-
PDF
- Split View
-
Views
-
Cite
Cite
Ellen Loyens, Dimitri De Bundel, Heidi Demaegdt, Siew Yeen Chai, Patrick Vanderheyden, Yvette Michotte, Paul Gard, Ilse Smolders, Antidepressant-like effects of oxytocin in mice are dependent on the presence of insulin-regulated aminopeptidase, International Journal of Neuropsychopharmacology, Volume 16, Issue 5, June 2013, Pages 1153–1163, https://doi.org/10.1017/S1461145712001149
Close - Share Icon Share
Abstract
Oxytocin is a neuromodulator with antidepressant-like effects. In vitro, oxytocin is rapidly cleaved by insulin-regulated aminopeptidase (IRAP). Oxytocin metabolites are known to exert strong central activities that are different from the effects of the parent molecule. Our goal is to investigate in vivo whether IRAP deletion modifies the antidepressant-like effects of oxytocin. Male and female C57Bl/6 mice, IRAP wild-type (IRAP+/+) and knock-out (IRAP−/−) mice were injected subcutaneously with saline, oxytocin or oxytocin combined with angiotensin IV. One hour after injection, immobility was timed during a 5 min forced swim that was preceded by an open field to study locomotor behaviour. Oxytocin induced antidepressant-like effects in male (0.25 mg/kg oxytocin) and female (0.15 mg/kg oxytocin) C57Bl/6 mice subjected to the forced swim test. Oxytocin did not influence locomotor behaviour in mice, as shown with the open field. These findings were reproduced in transgenic male (aged 3–6 months) and female (aged 12–18 months) IRAP+/+ mice. However, the major findings of our study were that the antidepressant-like effect was reversed in angiotensin IV treated IRAP+/+ mice and was completely absent in age- and gender-matched IRAP−/− mice. The lack of an antidepressant-like effect of oxytocin in young male and middle-aged female IRAP−/− mice attributes an important role to IRAP in mediating this effect.
Introduction
Oxytocin is a cyclic nonapeptide synthesized in cell bodies of the paraventricular and supraoptic nuclei of the hypothalamus (Rotzinger et al.2009). Via axonal fibre systems, oxytocin is transported from the hypothalamus to the posterior pituitary where it is released into the circulation as a hormone. The peripheral biological actions of oxytocin consist of facilitating uterine contractility and milk ejection in order to regulate parturition and lactation (Klenerova et al.2009). Oxytocin can also act as a neuromodulator in the central nervous system since the axonal terminals innervate different brain areas involved in the regulation of behavioural responses (Gimpl & Fahrenholz, 2001). These include the modulation of memory processes (Burbach et al.1983), social recognition (Ferguson et al.2001; Neumann, 2008) and anxiety responses (Ring et al.2006; Slattery & Neumann, 2010).
Moreover, oxytocin has been shown to exert antidepressant-like effects in mice (Arletti & Bertolini, 1987b) and in rats (Arletti et al.1995), assessed by the forced swim test. In line with these studies, the oxytocin analogue carbetocin led to a reduced immobility behaviour in rats and mice, which is characteristic of an antidepressant-like effect (Chaviaras et al.2010; Loyens et al.2012). On the other hand, the antidepressant-like effect of oxytocin in the mouse-tail suspension test could not be mimicked by an oxytocin receptor agonist nor be blocked by an oxytocin receptor antagonist (Ring et al.2010). This study suggested that the proteolytic conversion of oxytocin into active metabolites, which possibly bind to another receptor than the oxytocin receptor, could be responsible for the reduced immobility time (Ring et al.2010).
It has been shown that oxytocin can be degraded into smaller active oxytocin fragments (Burbach & Lebouille, 1983; Burbach et al.1980) by membrane-bound aminopeptidases and endopeptidases in purified rat synaptic membranes (Stancampiano et al.1991). These findings were confirmed in vivo after microinjection of oxytocin in the rat hippocampus (Stancampiano & Argiolas, 1993). In more recent in vitro studies, the aminopeptidase activity that degrades oxytocin has been identified as insulin-regulated aminopeptidase (IRAP; Lew et al.2003; Matsumoto et al.2000; Tsujimoto et al.1992). IRAP is a membrane-associated zinc-dependent metallopeptidase of the M1 family with characteristic extracellular catalytic and transmembrane domains but a unique cytoplasmatic amino-terminal tail (Chai et al.2004; Lukaszuk et al.2008). The catalytic site of IRAP is bound by the potent and competitive inhibitor angiotensin IV (Lew et al.2003), thereby possibly prolonging the half-life of circulating neuropeptides as oxytocin (Lew et al.2003).
An angiotensin IV × oxytocin interaction has been shown in BKW albino mice, where angiotensin IV could reverse the reduction of immobility time induced by oxytocin during a forced swim test (Gard et al.2007). These findings were confirmed in the present study in C57Bl/6 mice and IRAP+/+ mice.
The findings that oxytocin is a substrate of IRAP in vitro (Lew et al.2003; Matsumoto et al.2000, 2001), and that angiotensin IV is able to reverse the antidepressant-like effect of oxytocin in mice (Gard et al.2007), suggest the possibility that IRAP is involved in the in vivo oxytocin-mediated antidepressant-like effect. To test this hypothesis, we here compared the antidepressant-like effect of oxytocin in mice in which the IRAP gene was deleted, with wild-type and age-matched littermate controls.
We are the first to conclusively demonstrate that the antidepressant-like effect of oxytocin observed in IRAP+/+ mice was completely absent in IRAP−/− mice.
Method
Animals
Male C57Bl/6 mice (Charles River Laboratories, France) and IRAP wild-type (IRAP+/+) and knock-out (IRAP−/−) mice were used in this study. IRAP mice were generated by Ozgene (Ozgene Ptd Ltd, Australia) as described before (Albiston et al.2010) and bred at the animal house of the Vrije Universiteit Brussel. Mice used in the experiments were offspring from heterozygous IRAP breeding pairs on a C57Bl/6 background. The mice were housed under a constant light and dark cycle (lights on 07:00 hours) and were given free access to food and water. Genotypes were determined by polymerase chain reaction with the REDExtract-N-Amp Tissue PCR Kit (Sigma, USA) following the manufacturer's instructions, with the following primer pairs: for IRAP+/+ 5′ GATAAGATAGAAGTAGGGGAGA 3′ and 5′ CAATAGAGGTACAGTCACCA 3′ and for IRAP−/− 5′ CAATAGAGGTACAGTCACCA 3′ and 5′ GGAGAATAAGGGCTGTGAGAGA 3′. All experiments were approved by the Ethical Committee for Animal Experiments of the Faculty of Medicine and Pharmacy of the Vrije Universiteit Brussel.
Forced swim test
C57Bl/6 mice
Male and female C57Bl/6 mice of different ages (3 months and 14 months) were used in the forced swim test to assess the antidepressant-like effects of oxytocin (Polypeptide groups, France). Mice were placed individually into a glass cylinder (diameter 15 cm) containing water (25 °C; depth 14 cm) and were unable to escape or touch the bottom of the cylinder. Mice underwent a 5 min forced swim 1 h after subcutaneous injection of either saline (10 ml/kg), oxytocin (0.05, 0.15, 0.25, 0.35 or 0.5 mg/kg), angiotensin IV (0.5 mg/kg) or angiotensin IV (0.5 mg/kg) in combination with oxytocin (0.25 mg/kg). Periods of immobility were timed during the forced swim by an investigator blinded to the genotype and treatment.
IRAP mice
Male and female IRAP+/+ and IRAP−/− mice of different ages were used. Young mice were aged 3–6 months and middle-aged mice were aged 12–18 months. These mice underwent the same forced swim test protocol as C57Bl/6 mice, as described earlier. Before testing oxytocin in these transgenic mice, a separate batch of IRAP+/+ and IRAP−/− mice was injected with the tricyclic antidepressant imipramine (Sigma-Aldrich Chemie, Belgium; 10 mg/kg i.p. injection) to investigate whether the immobility time in these mice could be changed by using a well-known antidepressant. After this positive control experiment, IRAP+/+ and IRAP−/− mice were injected with oxytocin to assess the antidepressant-like activity of this nonapeptide. Young male IRAP+/+ mice were also treated with angiotensin IV (0.5 mg/kg) alone or in combination with oxytocin (0.25 mg/kg).
Locomotor behaviour in open field test
To unravel possible attenuation of immobility time within the forced swim test due to drug-induced increases in locomotor activity, control mice and oxytocin-treated mice were subjected to an open field test 50 min after injection. With a plexiglass arena of 60 × 60 cm and the Noldus EthoVision video tracking system (Noldus, The Netherlands), the total distance moved (cm) and the mean velocity (cm/s) of each animal was quantified during a 10 min test period. We also measured the time spent in central zone (s) as a first indication of possible differences in anxiety.
Membrane preparation from cortical tissue
IRAP+/+ and IRAP−/− mice were killed by cervical dislocation, the brain was removed and the cortex dissected. The tissue was homogenized in 50 mm Tris-HCl (pH = 7.4) using a Polytron (2 × 5 s, maximum speed) and Potter homogenizer (10 strokes; 1000 rpm) and then centrifuged (30 min; 30 000 g; 4 °C). The pellet was resuspended in 50 mm Tris-HCl, centrifuged (30 min; 30 000 g; 4 °C) and the supernatant was removed. The resulting cortex cell membrane-containing pellets were stored at −20 °C until use. The protein content was determined with the BCA protein kit from Pierce (Perbio Sciences, Belgium).
Measuring aminopeptidase activity in cortical membranes
As previously described (Demaegdt et al.2004), determination of the cystinyl aminopeptidase catalytic activity was based on the cleavage of the substrate l-leucine-p-nitroanilide (L-Leu-pNA; Sigma, USA) into l-leucine and p-nitroaniline. The latter component displays a characteristic light absorption maximum at 405 nm. To this end, pellets were thawed and resuspended using a sonicator (2 × 10 s) in enzyme buffer containing 50 mm Tris-HCl (pH = 7.4), 150 mm NaCl, 0.1% bovine serum albumin and 100 µm phenylmethanesulfonyl fluoride. The incubation mixture comprised 50 µl membrane homogenate (corresponding to 50 µg proteins), 200 µl L-Leu-pNA (1.5 mm) and 50 µl enzyme buffer. The membrane homogenate was incubated at 37 °C in 96-well plates (Medisch Labo Service, Belgium) and the formation of p-nitroaniline was followed by measuring the absorption at 405 nm between 5 and 60 min in a Tecan M200 multi-well reader (Mechelen, Belgium).
Determination of plasma oxytocin levels
Oxytocin measurements were performed by the Bioanalysis Department, SGS Life Science Services (Belgium). Basal oxytocin levels and oxytocin levels following exogenous oxytocin (0.15 mg/kg) treatment were measured quantitatively in mouse plasma using a commercially available ELISA kit (Cusabio Biotech Ltd, China) according to the manufacturer's instruction. All samples were tested in duplicate. Results were expressed in µIU/ml.
Data analysis
Data are expressed as means±s.e.m. Statistical analysis was performed using GraphPad Prism 4.0 software. Data were analysed using the non-parametric Kruskal–Wallis test with Dunn's post hoc test (α = 0.05) to compare more than two groups or by using the Mann–Whitney test (α = 0.05) to compare two groups. The number of mice per group (n) is shown in the Figs.
All ex vivo enzyme measurements were performed at least three times with duplicate determinations each. The Km and Vmax values for female IRAP+/+ mice were calculated through nonlinear regression of Michaelis–Menten curves. To compare aminopeptidase activities, Km and Vmax values, a Mann–Whitney test was carried out (α = 0.05).
Results
Antidepressant-like effect of oxytocin in young male C57Bl/6 mice
To determine at which concentration oxytocin exerts antidepressant-like activities in our forced swim test set-up, we performed a dose–response curve in young male C57Bl/6 mice. Oxytocin doses of both 0.15 and 0.25 mg/kg showed a significantly reduced immobility time in comparison with saline-treated controls (Fig. 1a; p < 0.05, Kruskal–Wallis test with Dunn's post hoc test). The administration of lower (0.05 mg/kg) or higher (0.35 and 0.5 mg/kg) doses oxytocin did not influence immobility time, indicating a U-shaped dose–response curve for oxytocin in the forced swim test (Fig. 1a, Kruskal–Wallis test with Dunn's post hoc test). The effective doses of oxytocin had no effect on the mean velocity (Fig. 1b) or on the total distance moved (Fig. 1c) of mice, as shown in the open field (Kruskal–Wallis test with Dunn's post hoc test). These data exclude increases in locomotor behaviour due to oxytocin administration.

Dose–response curve of oxytocin (OT) in young male C57Bl/6 mice subjected to (a) the forced swim test to assess the antidepressant-like effect by measuring immobility and the open field, (b) to determine mean velocity and (c) total distance moved. * p < 0.05, Kruskal–Wallis test with Dunn's post hoc test, statistically significant difference from saline control.
Antidepressant-like effect of oxytocin in young male IRAP+/+ mice is absent in IRAP−/− mice
Young male IRAP+/+ and IRAP−/− mice were subjected to a treatment with imipramine to verify that the immobility time of these transgenic animals in the forced swim test could be modified by a well-known antidepressant. Figure 2a shows that 10 mg/kg imipramine reduced immobility time from each group (p < 0.05, Mann–Whitney test), demonstrating that antidepressant-like effects can be obtained in young male mice. A separate set of IRAP+/+ and IRAP−/− mice was used for oxytocin experiments to test whether the results in young male C57Bl/6 were reproducible in these transgenic mice. The antidepressant-like effect in IRAP+/+ mice was only significant at the 0.25 mg/kg oxytocin dose (Fig. 2b, left panel; p < 0.05, Kruskal–Wallis test with Dunn's post hoc test). Most interestingly, the immobility time in IRAP−/− mice did not change after oxytocin administration, demonstrating that oxytocin failed to exert an antidepressant-like effect (Fig. 2b, right panel; Kruskal–Wallis test with Dunn's post hoc test). An oxytocin dose 0.25 mg/kg administered to IRAP+/+ and IRAP−/− mice did not change mean velocity (Fig. 2c) nor total distance moved (Fig. 2d; Kruskal–Wallis test with Dunn's post hoc test). None of the oxytocin doses that were administered changed the time spent in central zone (data not shown, Kruskal–Wallis test with Dunn's post hoc test). No genotype-related effects were observed between saline-treated IRAP+/+ and IRAP−/− mice (Fig. 2a–d; Mann–Whitney test). These results show that IRAP is required for the antidepressant-like effects of oxytocin in young male IRAP+/+ mice.
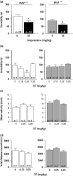
Young male insulin-regulated aminopeptidase wild type (IRAP+/+; left panel) and knockout (IRAP−/−; right panel) mice were treated with (a) 10 mg/kg imipramine (* p < 0.05, Mann–Whitney test, statistically different from saline control) and (b) oxytocin (OT; 0.15, 0.25 and 0.35 mg/kg) before subjecting them to the forced swim test. OT (0.25 and 0.35 mg/kg) was also tested in the open field (c, d). * p < 0.05, Kruskal–Wallis test with Dunn's post hoc test, statistically significant difference from saline control.
Antidepressant-like effect of oxytocin is reversed by angiotensin IV in young male C57Bl/6 mice and young male IRAP+/+ mice
Young male C57Bl/6 mice were treated with angiotensin IV to investigate whether IRAP ligand binding would reverse the oxytocin-induced antidepressant-like effect. Figure 3a shows that 0.5 mg/kg angiotensin IV alone had no effect on immobility time, while this IRAP ligand reversed the antidepressant-like effect of 0.25 mg/kg oxytocin (p < 0.001, Kruskal–Wallis test with Dunn's post hoc test). None of the treatments changed mean velocity (Fig. 3b) nor total distance moved (Fig. 3c). These data confirm the role of IRAP as a mediator of oxytocin-induced antidepressant-like effects.

Young male C57Bl/6 mice were treated with 0.5 mg/kg angiotensin IV (Ang IV) alone or in combination with 0.25 mg/kg oxytocin (OT) before subjecting them to the forced swim test (a) and the open field (b, c). *** p < 0.001, Kruskal–Wallis test with Dunn's post hoc test, statistically significant from saline control.
To exclude possible strain differences, young male IRAP+/+ mice were injected with the same doses of angiotensin IV and oxytocin. The antidepressant-like effect of oxytocin was also reversed in these mice after treatment with angiotensin IV (Fig. 4a; p < 0.05, Kruskal–Wallis test with Dunn's post hoc test). None of the treatments changed mean velocity (Fig. 4b) nor total distance moved (Fig. 4c). These data with oxytocin are comparable to the data obtained in young male IRAP−/− mice, adding proof to the concept that IRAP is required for oxytocin to induce antidepressant-like effects.

Young male insulin-regulated aminopeptidase wild type (IRAP+/+) mice were treated with 0.5 mg/kg angiotensin IV (Ang IV) alone or in combination with 0.25 mg/kg oxytocin (OT) before subjecting them to the forced swim test (a) and the open field (b, c). * p < 0.05, Kruskal–Wallis test with Dunn's post hoc test, statistically significant from saline control; $p < 0.05, Kruskal–Wallis test with Dunn's post hoc test, statistically significant from mice treated with 0.25 mg/kg OT.
No antidepressant-like effect of oxytocin in young female C57Bl/6 mice, young female IRAP+/+ nor IRAP−/− mice
Experiments performed with young male C57Bl/6 mice were repeated in young female C57Bl/6 mice in order to investigate whether gender could influence the outcome of an oxytocin treatment. As shown in Fig. 5, the doses of oxytocin that were effective in young male mice (0.15 and 0.25 mg/kg) did not alter immobility time (Fig. 5a), mean velocity (Fig. 5b) or total distance moved (Fig. 5c) in young female mice (Kruskal–Wallis test with Dunn's post hoc test).

Oxytocin (OT) treatment (0.15 mg/kg and 0.25 mg/kg) of young female C57Bl/6 mice subjected to the forced swim test (a) and the open field (b, c).
Injecting female IRAP+/+ and IRAP−/− mice with 10 mg/kg imipramine led to significantly reduced immobility times in both groups, demonstrating that antidepressant-like effects can be obtained in young female mice (Fig. 6a; p < 0.01, p < 0.05 for IRAP+/+ and IRAP−/− groups respectively, Mann–Whitney test). As in young female C57Bl/6 mice, the antidepressant-like effect of oxytocin was absent in IRAP+/+ and IRAP−/− mice (Fig. 6b; Kruskal–Wallis test with Dunn's post hoc test). Mean velocity (Fig. 6c) and total distance moved (Fig. 6d) remained unchanged after oxytocin treatment (Kruskal–Wallis test with Dunn's post hoc test). No genotype-related effects were observed between saline-treated IRAP+/+ and IRAP−/− mice (Fig. 6a–d; Mann–Whitney test).

Effects of 10 mg/kg imipramine (a) and 0.15, 0.25 and 0.35 mg/kg oxytocin (OT; b) in young female insulin-regulated aminopeptidase wild type (IRAP+/+; left panel) and knockout (IRAP−/−; right panel) mice on the duration of immobility in the forced swim test. OT was also tested in the open field for its effect on mean velocity (c) and total distance moved (d). * p < 0.05, ** p < 0.01, Mann–Whitney test, statistically significant difference from saline control.
Antidepressant-like effect of oxytocin in middle-aged female C57Bl/6 mice
Since the antidepressant-like effect was absent in young female mice and since endogenous brain oxytocin levels are shown to be increased in aged rats (Keck et al.2000; Melis et al.1992; Stancampiano et al.1994), we tested middle-aged female C57Bl/6 mice in our forced swim test set-up. In comparison with saline-treated controls, 0.15 mg/kg oxytocin significantly reduced immobility time while higher doses (0.25 and 0.35 mg/kg) did not have an effect [Fig. 7a; p < 0.05, one-way analysis of variance (ANOVA) with Bonferroni's post hoc test]. The effective oxytocin dose did not change mean velocity (Fig. 7b) or total distance moved (Fig. 7c) in these mice (Kruskal–Wallis test with Dunn's post hoc test).

Oxytocin (OT) treatment (0.15, 0.25 and 0.35 mg/kg) of middle-aged female C57Bl/6 mice subjected to the forced swim test (a) and the open field (b, c). * p < 0.05, Kruskal–Wallis test with Dunn's post hoc test, statistically significant difference from saline control.
Antidepressant-like effect of oxytocin in middle-aged female IRAP+/+ mice is abolished in age-matched IRAP−/− mice
The treatment of middle-aged female IRAP+/+ mice with 0.15 mg/kg oxytocin significantly reduced immobility time when compared to saline-treated controls (Fig. 8a, left panel; p < 0.01 Mann–Whitney test). This antidepressant-like effect of oxytocin was absent in middle-aged female IRAP−/− mice (Fig. 8a, right panel; Mann–Whitney test). No changes were detected in mean velocity (Fig. 8b) or total distance moved (Fig. 8c) in these mice (Mann–Whitney test). Although oxytocin induced an antidepressant-like effect in middle-aged female IRAP+/+ mice, this treatment did not significantly change the time spent in central zone (data not shown, Kruskal–Wallis test with Dunn's post hoc test). No genotype-related effects were observed between saline-treated IRAP+/+ and IRAP−/− mice (Fig. 8a–c; Mann–Whitney test).
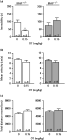
Middle-aged female insulin-regulated aminopeptidase wild type (IRAP+/+; left panel) and knockout (IRAP−/−; right panel) mice were injected with 0.15 mg/kg oxytocin (OT) and subjected to the forced swim test (a) and to the open field for its effect on mean velocity (b) and total distance moved (c). ** p < 0.01, Mann–Whitney test, statistically significant difference from saline control.
Cortical aminopeptidase activity in female IRAP+/+ mice
The cortical IRAP enzyme activity was measured ex vivo in young and middle-aged female mice to investigate whether changes in enzyme activity could account for the observed difference in oxytocin-mediated antidepressant-like effects.
As shown in Fig. 9a, the aminopeptidase activity in young female IRAP+/+ mice was proportional to the membrane concentration. Further enzymatic assays were carried out with a membrane concentration corresponding to 50 µg protein per assay.

(a) Enzymatic cleavage of l-leucine-p-nitroanilide (L-Leu-pNA) as a function of the membrane concentration in young female insulin-regulated aminopeptidase (IRAP) wild type (IRAP+/+), mice. 1.5 mm L-Leu-pNA was incubated at 37 °C with increasing membrane concentrations of young female mice (expressed as the corresponding amount of protein/incubation). Rate constants for L-Leu-pNA cleavage (v) were calculated by linear regression analysis of the absorption (at 405 nm) vs. time curves with measurements made every 5 min (between 10 and 50 min). Fixed p-nitroaniline concentrations were used as standard to convert absorption into concentration. Data are the mean±s.e.m. of three independent experiments. (b) Enzymatic cleavage of L-Leu-pNA as a function of the substrate concentration in young female IRAP+/+ (■) and knockout (IRAP−/−; □) mice and in (c) middle-aged female IRAP+/+ (●) and IRAP−/− (○) mice. Membranes (corresponding to 50 µg protein/incubation) were incubated at 37 °C with increasing concentrations of L-Leu-pNA (mm). The rate constants of L-Leu-pNA (enzyme activity expressed as a percentage of control) were determined as in Fig 7a. The Km and Vmax values are calculated through nonlinear regression analysis of Michaelis–Menten curves. The values are the mean±s.e.m. of three independent experiments.
The aminopeptidase activity as a function of the substrate concentration was shown in young female IRAP+/+ and IRAP−/− mice (Fig. 9b) and middle-aged female IRAP+/+ and IRAP−/− mice (Fig. 9c). Although the substrate L-Leu-pNA is not specific for measuring IRAP activity, we were able to distinguish between IRAP and non-IRAP enzyme activity by comparing both genotypes. In young (Fig. 9b) and middle-aged (Fig. 9c) female IRAP+/+ mice, 70% of the aminopeptidase activity was represented by IRAP. Aminopeptidase-N, non-IRAP and non-aminopeptidase N accounted for the remaining enzymatic activity and did not differ between these two groups (Mann–Whitney test). The Km and Vmax values for IRAP+/+ mice were calculated through nonlinear regression of Michaelis–Menten curves. The Km and Vmax values did not differ between young (Fig. 9b) and middle-aged (Fig. 9c) female IRAP+/+ mice (Mann–Whitney test). These data indicate that the IRAP enzyme activity, the affinity between IRAP and its possible substrate oxytocin and the distribution of IRAP in cortical membranes, are not influenced by ageing.
Plasma oxytocin levels in young and middle-aged female IRAP+/+ and IRAP−/− mice
Basal plasma oxytocin levels were measured in young and middle-aged female IRAP+/+ and IRAP−/− mice in order to investigate whether altered plasma levels could account for the differences in oxytocin-mediated antidepressant-like activity seen in these mice. As shown in Fig. 10, oxytocin levels in young mice appear to be higher than the levels in middle-aged mice. However, no significant differences in plasma oxytocin levels between these groups of mice could be shown, indicating that plasma oxytocin levels in female IRAP+/+ mice are not influenced by ageing (Mann–Whitney test).

Plasma oxytocin (OT) levels in baseline conditions and following 0.15 mg/kg OT treatment (µIU/ml) were measured in young and middle-aged female insulin-regulated aminopeptidase wild type (IRAP+/+) and knockout (IRAP−/−) mice by a commercially available ELISA kit (Mann–Whitney test).
Plasma oxytocin levels were also measured in oxytocin-treated young and middle-aged female IRAP+/+ and IRAP−/− mice (1 h after injection with 0.15 mg/kg oxytocin) to investigate whether possible genotype-related differences in the processing of oxytocin could be associated with different plasma levels of oxytocin. As shown in Fig. 10, no significant differences were shown between oxytocin-treated groups, indicating that the possible metabolization of oxytocin is not affected in the presence of IRAP (Mann–Whitney test).
Discussion
The major finding of our study is that the presence of IRAP is required for oxytocin to exert antidepressant-like effects since these effects are reversed by angiotensin IV in IRAP+/+ mice and because they are absent in IRAP−/− mice. The antidepressant-like activity of oxytocin that we found in young male C57Bl/6 mice and young male IRAP+/+ mice is in line with the results of an earlier study, which demonstrated that 0.25 mg/kg oxytocin decreased immobility time in young male BKW mice subjected to the forced swim test. Here, the administration of angiotensin IV abolished the oxytocin-mediated effect, suggesting that inhibition of IRAP could account for the decreased immobility time (Gard et al.2007).
We are the first to use IRAP−/− mice in these settings and we attribute an important role to IRAP in mediating the in vivo antidepressant-like activity of oxytocin. A first obvious explanation for these effects seems the cleavage of oxytocin by this aminopeptidase. That oxytocin fragments are able to decrease immobility time has been shown in a previous study in mice in which the antidepressant-like activity of oxytocin was prevented by a protease inhibitor cocktail (Arletti & Bertolini, 1987a). Although these metabolites are not yet identified in vivo, these authors concluded that oxytocin metabolites rather than the intact nonapeptide are crucial for the antidepressant-like effects.
To date, only in vitro studies have shown that oxytocin is rapidly cleaved by IRAP. The incubation of oxytocin with human embryonic kidney 293 cells transfected with human IRAP (Lew et al.2003) or Chinese hamster ovary cells expressing recombinant placental-leucine aminopeptidase with oxytocin (Matsumoto et al.2000), resulted in a sequential cleavage of N-terminal peptide bonds. In an ex vivo study with adipocytes and muscle cells of IRAP+/+ mice, the same degradation profile of oxytocin was seen (Wallis et al.2007). Moreover, the possible oxytocin × IRAP interaction is supported by recent work showing that angiotensin IV elevates oxytocin levels in the rat amygdala, thereby leading to an anxiolytic-like effect (Beyer et al.2010). However, absolute evidence that oxytocin is an in vivo substrate of IRAP is still lacking.
In the present study, we clearly demonstrated that IRAP+/+ mice are responsive to behavioural effects induced by oxytocin and that this peptide is able to produce antidepressant-like effects via subcutaneous administration. The finding that the antidepressant-like effect is not present in IRAP−/− mice and that this effect is completely reversed by angiotensin IV in IRAP+/+ mice suggests that IRAP degrades oxytocin and that the formed oxytocin metabolites can possibly cause the antidepressant-like effects. Nevertheless, other possible mechanisms of interaction between IRAP and oxytocin or IRAP and oxytocin receptors cannot be excluded and need future investigations.
The lack of oxytocin-induced anxiolytic effects in our study could be explained by the dose of oxytocin that is lower in comparison with previous studies. Indeed, 3–30 mg/kg oxytocin has been shown to exert anxiolytic effects in rats subjected to the four-plate test (Ring et al.2006). In addition, although the open field is a well-validated anxiety test in mice, further investigating the anxiolytic properties of oxytocin in IRAP+/+ and IRAP−/− mice subjected to the four-plate test or the elevated plus maze is required.
In female animals, the antidepressant-like effect was only present in middle-aged IRAP+/+ mice and not in young IRAP+/+ mice. Although this is not the main focus of our study, we could not detect changes in their cortical IRAP enzyme activity, in their basal plasma oxytocin levels or in their plasma oxytocin levels after oxytocin injection, which is not unexpected in that IRAP is not the rate-limiting enzyme for the degradation of circulating oxytocin (Wallis et al.2007). It would be most interesting to also measure the endogenous brain oxytocin levels in middle-aged vs. young female mice. Unfortunately, we were unable to reliably measure these brain oxytocin concentrations (data not shown). A second drawback in the experiments with female mice is that their oestrous cycle was not matched, although it has been published that oxytocin levels and oxytocin receptor levels fluctuate during the different phases of the oestrous cycle (Larcher et al.1995; Murata et al.2000, 2003). However, the variation in our experiments performed with female mice is very low and the results are reproducible, suggesting that the oxytocin-induced antidepressant-like effects are reliable.
In summary, we are the first to demonstrate that the antidepressant-like effect of oxytocin observed in IRAP+/+ mice was completely absent in IRAP−/− mice, conclusively demonstrating that an interaction between oxytocin and IRAP is necessary for the antidepressant-like effects of oxytocin. Oxytocin fragments formed by IRAP, rather than the intact nonapeptide could be responsible for the antidepressant-like effects. Nevertheless, other interaction mechanisms between IRAP and oxytocin/oxytocin receptors could also be responsible and require future investigation. Finally, our study suggests that IRAP is a potential therapeutic target in research on depression.
Acknowledgements
Financial support was provided by the Queen Elisabeth Medical Foundation (Geneeskundige Stichting Koningin Elisabeth, Belgium) and a research grant from the Vrije Universiteit Brussel (VUB; GOA61). P.V. is holder of a VUB research fellowship. The authors thank E. Tshimanga and K. Slimani (SGS Life Science Services) for technical assistance.
Statement of Interest
None.
References



