-
PDF
- Split View
-
Views
-
Cite
Cite
Maria Mavrikaki, Nicoletta Schintu, George G Nomikos, George Panagis, Per Svenningsson, Ropinirole regulates emotionality and neuronal activity markers in the limbic forebrain, International Journal of Neuropsychopharmacology, Volume 17, Issue 12, December 2014, Pages 1981–1993, https://doi.org/10.1017/S1461145714000728
Close - Share Icon Share
Abstract
Restless legs syndrome (RLS) and Parkinson's disease (PD) are movement disorders usually accompanied by emotional and cognitive deficits. Although D3/D2 receptor agonists are effective against motor and non-motor deficits in RLS and PD, the exact behavioral and neurochemical effects of these drugs are not clearly defined. This study aimed to evaluate the effects of acute ropinirole (0, 0.1, 1 or 10 mg/kg, i.p.), a preferential D3/D2 receptor agonist, on intracranial self-stimulation (ICSS), spontaneous motor activity, anxiety- and depression-like behaviors, spatial reference and working memory in rats as well as on certain markers of neuronal activity, i.e. induction of immediate early genes, such as c-fos and arc, and crucial phosphorylations on GluA1 subunit of α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors and NA1, NA2A and NA2B subunits of N-methyl-D-aspartate (NMDA) receptors. Ropinirole decreased ICSS thresholds and induced anxiolytic- and antidepressive-like effects without affecting motor activity or spatial memory. The effects on emotionality were associated with a decrease in p-Ser897-NA1 and an increase in p-Tyr1472-NA2B in the ventral striatum as well as an increased induction of c-fos messenger RNA (mRNA) in the prefrontal cortex (PFC) and decreased expression of arc mRNA in the striatum and the shell of the nucleus accumbens. Our data indicate that ropinirole significantly affects emotionality at doses (1–10 mg/kg, i.p.) that exert no robust effects on locomotion or cognition. The data reinforce the use of D3/D2 receptor agonists in the treatment of RLS and PD patients characterized by emotional deficits and suggest that altered NMDA-mediated neurotransmission in the limbic forebrain may underlie some of ropinirole's therapeutic actions.
Introduction
Restless legs syndrome (RLS) and Parkinson's disease (PD) are movement disorders which are mainly characterized by motor deficits, although co-morbid emotional and cognitive deficits are also observed (Aarsland et al., 2011; Benes et al., 2011; Goetz, 2011; Svenningsson et al., 2012). RLS and PD patients are widely treated with dopaminergic drugs, including agonists of the D2-like receptor family (Jost and Angersbach, 2005; Perez-Lloret and Rascol, 2010; Comella, 2014). Ropinirole is a non-ergoline dopamine receptor agonist (Eden et al., 1991) and is considered a preferential D3 receptor agonist (Reavill et al., 2000; Millan et al., 2002; Jost and Angersbach, 2005). Its affinity for D3 receptors is 10–20 fold higher than for the other receptors of the D2-like family, the D2 and D4 receptors (Reavill et al., 2000; Rogers et al., 2000; Jost and Angersbach, 2005), whereas it has no affinity for the D1 receptors (Eden et al., 1991; Millan et al., 2002). In addition, ropinirole acts as a weak agonist of the α2 (Eden et al., 1991; Millan, 2010), 5-HT2 (Eden et al., 1991; Millan, 2010) and 5-HT1A receptors (Millan et al., 2002; Millan, 2010). Ropinirole has a relatively low propensity to induce dyskinesia compared to other D2-like receptor agonists (Carta et al., 2008).
D3 receptors are expressed both presynaptically and post-synaptically (Zapata et al., 2007) and are selectively expressed in forebrain limbic structures (Carr et al., 2002), including the nucleus accumbens (NAc) and the prefrontal cortex (PFC) (Southam et al., 2007), regions critical for emotionality. Indeed, the mesolimbic dopaminergic pathway regulates motivational states (Nestler and Carlezon, 2006) and the mesocortical dopaminergic pathway is involved in memory and cognitive functions (Floresco and Magyar, 2006).
At a cellular level, the dopaminergic system modifies rapid neurotransmission, including regulation of the major inhibitory and excitatory neurotransmission of γ-aminobutyric acid (GABA) and glutamate, respectively (Svenningsson et al., 2004; Beaulieu et al., 2007). Furthermore, it has been demonstrated that disruption of the bidirectional pathway of neuronal activity related to trafficking of ionotropic glutamatergic α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate receptors (NMDARs) could be associated with neuropsychiatric disorders (Gao and Wolf, 2007). Thus, we hypothesize that ropinirole could induce post-translational modifications of AMPA and NMDA receptors.
Crucial phosphorylation sites (including Ser831 and Ser845) on the GluA1 subunit of the AMPA receptor have been shown to regulate the trafficking of the receptor (Lee, 2006). Furthermore, NMDA receptor trafficking is also dependent on the phosphorylation state of different subunits (Lau and Zukin, 2007). Specifically, phosphorylation of Ser896, by PKC, and Ser897, by PKA, on the NA1 subunit are required for synaptic localization (Lau and Zukin, 2007). In addition, dephosphorylation of the Tyr1472 on the NA2B subunit leads to internalization of NMDA receptors (Lau and Zukin, 2007).
Neurotransmitters induce short-term actions via ion channels and metabotropic receptors and long-term actions via regulation of gene expression. The levels of immediate early genes (IEGs), such as c-fos and arc (activity-regulated cytoskeleton-associated protein or Arg3.1), are generally low in cells that do not receive any stimuli (Martin and Magistretti, 1998), but are rapidly and transiently activated in response to multiple environmental stimuli, action potentials or pharmacological agents (Robertson et al., 1991; Cole and Di Figlia, 1994; Panagis et al., 1997; Martin and Magistretti, 1998; Kovacs et al., 2001). Thus, IEG expression constitutes an indirect biochemical indication of synaptic neurotransmission and activity (Dassesse et al., 1999). While up-regulation of c-fos messenger RNA (mRNA) indicates coinciding neuronal activity, arc is implicated in synaptic plasticity (Zachariou et al., 2006; Moro et al., 2007; Bramham et al., 2008, 2010; Molteni et al., 2009). arc is related to cytoskeletal structural and functional reorganization (Dassesse et al., 1999; Molteni et al., 2009) and in protein synthesis related to synaptic plasticity (Bramham et al., 2010). This correlation is supported by the fact that arc mRNA is transferred in the dendrites and is accumulated in synaptic terminals with robust recent neuronal activity (Pei et al., 2000; Moro et al., 2007; Bramham et al., 2010), where it selectively modifies the expression of AMPA receptors in excitatory synapses, increasing their endocytosis (Bramham et al., 2008, 2010; Molteni et al., 2009), but decreasing their synaptic expression leading to long-term depression (LTD) (Bramham et al., 2008, 2010). Behaviorally, arc has been implicated in learning and memory, information processing (Bramham et al., 2008; Molteni et al., 2009) as well as in depression (Molteni et al., 2009) and emotional aspects of behavior (Eriksson et al., 2012).
Considering the selective expression of D3 receptors in the limbic forebrain, the present study aimed to carefully assess dose-dependent effects of ropinirole, on emotional aspects of behavior, such as brain stimulation reward, anxiety- and depression-like responses. We also aimed to investigate possible confounding effects of ropinirole on motor activity as well as potential impairments in cognition. Furthermore, the study was designed to evaluate ropinirole's effects on markers of neuronal activity in the limbic forebrain, which may at least partially underlie the observed behavioral responses. Specifically, we investigated crucial phosphorylations on the GluA1 subunit of AMPAR and the NA1, NA2A and NA2B subunits of NMDARs as well as the induction of IEGs expression, such as c-fos and arc.
Materials and methods
Male Sprague–Dawley rats weighing 220–350 g were used. Animals were housed 3–5 per cage except for the rats that were used for intracranial self-stimulation (ICSS) and radial arm maze (RAM) studies, which were housed in individual cages. All animals were maintained on a 12 h light −12 h dark cycle with free access to food and water. Experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and approved by the local ethical committee at Karolinska Institutet.
Ropinirole (Sigma-Aldrich, USA) was dissolved in 0.9% NaCl and injected intraperitoneally (i.p.) at a dose of 0.1, 1 and 10 mg/kg of body weight.
Behavioral studies
Motor activity
Motor activity was measured in square open field arenas (68 × 68 × 45 cm), equipped with two rows of eight photocells along two adjacent sides, placed 4 and 12.5 cm above the floor. The open field was enclosed in a ventilated, sound-attenuating box kept in dark. Measurements were performed between 10:00 and 17:00 h. Three days prior to the start of the behavioral experiment, the animals were habituated to the testing apparatus for 30 min daily. Each animal (n = 10) received three doses of ropinirole (0.1, 1 or 10 mg/kg, i.p.) or vehicle in a randomized order with a 3-d interval in-between. Total locomotor, peripheral, corner activity and rearing were measured 15 min after ropinirole administration in 30 min sessions. No measurements were performed during the wash-out periods.
Intracranial self-stimulation (ICSS)
In the ICSS study, rats (n = 7) were implanted with a monopolar stimulation electrode aimed at the medial forebrain bundle (MFB) at the level of lateral hypothalamus (LH) (2.56 mm posterior to bregma, 1.8 mm lateral from midsagittal suture, and 8.6 mm below the outer flat skull) according to Paxinos and Watson (2007). Surgery followed previously described procedures (Mavrikaki et al., 2009). Rats were trained in operant chambers equipped with a stainless-steel lever. Each bar-press triggered a constant current stimulator (Med Associates, USA) that delivered a 0.4-s train of rectangular cathodal pulses of constant duration (0.1 ms) and intensity (250 μA) and variable frequency (15–100 Hz, i.e. 6–40 number of pulses/0.4 s). During the initial acquisition phase, the animals were trained to self-stimulate using stimulation parameters that maintained near maximal bar-pressing rates. As soon as self-stimulation had been acquired, rats were trained to self-stimulate using four alternating series of descending (two trials/pulse frequency) and ascending (two trials/pulse frequency) pulse frequencies. Each pulse frequency was varied of approximately 0.1 log units and was tested within 60 s trials, followed by a 30 s extinction period. For each trial, there was a ‘priming’ phase during which the animals received three trains of stimulation at the frequency which was available for the consequent trial. A rate-frequency determination session lasted about 45 min. Each measurement consisted of a pre-drug rate-frequency determination as well as a post-drug rate-frequency determination. Analysis was performed on two aspects of data obtained from the rate-frequency curve: the ICSS threshold and the maximum rate of responding or asymptote (Mavrikaki et al., 2009). Rats were treated with various doses of ropinirole (0.1, 1 and 10 mg/kg, i.p.) or vehicle in a randomized order with a 3-d interval in-between. After a 15-min post-injection interval, rats were placed in the operant chamber and the post-drug session began. Stimulation sites were post-mortem confirmed by histology.
Elevated plus maze (EPM)
The elevated plus maze (EPM) apparatus consisted of two open arms and two closed arms opposite to each other (24 cm length × 15 cm width), elevated 70 cm from the floor. The closed arms were surrounded by gray walls (23 cm height). Rats (n = 10 per group in a between subjects design, total n = 40) were treated with ropinirole (0.1, 1 or 10 mg/kg, i.p.) or vehicle and 15 min later they were placed in the center of the maze facing an open arm. The experiment was performed with normal room lights. Time spent in open and closed arms, as well as in the center was measured for 5 min using the Anymaze software (version 4.75). Number of entries in open and closed arms and ethological parameters, such as total stretch attend postures (SAP), total head dips (HD) as well as % protected SAP and % protected HD were also counted. Protected SAP and protected HD were defined as the postures performed from the protective zone, which includes the closed arms and the central area.
Forced swim test (FST)
Forced swim test (FST) was based on a revised version of Porsolt's protocol (Porsolt et al., 1978). Naïve rats (n = 10 per group in a between subjects design, total n = 40) were forced to swim for 15 min in opaque Plexiglas cylinders (high 60 cm × 20 cm diameter) containing 30 cm of water maintained at 23–24°C. Twenty-four hours following the pretesting phase, rats were treated with ropinirole (0.1, 1 or 10 mg/kg, i.p.) or vehicle and 15 min later they were placed in the cylinder. The total duration of immobility, climbing and swimming were measured for 5 min according to Chiba et al. (2010).
Radial arm maze (RAM)
In the RAM study, Sprague–Dawley rats (n = 10) weighing ∼250 g at their arrival were used. For one week after their arrival they had free access to food and water. Afterwards, they were kept at 85–90% of their free feeding body weight and had continuous access to water. The food was provided after training or testing or at the same time when there was no experimental procedure.
The RAM apparatus consisted of 8 arms (43 cm length × 15 cm width) extended from a common central arena (53 cm diameter). There were a variety of extra-maze visual cues including the laboratory equipment and posters, which helped rats to navigate. These visual cues were kept constant for all the experimental days.
Rats were habituated to the maze and food pellets (Noyes precision food pellets, Sandown Scientific) in accordance to protocols previously described by others (Kay et al., 2010). Once confirmed that each rat entered each arm, four baited arms (no more than two consecutive) were selected for each of them, and arms were counterbalanced between rats. Baited arms remained constant throughout their training and testing. Small food dishes were placed at the end of each arm and only in the reinforced arms the dishes contained a food pellet. In the remaining arms, a food pellet was placed outside the final wall so that the rats could smell the pellet, without having access to it.
During training, the rats were placed in the central arena and were given the required time to collect the four food pellets for a maximum period of 5 min. Three consecutive trials were performed. Arm entries and time spent to complete the trial were recorded using the RAM software. Accuracy was separated into working (re-entry in an arm that was already visited within a trial) and reference memory errors (entering in an unbaited arm). The training lasted 15–32 d, until each rat acquired an average of 71–75% of accuracy during the last 7 d. Two rats were excluded since they did not reach the expected criterion of accuracy. All the animals received all doses of ropinirole (0.1, 1 and 10 mg/kg, i.p.) or vehicle. A 3-d period was allowed between measurements and in the second day an additional training session took place in order to avoid extinction of the existing memory.
Molecular studies
Immunoblotting
Rats (n = 6 per group, total n = 24) were treated with ropinirole (0.1, 1 or 10 mg/kg, i.p.) or vehicle and sacrificed 20 min later using focused microwave irradiation (Muromachi Kikai, Japan) (Svenningsson et al., 2007). PFC and ventral striatum (VSTR) were immediately dissected and stored at −80°C until used. Frozen tissue samples were sonicated in 1% sodium dodecyl sulfate (SDS) and boiled for 10 min. Small aliquots of the homogenate were retained for protein determination by the bicinchoninic acid protein assay (BCA-kit, Pierce, Rockford, IL, USA). Equal amounts of protein (40 μg) were loaded on 9% acrylamide gels and the proteins were separated by SDS-PAGE and transferred to Immobilon-P (Polyvinylidene Difluoride membranes, Sigma-Aldrich, USA). The membranes were blocked for 1 h at room temperature with 5% (w/v) fat free milk in TBS-Tween 20. Immunoblotting was carried out with phosphorylation-state-specific antibodies against p-S831-GluA1(1:500, Millipore), p-S845-GluA1(1:1000, Upstate/Millipore), p-Ser896-NA1 (1:500, Upstate/Millipore), p-Ser-897-NA1 (1:500, Cell signaling), p-Ser1232-NA2A (1:500, Tocris), p-Ser1303-NA2B (1:200, Upstate) and p-Tyr1472-NA2B (1:500, Calbiochem) or antibodies that are not phosphorylation state specific, total GluA1 (1:1000, Millipore), NA1 (1:1000, Upstate), NA2A (1:500, Calbiochem), NA2B (1:500, Calbiochem) and α-actin (1:4000, Sigma-Aldrich) in 5% fat free dissolved in TBS-Tween 20. Antibody binding was revealed by HRP conjugated goat anti-rabbit or mouse IgG (1:6000, Thermo Scientific) and the enhanced chemiluminescence detection method (PerkinElmer) with Kodak BioMax film. Bands were quantified by densitometry with the Scion Image Software. The level of phosphorylated proteins was normalized to total level.
In situ hybridization
In situ hybridization studies were carried out as previously described (Eriksson et al., 2012) in rats treated with ropinirole (0.1, 1 or 10 mg/kg, i.p.) or vehicle (n = 5 per group, total n = 20) and sacrificed 30 min later by decapitation. Brains were rapidly removed, frozen and stored at −80°C. 12 μm coronal cryostat sections were postfixed in 4% paraformaldehyde for 5 min at room temperature, rinsed twice in 4 × sodium chloride–sodium citrate buffer (SSC) and placed into 0.25% acetic anhydride in 0.1 m triethanolamine/4 × SSC for 10 min. After dehydration in graded alcohols, the sections were hybridized overnight at 55°C with 35S-labelled antisense probe for arc or c-fos mRNA in 100 μl of hybridization solution (20 mmTris-HCl/1 mm ethylenediaminetetraacetic (EDTA)/300 mmNaCl/50% formamide/10% dextran sulphate/1 × Denhardt's/250 g/ml yeast tRNA/100 g/ml salmon sperm DNA/0.1% SDS/0.1% sodium thiosulphate). The slides were washed in 4 × SSC, RNAse A (20 min, at 37°C), 2 × SSC, 1 × SSC, 0.5 × SSC at room temperature, and 0.1 × SSC at 65°C, before being dehydrated in graded alcohols. The slides were then exposed on X-ray films for at least 30 d. Densitometric measurements were obtained from autoradiograms using Scion Image Software after subtraction of non-specific binding.
Statistics
Data were statistically analyzed using one-way analysis of variance (ANOVA) with or without repeated measures (rm), considering the experimental design, followed by correlated t test using Bonferroni's adjustment for multiple comparisons. Immunoblotting and in situ hybridization data were further analyzed using least significant difference (LSD) post-hoc test due to the small number of samples.
Results
Behavioral studies
Motor activity
Ropinirole affected rearing and time spent in corners more than locomotion and peripheral activity (Fig 1). Specifically, rm-ANOVAs revealed a significant drug effect on locomotor/horizontal and peripheral activity [F(3,27) = 6.549, p < 0.05 and F(3,27) = 5.548, p < 0.01], although the Bonferroni test for multiple comparisons demonstrated no significant differences between groups (Fig. 1a, b). rm-ANOVA demonstrated a significant drug effect on corner time [F(3,27) = 10.54, p < 0.001], which was significant for the dose of 10 mg/kg according to the Bonferroni ptest (p < 0.001, Fig. 1c). rm-ANOVA revealed a significant drug effect on rearing [F(3,27) = 4.536, p = 0.05], which was significant for 1 and 10 mg/kg (p < 0.05, Fig. 1d).

Effects of ropinirole on motor activity. Effects of ropinirole on (a) locomotor activity, (b) peripheral activity, (c) corner time, (d) rearing. Asterisks (*) signify a statistically significant effect compared to 0 mg/kg: * p < 0.05, *** p < 0.001, according to Bonferroni post-hoc for multiple comparisons.
ICSS
Ropinirole decreased ICSS threshold (Fig. 2). rm-ANOVA demonstrated a significant drug effect on ICSS threshold [F(3,24) = 5.683, p < 0.01, Fig. 2a] but no significant effects on asymptote (Fig. 2b). Bonferroni test indicated a significant decrease in ICSS threshold at the dose of 10 mg/kg (p < 0.05, Fig. 2a, b).
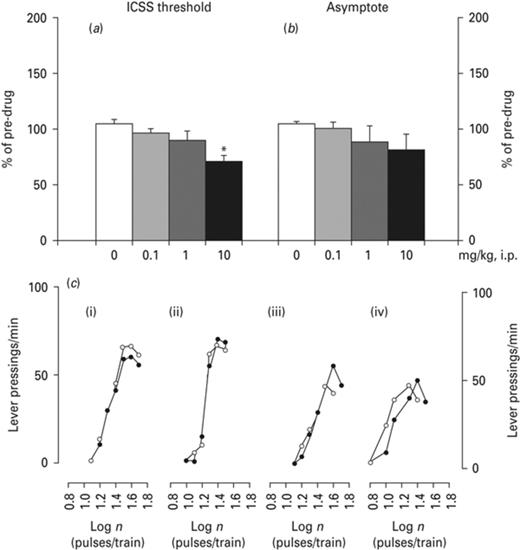
Effects of ropinirole on intracranial self-stimulation (ICSS). Effects of ropinirole on (a) ICSS threshold and (b) asymptote. Rate-frequency functions from representative animals for each drug treatment (rate of lever pressing as a function of stimulation frequency). Each plot represents data from a single animal under predrug and drug conditions. (c) Rate frequency functions were obtained by logarithmically decreasing the frequency of the stimulation pulses from a value that sustained maximal lever pressing to one that failed to sustain lever pressing. (i) Vehicle, (ii) 0.1 mg/kg, (iii) 1 mg/kg and (iv) 10 mg/kg of ropinirole. -●-pre-injection; -○-post-injection. Asterisks (*) signify a statistically significant effect compared to 0 mg/kg; p < 0.05, according to Bonferroni post-hoc for multiple comparisons.
EPM
Ropinirole affected the time spent in the open and closed arms, the % of time spent in the open arms as well as the % of protected SAP and HD (Fig. 3). One-way ANOVA indicated significant effects of ropinirole on the open arm time [F(3,36) = 6.085, p < 0.01] and the % of open arm time [F(3,36) = 6.32, p < 0.01]. The Bonferroni test indicated a significant increase of the time spent in open arms at the dose of 1 mg/kg (p < 0.05) and 10 mg/kg (p < 0.01, Fig. 3a, g). One-way ANOVA demonstrated a significant drug effect on the time spent in closed arms [F(3,36) = 10.81, p < 0.001] (Fig. 3b), which was significant at the dose of 1 and 10 mg/kg (p < 0.001). However, one-way ANOVA indicated that there was no significant drug effect considering the time spent in the center (Fig. 3c), total arm entries (Fig. 3d), total SAP (Fig. 3e) and HD (Fig. 3f). One-way ANOVA indicated a significant drug effect on % of protected SAP [F(3,36) = 9.139, p < 0.001] (Fig. 3h), which was significant at the dose of 1 mg/kg (p < 0.01) and 10 mg/kg (p < 0.001). Finally, one-way ANOVA indicated a significant effect of ropinirole in the % of protected HD [F(3,36) = 9.275, p < 0.001] (Fig. 3i), which reached significance only at the dose of 10 mg/kg (p < 0.001), according to the Bonferroni test.
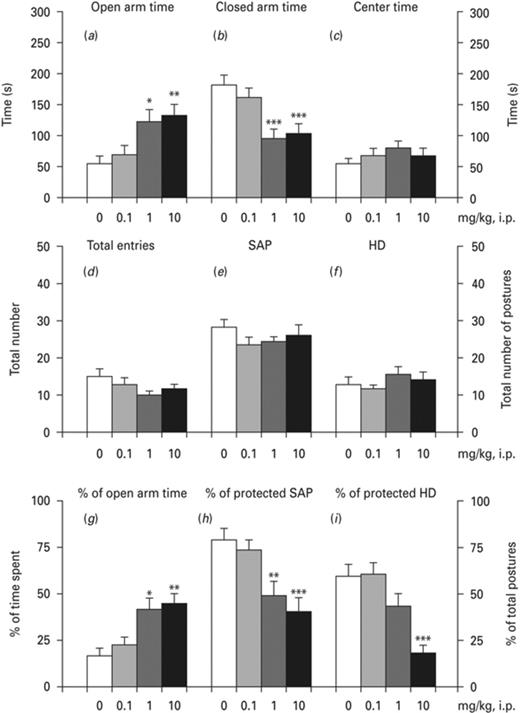
Effects of ropinirole on elevated plus maze (EPM). Effects of ropinirole on (a) time spent in open arms, (b) time spent in closed arms, (c) time spent in the center, (d) total number of entries in open and closed arms, (e) total stretch attend postures (SAP), (f) total number of head dips (HD), (g) the % of time spent in open arms, (h) the % of protected SAP and (i) the % of protected HD. Asterisks (*) signify a statistically significant effect compared to 0 mg/kg; *p < 0.05, **p < 0.01, *** p < 0.001, according to Bonferroni post-hoc for multiple comparisons.
FST
Ropinirole decreased immobility time and increased climbing and swimming (Fig. 4). One-way ANOVA indicated a significant effect of ropinirole on immobility [F(3,36) = 15.60, p < 0.001] (Fig. 4a). The Bonferroni test indicated a statistically significant decrease in immobility time at the dose of 1 mg/kg (p < 0.01) and 10 mg/kg (p < 0.001). Furthermore, one-way ANOVA demonstrated a significant effect of ropinirole on climbing [F(3,36) = 7.284] (Fig. 4b). The Bonferroni test indicated that only the dose of 10 mg/kg significantly increased climbing time (p < 0.05). Finally, one-way ANOVA revealed a significant effect of ropinirole on swimming [F(3,36) = 6.036, p < 0.05] (Fig. 4c), which reached significance at the doses of 1 mg/kg (p < 0.05) and 10 mg/kg (p < 0.01).
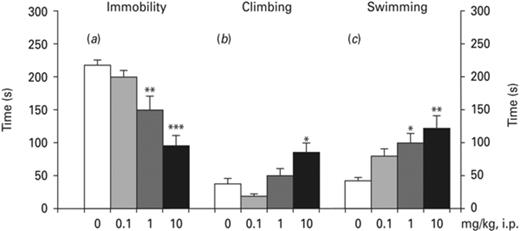
Effects of ropinirole on forced swim test (FST). Effects of ropinirole on (a) immobility, (b) climbing and (c) swimming. Asterisks (*) signify a statistically significant effect compared to 0 mg/kg; * p < 0.05, ** p < 0.01, ***p < 0.001, according to Bonferroni post-hoc for multiple comparisons.
RAM
Ropinirole did not significantly affect spatial working or reference memory (Fig. 5). rm-ANOVA analysis revealed that ropinirole did not affect accuracy (Fig. 5a), working memory errors (Fig. 5b), the % of working memory errors (Fig. 5e), reference memory errors (Fig. 5c) or the % of reference memory errors (Fig. 5f). In contrast, rm-ANOVA indicated that ropinirole influenced the mean time spent to complete a trial [F(3,25) = 4.245, p < 0.05] (Fig. 5d). This effect was significant at the dose of 10 mg/kg (p < 0.05), according to the Bonferroni test. In our locomotor activity study, rm-ANOVA demonstrated no significant differences in horizontal activity at the time-points 0–5 min (Fig. 5g), 5–10 min (Fig. 5h) or 10–15 min (Fig. 5i) which corresponds to trial 1, trial 2 and trial 3, respectively, in the RAM study.
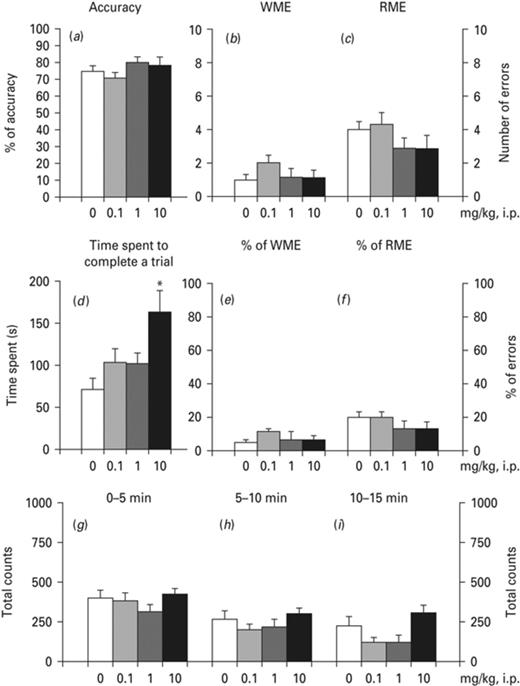
Effects of ropinirole on radial arm maze (RAM). Effects of ropinirole on (a) total accuracy, (b) total number of working memory errors, WME, (c) total number of reference memory errors, RME, (d) mean time spent to complete a trial, (e) % of WMEs and (f) % of RMEs. Effects of ropinirole on horizontal activity according to the locomotor activity study: (g) 0–5 min, (h) 5–10 min and (i) 10–15 min. Asterisks (*) signify a statistically significant effect compared to 0 mg/kg; *p < 0.05, according to Bonferroni post-hoc for multiple comparisons.
Molecular studies
Immunoblotting
Ropinirole did not affect the total levels of the NMDAR and AMPAR subunits but demonstrated selective post-translational modifications on NA1 and NA2B subunits of NMDARs in the VSTR (Fig. 6). One-way ANOVA indicated that ropinirole did not significantly affect the total levels of NA1, NA2A, NA2B, GluA1 and a-actin as well as p-Ser896-NA1, p-Ser1232-NA2A, p-Ser1303-NA2B, p-Ser831-GluA1, p-Ser845-GluA1 in PFC (data not shown) and VSTR. Interestingly, ropinirole significantly reduced p-Ser897-NA1 in the VSTR [F(3,19) = 4.168, p < 0.05], but not in the PFC (data not shown). Ropinirole's effect on p-Ser897-NA1 in the VSTR reached significance at the doses of 1 and 10 mg/kg (p < 0.05, Fig. 6) in accordance to the LSD test for multiple comparisons. Furthermore, ropinirole selectively increased p-Tyr1472-NA2B in the VSTR [F(3,20) = 3.202, p < 0.05, Fig. 6], but not in the PFC (data not shown). This increase reached significance only at the dose of 1 and 10 mg/kg (p < 0.05) according to the LSD test (Fig. 6).
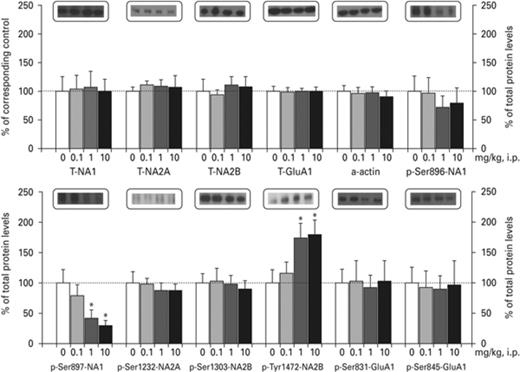
Effects of ropinirole on N-methyl-D-aspartate (NMDA) and α-Amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor phosphorylation in the ventral striatum (VSTR). Asterisks (*) signify a statistically significant effect compared to 0 mg/kg; *p < 0.05, according to LSD post-hoc.
In situ hybridization
Ropinirole induced a selective pattern of IEG expression in the limbic forebrain (Fig. 7). Specifically, ropinirole significantly increased c-fos mRNA in the PFC [F(3,18) = 4.390, p < 0.05]. This effect reached significance at the dose of 10 mg/kg (p < 0.01) according to the LSD test (Fig. 7a). In contrast, no significant differences were found when analyzing the dorsal striatum and NAc shell or core (data not shown).
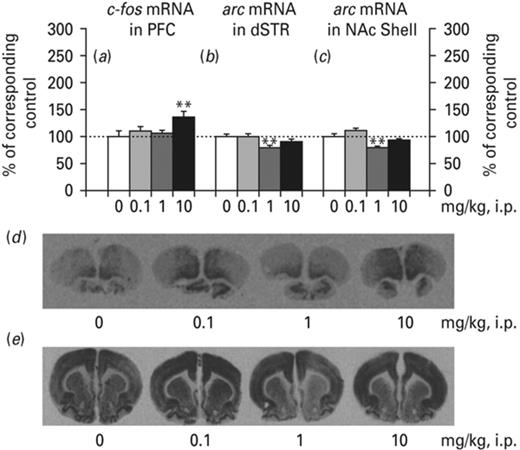
Effects of ropinirole on c-fos and arc messenger RNA (mRNA). Ropinirole significantly increased c-fos mRNA in the prefrontal cortex (PFC) (a and d) and decreased arc mRNA the dorsal striatum (dSTR) (b and e) and nucleus accumbens (NAc) shell (c and e). Asterisks (*) signify a statistically significant effect compared to 0 mg/kg; **p < 0.01, according to least significant difference (LSD) post-hoc.
arc mRNA in the dorsal striatum was found to be significantly decreased [F(3,18) = 4.093, p < 0.05], and this effect reached significance at the dose of 1 mg/kg (p < 0.01) according to the LSD test (Fig. 7b). One-way ANOVA indicated significant differences between groups in arc mRNA in NAc core [F(3,18) = 3.301, p < 0.05]; however, post-hoc analysis indicated no significant differences of ropinirole. One-way ANOVA indicated significant differences between groups in arc mRNA in NAc shell [F(3,18) = 7.081, p < 0.01] and this effect reached significance at the dose of 1 mg/kg (p < 0.01), according to the LSD test (Fig. 7). In contrast, no significant differences were found when analyzing arc mRNA in the PFC (data not shown).
Discussion
The present study demonstrates that ropinirole significantly decreased ICSS threshold, immobility time and time spent in the closed arms in the EPM, but increased active behaviors in the FST and time spent in open arms, without exerting significant effects on locomotion or spatial reference and working memory. Thus, ropinirole demonstrated reinforcing, anxiolytic- and antidepressive-like properties, affecting multiple aspects of emotionality, without exerting any major confounding motor or cognitive effects. The reward facilitating, anxiolytic- and antidepressive-like profile of ropinirole was accompanied by selective effects on neuronal activity markers in the limbic forebrain. Our data demonstrate a selective decrease in p-Ser897-NA1 and increase in p-Tyr1472-NA2B in the ventral striatum as well as an increased c-fos mRNA in the PFC and decreased arc mRNA in the striatum and the shell of the nucleus accumbens.
The present study indicates that ropinirole did not significantly affect general locomotor or peripheral activity, but significantly decreased rearing, an exploratory behavior, in a wide range of doses tested. The highest dose of ropinirole demonstrated a tendency for increased locomotor activity, while lower doses tended to decrease locomotion. The tendency for hyperlocomotion that we observed at the highest dose is in accordance with previous findings that indicated that 10 mg/kg of ropinirole increased locomotion in habituated animals (Millan et al., 2004). In addition, another study indicated that D3 receptor stimulation leads to hypolocomotion (Millan et al., 2000). However, the time spent in the corner was significantly reduced in response to the highest dose tested, which might indicate an anxiolytic-like profile, since time spent in the corners of an open field reflects anxiety-like responses (Zhang et al., 2011). This hypothesis was further assessed in the EPM study (see below).
In contrast to the absence of significant effects on locomotion, ropinirole demonstrated robust and dose-dependent effects on emotional processes. Specifically, ropinirole at the highest dose tested, significantly decreased ICSS threshold, which indicates reward-facilitating effects. Interestingly, we also observed a tendency for decreased asymptote, although this effect did not reach significance. The effects of ropinirole on reward function are in accordance with previous studies that assessed the effects of the selective D3 receptor agonist 7-OH-DPAT on ICSS paradigm and demonstrated decreases both at ICSS threshold and ICSS asymptote (Panagis and Spyraki, 1996). Furthermore, recent studies in rhesus monkeys demonstrated reinforcing effects of ropinirole (Freeman et al., 2012). Accordingly, overexpression of the D3 receptor in mice disrupts motivation (Simpson et al., 2014). Anhedonia constitutes one of the major depressive symptoms that 40% of PD patients experience (Tadaiesky et al., 2008). Thus, the facilitating effects of ropinirole on brain reward processes could be beneficial for this subpopulation of PD patients.
In addition to the effects on brain reward function, ropinirole demonstrated a clear anxiolytic- and antidepressive-like profile in the doses of 1 and 10 mg/kg. In these doses, ropinirole significantly increased the time spent in the open arms of the EPM and decreased the time spent in the closed arms, which indicates a clear anxiolytic effect. Our data are in accordance with previous findings that demonstrated an anxiolytic-like profile of ropinirole and other D3 receptor agonists (Rodgers et al., 1996; Rogers et al., 2000; Rogoz et al., 2004). However, to the best of our knowledge, this is the first study that assessed also ethological parameters. Our findings demonstrate that ropinirole significantly decreased protected HD at 10 mg/kg, which indicates not only anxiolytic-like behavior, but also effects on decision making probably related to increased impulsivity. This observation corresponds nicely to clinical data, showing that RLS or PD patients treated with ropinirole display lack of impulse control (Ahlskog, 2011).
In the FST, ropinirole significantly and dose-dependently decreased immobility time and increased swimming at the higher doses tested, but only 10 mg/kg significantly increased climbing. It is accepted that the different active behaviors are related to different neurotransmitters system function (Reneric and Lucki, 1998; Page et al., 1999). Specifically, the serotoninergic system has been implicated in swimming behavior and climbing might be related to function of the noradrenergic system. The present data demonstrate that ropinirole may affect serotoninergic neurotransmission, whereas noradrenergic neurotransmission might be affected only following high doses of ropinirole. Our data are in accordance with previous findings that indicated that ropinirole decreases immobility time (Millan et al., 2000, 2004; Rogers et al., 2000; Dhir and Kulkarni, 2007; Ghorpade et al., 2011), improves depressive symptoms in humans (Benes et al., 2011), and provide novel data considering the possible ensuing interactions between monoaminergic systems. Those emotional effects of ropinirole seem to be D2-like receptor mediated, since they can be blocked by D2-like receptor antagonists, such as haloperidol and sulpiride, but not by the D1 receptor antagonist SCH 23390 (Dhir and Kulkarni, 2007). Other studies indicated that the neurotrophic effects of ropinirole on mesencephalic dopamine neurons were blocked by the D3 antagonist nafadotride, but not the D2 antagonist sulpiride (Du et al., 2005). Thus, ropinirole mediates some of its effects selectively via the D3 receptors.
Furthermore, the present study demonstrated that ropinirole selectively modifies emotional processes without exerting confounding effects on motor or cognitive functions. Our data from the RAM study indicate that ropinirole did not affect the total accuracy to perform the task. Ropinirole did not affect working memory or reference spatial memory. Notably, ropinirole tended to improve reference memory, an effect that did not reach significance. However, ropinirole increased the time to complete the trial at the highest dose tested, an effect that might be related to, for example, disinterest in food or anorexia. Interestingly, D2 receptor activation has been shown to suppress appetite (Kern et al., 2012). The absence of cognitive effects is in accordance with previous studies, which demonstrated that ropinirole may not significantly affect cognitive aspects of behavior (Millan, 2010; Zaqmutt and Tarrants, 2012), and others which have shown that D3 receptors do not affect spatial learning (Xing et al., 2010). Our findings are also supported by a recent study (Simpson et al, 2014), which demonstrated that over-expression of the D3 receptor disrupts motivation but not cognition.
At the cellular level, our data demonstrate that ropinirole specifically affected the phosphorylation state of NMDA, but not AMPA, receptors. D2 receptor stimulation inhibits adenyl cyclase and decreases NMDA currents (Neve et al., 2004). In our immunoblotting studies, ropinirole selectively affected the phosphorylation state of the NA1 subunit in Ser897 in the VSTR. This critical phosphorylation is regulated by PKA activity (Lau and Zukin, 2007) and the dephosphorylation of this residue seems to be in accordance to ropinirole's D3 receptor agonist profile. Indeed, D3 receptor knockout mice demonstrate increased NMDA-induced phosphorylation of Ser897-NA1 (Jiao et al., 2007). Furthermore, ropinirole selectively increased the phosphorylation of NA2B subunit in Tyr1472, a Fyn-dependent phosphorylation site (Lau and Zukin, 2007), in the VSTR. Since AKT regulates Fyn kinase activity, it is intriguing that D3 receptor agonism activates AKT in striatum (Salles et al., 2013). Considering the major contribution of VSTR in emotional processes, we speculate that the altered phosphorylation pattern of NMDA receptors might be related to the observed emotional phenotype.
Ropinirole significantly affected the induction of IEGs in the PFC and the striatum. Specifically, ropinirole, at 10 mg/kg, increased c-fos mRNA in the PFC, an effect that might be related to its reward-facilitating, anxiolytic and/or antidepressive-like profile. Our data are in accordance with previous findings that indicated that high doses of the selective D3 receptor agonist 7-OH-DPAT increases c-fos mRNA in the PFC (Ishibashi et al., 2002). In addition, ropinirole (1 mg/kg) significantly decreased arc mRNA in the dorsal striatum and in the shell part of nucleus accumbens (NAc), an effect that might be related to the tendencies for hypolocomotion observed in the present study. Acute administration of quinpirole, a D2/D3 receptor agonist, or caffeine, an adenosine receptor antagonist has been shown to suppress the expression of IEGs, including arc, selectively in the striatum (Svenningsson et al., 1995; Dassesse et al., 1999), further supporting that D2/D3 receptor stimulation can lead to alterations in arc mRNA.
In summary, the present study demonstrates that ropinirole markedly affected emotional processes, without significantly affecting motor activity and/or cognition. Furthermore, our data demonstrate that ropinirole regulated NMDA receptor phosphorylation as well as affected the induction of IEGs in the D3 receptor-rich regions of PFC and VSTR, which may at least partially underlie the reward facilitating, anxiolytic- and/or antidepressive-like effects of the drug. To conclude, our data support the notion that D3 receptor agonists, including ropinirole, could be an efficient treatment for several emotional deficits that usually accompany PD and RLS. Since ropinirole is usually given chronically to PD and RLS patients, it will be important in the future to study whether these effects persist also by repeated administration.
Acknowledgements
We thank the Swedish Research Council for their support with a grant to PS. We also thank the Alexander S. Onassis Public Benefit Foundation for their support with a scholarship to MM.
Statement of Interest
The authors declare no conflict of interest.



