-
PDF
- Split View
-
Views
-
Cite
Cite
Haowei Shen, Peter W. Kalivas, Reduced LTP and LTD in prefrontal cortex synapses in the nucleus accumbens after heroin self-administration, International Journal of Neuropsychopharmacology, Volume 16, Issue 5, June 2013, Pages 1165–1167, https://doi.org/10.1017/S1461145712001071
Close - Share Icon Share
Abstract
Addiction changes prefrontal cortex regulation of the nucleus accumbens, including reduced ability to induce long-term potentiation (LTP) and long-term depression (LTD). This important potential mechanism of impaired prefrontal regulation of behaviour has been shown only for cocaine. Here we show that animals trained to self-administer heroin demonstrate impaired LTP and LTD in the core of the nucleus accumbens following in vivo stimulation of the prelimbic prefrontal cortex. These data indicate that compromised synaptic plasticity in prefrontal to accumbens projections is a common feature of at least two distinct classes of addictive drug.
Introduction
Drug addiction has been proposed to arise in part from impairments in the capacity of the prefrontal cortex (PFC) to regulate the motivation to relapse to drug use (Goldstein & Volkow, 2011). The nucleus accumbens serves as the main portal of entry whereby glutamatergic efferents from the PFC enter the basal ganglia to help guide behaviour (Yin et al.2008). A key finding demonstrating a deficiency in the projection from the PFC to the accumbens is the inability to induce classic forms of synaptic plasticity, such as long-term potentiation (LTP) or long-term depression (LTD; Huang et al.2011; Kasanetz et al.2010; Martin et al.2006; Moussawi et al.2009). However, this critical observation supporting a functional pathology in prefrontal to accumbens communication as a cardinal feature of drug addiction has only been obtained in animals trained to self-administer or administered non-contingent cocaine. Indeed, given the fact that distinct cellular adaptations are induced between different classes of addictive drug in the prefrontal to accumbens projection indicate that the impaired synaptic plasticity may not be a universal feature of addiction. For example, particularly relevant to synaptic plasticity, while withdrawal from cocaine administration results in accumbens spiny neurons showing evidence of LTP-like changes, such as increased spine head diameter and AMPA receptor-mediated current, withdrawal from opioids produces opposite LTD-like morphological and electrophysiological changes (Robinson & Kolb, 2004; Shen et al.2011; Wolf, 2010). However, supporting the possibility of heroin-induced changes in the PFC to accumbens projection, a number of studies find heroin-induced changes in synaptic plasticity in the PFC (van den Oever et al.2008, 2010).
Here we endeavoured to determine if the self-administration of heroin, a drug of abuse with a distinct binding site and many different behavioural actions from cocaine (Badiani et al.2011), also produces a marked impairment in the ability to induce synaptic plasticity in the glutamate projection from the PFC into the nucleus accumbens.
Method
All procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Assessment and Accreditation of Laboratory Animal Care. Male Sprague–Dawley rats were trained to lever press for i.v. heroin administration using a fixed ratio-1 schedule of reinforcement for 2 wk and then underwent 2 wk of extinction training (see Supplementary Fig. S1 and Supplementary material for detailed surgical and behavioural methods). Rats readily acquired heroin self-administration and demonstrated a stable level of <10 active lever pressing following 2 wk of extinction training. Yoked saline animals served as controls.
Following 2 wk extinction training, the rats were anaesthetized and mounted in a stereotaxic apparatus (see Supplementary material for detailed surgical and electrophysiological methods). A concentric bipolar stimulating electrode was placed into the prelimbic PFC (PL) and a glass recording electrode into the dorsomedial core subcompartment of the accumbens (NAcore). Our previous studies have demonstrated that the field potentials measured in this study arise primarily from glutamatergic field excitatory post-synaptic potentials that are eliminated by AMPA/kainate receptor antagonist CNQX (Moussawi et al.2009; Shen et al.2011). Field potential amplitude (estimated as described in Supplementary material) was stimulated at 40% of the minimum current intensity that evoked a maximum field response every 30 s, at a 10 kHz sampling frequency, and then averaged every 1 min.
Results
We previously demonstrated no difference in the input/output curves for field potential amplitude between heroin and yoked saline animals (Shen et al.2011). We induced LTP in yoked saline animals by stimulating at the minimum current intensity that evoked a maximum field response (based on the input–output curve) using two bursts of 100 pulses at 50 Hz with a 10- to 20-s interburst interval. Figure 1a shows that, in contrast to yoked saline animals, blunted LTP was elicited in heroin-trained animals using this stimulation protocol. While a small potentiation was observed immediately after stimulating the PL, the potentiation was of reduced amplitude in heroin animals and did not endure for 60 min. The LTD protocol involved stimulating at the minimum current intensity that evoked a maximum response using three trains of 900 pulses at 5 Hz with a 5-min inter-train interval. Figure 1b shows that this stimulation protocol elicited robust LTD in yoked saline animals, but produced significantly less LTD in animals extinguished from heroin self-administration.
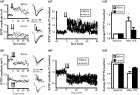
Reduction in both long-term potentiation (LTP) and long-term depression (LTD) in prelimbic prefrontal cortex (PL) to the core subcompartment of the accumbens (NAcore) synapses in rats extinguished from heroin self-administration. (a) Reduced LTP in heroin animals. a(1) Shows individual examples of LTP or lack thereof in saline and heroin rats, respectively. The individual traces correspond to the animals shown. Double-arrow indicates time of high frequency electrical stimulation (HFS) in the PL to induce LTP. a(2) Shows the normalized mean±s.e.m. Data were normalized to the average baseline field amplitude. Data were analysed using a two-way analysis of variance (ANOVA) with repeated measures over time to reveal a significant effect of heroin (n = 10) vs. yoked saline (n = 7); F1,79 = 9.35, p < 0.001. a(3) Shows the mean±s.e.m. of the average field amplitude at baseline for 20 min before HFS and over the 60 min after HFS. A two-way ANOVA with repeated measures over time revealed a significant interaction (F1,15 = 8.79, p = 0.030). (b) Reduced LTD in heroin animals. b(1) Shows individual examples of LTP or lack thereof in saline and heroin rats, respectively. The individual traces correspond to the animals shown. Triple-arrow indicates time of low frequency electrical stimulation (LFS) in the PL to induce LTD. b(2) Shows the normalized mean±s.e.m. Data were normalized to the average baseline field amplitude. Data were analysed using a two-way ANOVA with repeated measures over time revealed a significant effect of heroin (n = 9) vs. yoked saline (n = 8); F1,79 = 5.72, p < 0.001. b(3) Shows the mean+s.e.m. of the average field amplitude at baseline and over the 60 min after LFS. A two-way ANOVA with repeated measures over time revealed a significant interaction (F1,14 = 8.24, p = 0.012). * p < 0.001, comparing heroin to saline after HFS or LFS;+p < 0.01, comparing post-stimulation to baseline. fEPSP, Field excitory post-synaptic potentials.
Discussion
These data demonstrate that the impaired synaptic plasticity in the PL projection to the NAcore previously shown in cocaine-withdrawn rats also occurred in animals extinguished from heroin self-administration. Given that heroin and cocaine are from distinct chemical classes, with distinct binding sites in brain and behavioural effects (Badiani et al.2011), our findings support the hypothesis that impaired synaptic plasticity in this projection may contribute to the prefrontal cortical impairments that diminish the capacity of human drug addicts to adaptively control relapse to drug use (Goldstein & Volkow, 2011). This view is consistent with a variety of measurements made using preclinical models of addiction that show enduring cellular changes at excitatory synapses in the nucleus accumbens (Wolf, 2010) and human imaging studies showing long-lasting reductions in prefrontal cortical activity (Goldstein & Volkow, 2011).
The loss of PL-NAcore LTD in cocaine treated animals appears to be potentiated by the duration of cocaine use and whether or not animals undergo extinction training (Kasanetz et al.2010; Knackstedt et al.2010). In contrast, the loss of LTP is independent of extinction training (Knackstedt et al.2010). Although cocaine-trained subjects show baseline LTP-like increases in AMPA receptor-mediated currents and dendritic spine head diameter in the nucleus accumbens (Moussawi et al.2009; Wolf, 2010), heroin animals show LTD-like baseline electrophysiological and morphological changes after withdrawal from heroin (Shen et al.2011). Therefore, while the basal state of the heroin and cocaine withdrawn accumbens is quite different, there may exist a shared mechanism related to addiction that underpins the deficitmin LTP and LTD after chronic use of addictive drugs. Taken together, these studies with heroin and cocaine indicate that impaired LTP and LTD in the prefrontal to accumbens synapses may be a common neuropathology between addictive drugs.
Supplementary material
For supplementary material accompanying this paper, visit http://dx.doi.org/10.1017/S1461145712001071
Supplementary information supplied by authors.
Acknowledgements
This work was supported by grants DA015369 (P.W.K.) and DA003906 (P.W.K.) from the National Institutes of Health.
Statement of Interest
None.
References



