence. Missing baseline prevalence data were multiply16 imputed in accordance with the pre-specified analysis plan, using simple linear regression of the baseline values against the 12-month values in a predictive equation that included all 24 randomization units. Analyses were conducted on the basis of intention-to-treat at the community level.
3 Results
The trial profile is displayed in Figure 1 and the baseline characteristics of the study population are displayed in Table 1 . Baseline characteristics were missing for one subkebele in the single antibiotic treatment plus intensive latrine promotion arm as the survey team was directed to the wrong subkebele for the baseline survey and sample collection. The correct subkebele could not be re-visited as the error was not detected until after the field team was unmasked. The two treatment groups were well balanced except for antibiotic coverage, which was higher in the single antibiotic treatment only arm compared to the single antibiotic treatment plus intensive latrine promotion arm: overall coverage for all study participants was 94.8% (95% CI 92.9-96.7%) compared to 89.2% (95% CI 85.3-93.1%), P= 0.008 (Student’s t test). Table 1 shows antibiotic coverage for the children who were examined clinically at baseline in each group and provided ocular swab samples for PCR detection of ocular C. trachomatis infection; these are similar to the group’s overall. The prevalence of ocular C. trachomatis infection in children aged 0–9 years (ocular swabs positive for C. trachomatis DNA by PCR) at baseline was similar in the single treatment and single treatment plus intensive latrine promotion arms: 43.0% (95% CI 31.1–54.8) and 45.5% (95% CI 34.1–56.8), respectively. All duplicate swabs were concordant at baseline. The ‘air swab’ controls to assess contamination between samples were positive for 1/55 and 2/55 in the treatment only and treatment plus intensive latrine promotion arms, respectively, at baseline. All air swabs were negative at the 12 and 24 month assessment (0/60 for both arms at both time points).
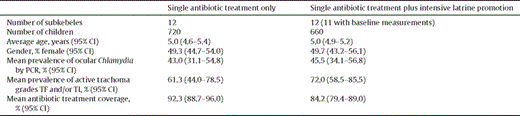
Mean baseline characteristics of children aged 0-9 years by treatment arm. (Data are for children in the sentinel state teams.).
The primary pre-specified outcome measure was prevalence of ocular C. trachomatis infection in children aged 0–9 years at 24 months adjusting for baseline prevalence. No subkebeles were missing the final prevalence measure at 24 months. Table 2 shows antibiotic treatment coverage, latrine coverage, and prevalence of ocular C. trachomatis infection at baseline, 12 months, and 24 months by subkebele. The mean prevalence of ocular C. trachomatis infection at baseline, 12 months, and 24 months in the single treatment only arm compared to the single treatment plus intensive latrine promotion arm was 43.0%, 15.5% and 14.6% compared to 45.5%, 12.8% and 14.8%. There was no evidence of a difference in the prevalence of ocular C. trachomatis infection in children aged 0–9 years at 24 months between the two arms (P = 0.93, imputing a single missing baseline prevalence using linear regression from the twelve month prevalence data, according to the pre-specified analysis plan). A post-hoc sensitivity analysis was conducted omitting the one subkebele with missing baseline prevalence data (out of 24 villages), and as for the primary pre-specified analysis, no evidence of a difference between the single treatment only and the single treatment plus intensive latrine promotion groups was seen (P = 0.54, analysis of covariance).
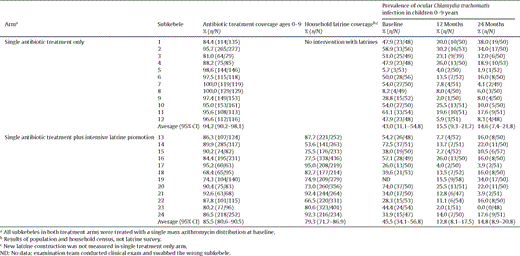
Intervention coverage and prevalence of ocular Chlamydia trachomatis infection at baseline, 12 and 24 months by subkebele and treatment arm.
As a secondary outcome, we also measured the prevalence of clinically active trachoma (grades TF and/or TI), shown in Table 3 . The mean prevalence of clinically active trachoma at baseline, 12 months and 24 months was 61.3%, 56.8% and 48.5% respectively in the single treatment only arm, compared to 72%, 53.5% and 45.8%, respectively, in the single treatment plus intensive latrine promotion arm. There was no difference in the prevalence of clinical signs between the single treatment and treatment plus latrine arms at 24 months P = 0.69 (analysis of covariance controlling for baseline clinical activity, including imputed value for one cluster with missing baseline data). Complete case analysis (i.e. deleting the cluster for which there was no baseline data) gave a similar finding P = 0.62 (analysis of covariance controlling for baseline).
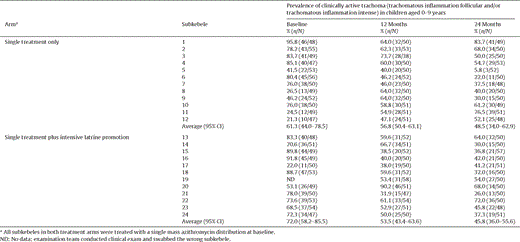
Prevalence of clinically active trachoma at baseline, 12 months and 24 months in children aged 0–9 years by treatment arm and subkebele.
There was a substantial difference in latrine coverage and usage between the two study arms. Approximately 30% of households surveyed in the single antibiotic treatment only arm had existing, non-programmatic latrines at 12 and 24 months. Coverage in the single antibiotic treatment plus intensive latrine promotion subkebeles was greater than in the single antibiotic treatment only subkebeles: 77.1% after 12 months and 80.8% after 24 months (Table 4 ). Overall, the proportion of households surveyed that had a latrine considered usable by our survey team 12 months into the study was 25/120 (20.8%) in the single treatment only arm and 82/122 (67.2%) in the single treatment plus intensive latrine promotion arm. In the subkebeles randomized to receive a single treatment only, the proportion of households that had a latrine with evidence of use (recent faeces in the pit) was 19/120 (15.8%) after 12 months. In contrast, the proportion of households in the single treatment plus intensive latrine promotion subkebeles that had a latrine with evidence of recent use at 12 months was 75/122 (61.5%). The proportion of households that had a latrine with recent use at 24 months was similar to recent use at 12 months: 30/120 (25.0%) in the single treatment only subkebeles and 74/120 (61.7%) in the single treatment plus intensive latrine promotion subkebeles.
4 Discussion
In the context of this study, there was no incremental benefit on the reduction in re-emergence of ocular C. trachomatis infection after a single mass treatment with oral azithromycin from an intensive latrine provision program. Because there was no evidence of emergence of ocular C. trachomatis infection in either intervention group, it is not possible to interpret these findings as evidence that latrine provision does or does not affect the rate of resurgence of ocular C. trachomatis infection after a single oral dose of azithromycin.
There are reasons why we may have expected a slower resurgence of ocular C. trachomatis infection. A series of studies in the 1990s demonstrated that the eye-seeking fly Musca sorbens was a likely mechanical vector of trachoma, since it fed on ocular discharge and moved from the eyes of one person to another frequently and directly in trachoma-endemic villages.2 A pilot study using a cross-over design showed that fly control with ultra-low volume insecticide spraying was effective in reducing clinical signs of trachoma in sprayed villages compared to unsprayed villages.17 A cluster-randomized control trial of fly control versus no intervention in a low prevalence area confirmed that ultra-low volume spraying with insecticide to control eye-seeking flies resulted in a significant reduction in clinical signs of trachoma.6 A second randomized study of intensive fly spray against control in a hyper-endemic area showed declines in clinical signs of trachoma in the intervention group compared to controls, although these relative differences were not statistically significant (P = 0.07)18. Studies on the ecology of Musca sorbens show that it breeds preferentially in human faeces.19 The ecological environment within a simple pit latrine is very different to that provided by single human faeces lying on the earth. An investigation on the productivity of simple pit latrines as breeding sites for domestic flies revealed that while they provide an ideal breeding site for the fly Chrysomya albiceps, they do not produce Musca sorbens.20 A cluster-randomized controlled trial of latrine provision in an area hypo-endemic for trachoma showed that villages randomized to receive latrines had a lower population of Musca sorbens and there were fewer fly–eye contacts than in control villages.6 There has been no randomized controlled trial that has demonstrated a significant reduction in either clinical signs of trachoma or microbiological evidence of trachoma infection as a result of latrine provision. However, there is an abundance of evidence from multivariate risk-factor analyses from Egypt, Tanzania, The Gambia, Burkina Faso, Sudan and Ethiopia that household ownership of a latrine is associated with a reduced risk of being an active trachoma case.21–26 The evidence has been considered sufficiently compelling to include latrine provision in national trachoma control programs, and hundreds of thousands of household latrines have been dug in support of trachoma control.27–29
In our study, the interventions were successfully delivered per protocol. The mean subkebele coverage with antibiotic was about 90% overall with 10/24 subkebeles reporting at least 95% coverage and just two with less than 80%. Antibiotic coverage was significantly higher in the antibiotic only arm by chance (94.8% compared to 89.2%), however coverage was extremely good in both arms and an additional one in twenty children receiving treatment in the antibiotic only arm at baseline would be unlikely to affect the outcome at 24 months. Participation in latrine promotion in the intensive latrine promotion arm was voluntary, and required a considerable investment of time and energy by the households for no compensation other than a free cement latrine slab. Assessment of latrine coverage and use at 12 and 24 months showed that the subkebeles randomized to receive household intensive latrine promotion had a mean latrine coverage of 77.1% compared to 29.2% in the villages randomized to treatment alone. Crucially, the use of latrines, as measured by the presence of recent faeces in the pit, was on average four times higher in the subkebeles randomized to intensive latrine promotion compared to those randomized to treatment alone (61.5% compared to 15.8%) at 12 months and over twice as high at 24 months (61.7% compared to 25.0%). Having a latrine in the household, and there being faeces in it, does not mean that everybody in the household had access to the latrine (i.e. was permitted to use it) or that everybody who had access used it all the time. In a study in The Gambia, it was found that 77% of households with a new pit latrine restricted access to the latrine, with the majority of the restrictions (94%) being for young children.30 Where simple pit latrines are the primary method for the safe disposal of human faeces, it is fair to expect there to be some human faeces in the environment even when latrine coverage is 100%. However, we are sure that latrine coverage, latrine access and latrine use in the villages randomized to receive a single treatment and intensive latrine promotion was substantially greater than that in the villages randomized to a single treatment only.
The study data show that children in the intervention and non-intervention villages had a similar prevalence of chlamydial infection at baseline (around 43%) and that this had declined to around 12–15% after 12 months. After 24 months, the prevalence of ocular C. trachomatis infection was similar to that seen at 12 months, around 14–15%. There had been no rapid emergence of infection in villages randomized to either a single treatment only or a single treatment plus intensive latrine promotion. Both sets of villages had remained at the same level. It was not possible to test the hypothesis that the rate of infection would be faster in the non-intervention villages, since there was no evidence of re-emergent infection at all. It is not possible to interpret these findings as evidence that latrine provision does or does not reduce transmission of ocular C. trachomatis and the hypothesis remains untested.
Trachoma is controlled with the integrated SAFE strategy, and intense latrine promotion is just a single component of that strategy. Additionally, eye-seeking flies are one route of transmission of ocular C. trachomatis, not the only one, and there are likely many more, particularly in hyper-endemic areas. The importance of flies in trachoma transmission will not be constant in time or space, moderated by biotic factors such as the climate and other breeding media and abiotic factors such as differing human behaviours. This points to the potential effect of fly control through sanitation also differing in time and space. Future studies on the role of latrines in trachoma control may look for an independent effect of latrines in post-hoc impact evaluations (which would be confounded by the choice of participants to build latrines or not) or in prospective studies that are powered to show a synergistic effect of the different SAFE components in combination. Testing single components of an integrated control strategy may not yield the results required to make evidence-based decisions.
Authors’ contributions
Concept and design: Gebre, Ayele, Lietman, Emerson, Porco; Acquisition of data: Stoller, Gebre, Ayele, Zerihun, Assefa, Habte, Keenan, House, Gaynor, Lietman, Emerson; Statistical analysis: Zhou, Porco, Keenan, Gaynor, Lietman; Obtaining funding: Gebre, House, Lietman, Emerson; Administrative, technical, or material support: Stoller, Gebre, Ayele, Zerihun, House, Emerson; Drafting of the manuscript: Stoller, Keenan, Lietman, Emerson; Revision of the manuscript for important intellectual content: all authors. Emerson is guarantor of the paper.
Funding
The National Institutes of Health (NEI U10 EY016214) was the main supporter of this trial. We thank the International Trachoma Initiative for their generous donation of azithromycin, the Bernard Osher Foundation, That Man May See, the Harper Inglis Trust, the Bodri Foundation, the South Asia Research Fund, and Research to Prevent Blindness.
Conflicts of interest
We declare that we have no conflicts of interest.
Ethical approval
Written consent to participate was obtained from adults and written assent obtained for children from the parent or guardian. Ethical approval for this study was obtained from the Committee for Human Research of the University of California, San Francisco (H9332-26016), Emory University Institutional Review Board (345-2005), and the Ethiopian Science and Technology Commission. The study was carried out in accordance with the tenets of the Declaration of Helsinki. A Data Safety and Monitoring Committee appointed by the National Institutes of Health-National Eye Institute met annually to oversee the design and implementation of the study. The study is registered at clinicaltrials.gov as trial number NCT00322972 where it is listed as specific aim 3 in the TANA study, and the intervention arms are F and G.
Acknowledgements
We gratefully acknowledge the contribution of the local health workers, community volunteers, and The Carter Center staff in conducting field work, supporting the sample collection, and coordinating logistics. The trachoma control program in Amhara is a joint program of the Amhara Regional Health Bureau and The Lions-Carter Center SightFirst Initiative.




Comments