-
PDF
- Split View
-
Views
-
Cite
Cite
C Mary Schooling, Man Ki Kwok, Jie V Zhao, The relationship of fatty acids to ischaemic heart disease and lifespan in men and women using Mendelian randomization, International Journal of Epidemiology, Volume 52, Issue 6, December 2023, Pages 1845–1852, https://doi.org/10.1093/ije/dyad108
Close - Share Icon Share
Abstract
Observationally, polyunsaturated fatty acids (PUFAs) have health benefits compared with saturated fatty acids (SFAs); randomized controlled trials suggest fewer benefits. We used uni- and multi-variable Mendelian randomization to assess the association of major fatty acids and their sub-species with ischaemic heart disease (IHD) overall and sex-specifically and with lifespan sex-specifically, given differing lifespan by sex.
We obtained strong (P <5x10-8), independent (r2<0.001) genetic predictors of fatty acids from genome-wide association studies (GWAS) in a random subset of 114 999 UK Biobank participants. We applied these genetic predictors to the Cardiogram IHD GWAS (cases = 60 801, controls = 123 504) and to the Finngen consortium GWAS (cases = 31 640, controls = 187 152) for replication and to the UK Biobank for sex-specific IHD and for lifespan based on parental attained age (fathers = 415 311, mothers = 412 937). We used sensitivity analysis and assessed sex differences where applicable.
PUFAs were associated with IHD [odds ratio 1.23, 95% confidence interval (CI) 1.05 to 1.44] and lifespan in men (-0.76 years, 95% CI -1.34 to -0.17) but not women (0.20, 95% CI -0.32 to 0.70). Findings were similar for omega-6 fatty acids and linoleic acid. Independent associations of SFAs, mono-unsaturated fatty acids or omega-3 fatty acids with IHD overall or lifespan in men and women were limited.
PUFAs, via specific subspecies, may contribute to disparities in lifespan by sex. Sex-specific dietary advice might be a start towards personalized public health and addressing inequities.
Based on observational studies, dietary polyunsaturated fatty acids (PUFA) have been recommended in place of saturated fatty acids (SFAs).
Randomized controlled trials have not consistently confirmed these benefits but have not examined fatty acids comprehensively nor assessed differences by sex.
Using Mendelian randomization, we also found little benefit of PUFAs or harms of SFAs for ischaemic heart disease or for lifespan in men or women, but evidence that PUFAs might decrease lifespan specifically in men, with omega-6 fatty acids or linoleic acid as the key drivers.
Introduction
Based on a comprehensive body of observational evidence accumulated over many years,1–3 monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) and their subtypes (omega-3 and omega-6 fatty acids) have long been considered part of a healthy diet, and have been recommended across the globe; however, limiting the intake of saturated fatty acids (SFAs) is often recommended.4 Systematic reviews and meta-analyses of randomized controlled trials (RCTs) have begun to raise questions about this advice. RCTs reducing SFAs have found replacement by PUFAs reduces cardiovascular events, with little benefit for cardiovascular or all-cause mortality.5 Evidence concerning replacement by MUFAs is limited.5 RCTs of PUFA omega-3 fatty acids have recently also found no overall benefit for mortality,6–9 but a specific omega-3-related pharmaceutical product, icosapent ethyl,10 may be protective.11 RCT findings are similar for PUFA omega-6 fatty acids although the evidence is more limited and less consistent.12,13 Whether specific omega-6 fatty acids, such as linoleic acid (LA), are protective is controversial, as some RCTs have unexpectedly suggested otherwise.14,15 As with previous studies of dietary items where RCTs did not substantiate observed benefits,16 the discrepancy is likely due to confounding by factors such as socioeconomic position which strongly determine both diet and health.
Effects of interventions or exposures may also vary by target population, for example by sex or ethnicity. Differences in lipid metabolism by sex have motivated investigations of ischaemic heart disease (IHD) since the 1940s.17 Recently, an MR study found a lipid relevant to cardiovascular disease, low-density lipoprotein (LDL) cholesterol, had a greater effect in men than women.18 The VITAL trial of omega-3 fatty acids recruited equal numbers of men and women to enable investigation of sex-specific effects, but was too small to be definitive.19 Fatty acids could have different effects by sex because of their role in steroidogenesis,20 particularly unsaturated fatty acids.21 It is also possible and consistent with evolutionary biology,22 that androgens promote reproduction at the expense of the drivers of longevity,23 such as cardiovascular disease.24,25
In this situation where definitive trial evidence is limited,5–9,12–15 a Mendelian randomization study (MR) provides an alternative. MR reduces confounding by comparing health outcomes according to genetically predicted attributes, because genetic endowment is largely independent of common confounders.26 MR also has the advantage of assessing specific physiological pathways rather than the effect of an intervention typically obtained from RCTs.27 Recent developments in MR have also facilitated the examination of the role of potentially linked exposures, using multivariable MR.28
Previous MR studies of fatty acids on IHD have been limited by sample size for the instruments,29–33 or by estimating direct effects of fatty acids allowing for key lipids,34 which does not correspond to the role of dietary fatty acids. Some studies have included highly pleiotropic genetic variants from the archaic Flores people in the FADS1/2 genes29–31,33 which likely strongly affect one specific fatty acid, i.e. the omega-6 PUFA arachidonic acid (AA),35 making interpretation difficult. A positive association of omega-6 fatty acids with IHD has most consistently been identified.31,33 However, previous MR studies have not always distinguished between fatty acids, considered whether effects differ by sex, considered the possibility of bias in disease specific studies from only recruiting survivors or considered the effect on all-cause mortality. Recently, larger genetic studies of major fatty acids have become available. Given their importance to the dietary guidelines, the empirical evidence and theoretical expectations, we investigated if PUFAs, SFAs, MUFAs and where appropriate and possible, their major subspecies, independently affected IHD and all-cause mortality sex-specifically, using the most recent and largest suitable genome wide association studies (GWAS).36 We also replicated where possible.
Methods
We conducted a two-sample MR study for IHD, and due to data availability, a one-sample MR study for all-cause mortality, proxied by parental lifespan, which is robust to selection bias arising from only selecting survivors. MR, as an instrumental variable analysis with genetic instruments, requires the assumptions of relevance, independence and exclusion-restriction. To address relevance, only strong (P <5x10-8), and independent (r2<0.001) genetic predictors were used. Independence is plausible given the random distribution of genotypes in the population. To address exclusion-restriction, sensitivity analysis was used. Genetic predictors from the FADS1 gene (GRCh37: chromosome 11: 61 567 099–61 596 790) and the FADS2 gene (GRCh37: Chromosome 11: 61 560 452–61 634 826) and their correlates were also specifically excluded in sensitivity analysis from fatty acid groups that do not include AA, i.e. MUFAs, SFAs and polyunsaturated omega-3 fatty acids, because of their known pleiotropic effects,37 partly via AA.35 Given concerns about bias in one-sample MR studies,38 we also included a one-sample MR study for IHD from the UK Biobank as an empirical test of the likely level of bias from using a one-sample study and to provide sex-specific estimates for IHD. Power was estimated using the approximation that the sample size for an MR study is the sample size for exposure on outcome divided by the r2 for instruments on exposure.39,40
Data sources
Genetic instruments for fatty acids
Genetic instruments for fatty acids were obtained from the largest available GWAS, i.e. the UK Biobank. The UK Biobank is a population-based cohort of half a million people, intended age 40 to 69 years, recruited across Great Britain from 2006 to 2010.41 The participants completed questionnaires, underwent tests and health assessments and provided samples for analysis and genotyping. Measurement of fatty acids from metabolomic analysis are available for approximately a third (121 657) of the UK Biobank participants, chosen at random.42 Almost all the samples (97%) pertain to the recruitment visit.42 Of these 121 657 participants, 114 999 people of European descent have quality-controlled associations with 12 321 875 genetic variants available.42 These genetic associations were adjusted for population structure, fasting time and sex.34
Genetic associations with IHD
Summary genetic associations with IHD were obtained from CARDIoGRAMplusC4D 1000 Genomes (cases = 60 801, controls = 123 504)43 mainly in individuals of European ancestry (77%), adjusted for study specific covariates and genomic control.43 The IHD case definition included myocardial infarction, chronic stable angina, acute coronary syndrome and coronary stenosis >50%.43 For replication, genetic associations with IHD were also taken from the Finngen consortium (cases = 31640, controls = 187152), based on a network of existing and new Finnish biobanks matched with national health records [https://www.finngen.fi/en/for_researchers]. Finally, to provide sex-specific estimates for IHD we used a wide definition of IHD from the UK Biobank [cases = 20857 (15056 men), controls = 340337 (151964 men)]. Quality-controlled genetic associations were obtained from linear regression adjusted for age, age2, sex, age*sex, age2*sex and first 20 principal components [http://www.nealelab.is/uk-biobank]. Estimates were converted to odds ratios using an approximation.44
All-cause mortality
Lifespan, as a proxy for all-cause mortality, was assessed from parental lifespan, given longevity is partly heritable.45 Lifespan was based on UK Biobank participant report of maternal and paternal attained age (age at death or current age) restricted to white Europeans.36 Fathers who died before age 46 years, mothers who died before age 57 years and participants who described themselves as adopted were excluded, giving 415 311 fathers and 412 937 mothers.36 Quality-controlled genetic associations with Martingdale residuals from Cox proportional hazards were adjusted for age, sex, assessment centre and array type.36 These estimates were converted into life-years using an established approximation.46
Statistical analysis
The univariable F statistic was estimated as beta for genetic instruments on exposure squared divided by its variance.47 Univariable MR estimates were obtained from an inverse variance weighted (IVW) meta-analysis of the genetic variant-specific Wald estimates (genetic association with outcome divided by genetic association with exposure) with the variance obtained using the first term of Feiller’s theorem with multiplicative random effects.48 Sensitivity analysis used methods with different assumptions for validity. The weighted median estimate is valid if >50% of the weight comes from valid genetic variants.49 The MR-Egger estimate is valid if the instrument strength is independent of the direct effect, i.e. the INSIDE assumption, but has less power.47 In one-sample MR, estimates can be biased towards the confounded estimate if the is less than about 97%.38 Multivariable MR estimates were obtained using IVW estimates unless the MR-Egger intercept indicated pleiotropy.50 Conditional F statistics were used to assess multivariable instrument strength and multivariable Q to assess pleiotropy.28 Given we did not consider covariance, the conditional F statistics are likely to be lower bounds and the multivariable Q statistic an upper bound. Differences by sex were assessed using a z test.51
Statistical analysis was conducted using R 4.1.2.52 and the R packages: MR-Base TwoSampleMR 0.5.653 to obtain independent variants and to align variants across studies, Mendelianrandomization 0.6.0 to obtain estimates, MVMR to obtain conditional F statistics and the multivariable Q,28 metafor 3.8–1 to test differences by sex and forestplotter to make plots. Sample code is provided in Supplementary File 2 (available as Supplementary data at IJE online).
Results
Estimates for SFAs, MUFAs and PUFAs on IHD and lifespan
About 50 genetic variants predicting each of PUFAs, MUFAs and SFAs were obtained (Supplementary File 3 Tables G1–G3, available as Supplementary data at IJE online). The mean F statistics were over 100, the minimum was >25.
Univariable estimates for SFAs, MUFAs and PUFAs
Considering each major type of fatty acid (PUFAs, MUFAs and SFAs) separately using univariable MR, estimates for PUFAs were most consistent across methods (Figure 1), with the least indication of pleiotropy and invalidity of the IVW estimates from the MR-Egger intercepts, although the MR-Egger estimates had wide confidence intervals, but the were generally adequate. Overall estimates for IHD from the onesample study in the UK Biobank were very similar indeed to the two-sample estimates from Cardiogram and Finngen, although the MR-Egger estimates looked slightly more extreme (Figure 1). Based on the IVW estimates, PUFAs were associated with higher risk of overall IHD and with shorter lifespan in specifically men but not women (P-value for sex difference 0.02). Correspondingly, the positive associations of PUFAs with IHD looked more marked in men than women. Genetic variants predicting PUFAs, MUFAs and SFAs included a FADS1/2 variant or a correlated variant (rs102275 and rs174564). Estimates for MUFAs and SFAs were very similar excluding those variants (Supplementary File 1 Figure S1, available as Supplementary data at IJE online).
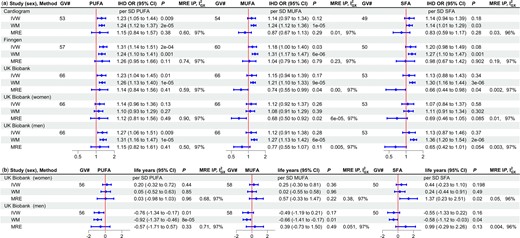
Univariable Mendelian randomization estimates for major types of fatty acids (PUFAs, MUFAs, SFAs) on a) ischaemic heart disease odds ratio and b) lifespan*. PUFAs, polyunsaturated fatty acids; MUFAs, monounsaturated fatty acids; SFAs, saturated fatty acids; GV#, number of genetic variants; IHD OR, ischaemic heart disease odds ratio; CI, confidence interval; MRE IP, MR-Egger intercept P-value; , measure of heterogeneity in gene-exposure estimates across GVs; IVW, inverse variance weighted; WM, weighted median; MRE, MR Egger; SD, standard deviation. *Life years were obtained as follows: first multiply by 2.5863 and 2.2869 in mothers and fathers, respectively, to account for the genetic associations used here being in children who only share half their genetic endowment with each parent, then years of life were estimated as 10 * log protection ratio, in accordance with a long-standing actuarial rule of thumb recently verified46
Multivariable MR estimates for SFAs, MUFAs and PUFAs
The conditional F statistics were adequate when considering PUFAs, MUFAs and SFAs in pairs (Supplementary File 1 Table S1, available as Supplementary data at IJE online) but possibly not when considering all three together. The MR-Egger intercepts did not indicate the multivariable IVW estimates were invalid. Considering MUFAs and PUFAs or SFAs and PUFAs together in multivariable MR, evidence of associations of MUFAs or SFAs with IHD or lifespan was limited (Supplementary File 1 Table S1). The estimates for PUFAs on IHD allowing for MUFAs or SFAs were similar using IVW or MR-Egger and were similar for Cardiogram and Finngen (Supplementary File 1 Table S1). Meta-analysing the IVW estimates for PUFAs on IHD for Cardiogram and Finngen from the multivariable analysis of SFAs and MUFAs, respectively, gave a positive association of PUFAs with IHD [odds ratio (OR) 1.23, 95% confidence interval (CI) 1.02 to 1.48], which was also evident for the UK Biobank. PUFAs were also associated with shorter lifespan in men.
Overall, these findings suggest that the univariable estimates for SFAs and MUFAs are not independent of PUFAs, and represent pleiotropy with PUFAs, particularly given some significant MR-Egger intercepts. Given the lack of evidence of consistent associations of SFAs or MUFAs with IHD or lifespan, only major subtypes of PUFA (omega-3 and omega-6) were considered further.
Estimates for omega-3 and omega-6 fatty acids on IHD and lifespan
About 45 variants predicting omega-3 and omega-6 fatty acids were obtained (Supplementary File 3 Tables G4 and G5, available as Supplementary data at IJE online); mean F statistics were over 100, the minimum was 26.2.
Univariable estimates for omega-3 and omega-6 fatty acids
Considering omega-3 and omega-6 fatty acids separately using univariable MR IVW estimates, associations were unclear for omega-3 fatty acids (Figure 2). The MR-Egger intercepts did not indicate invalidity of the IVW estimates and MR-Egger were close to 97%. Omega-6 fatty acids were associated with shorter lifespan in men but not women (Figure 2), P-value for sex difference 0.06. Repeating the analysis excluding a FADS1/2 variant gave similar estimates for omega-3 fatty acids (Supplementary File 1 Table S2, available as Supplementary data at IJE online).
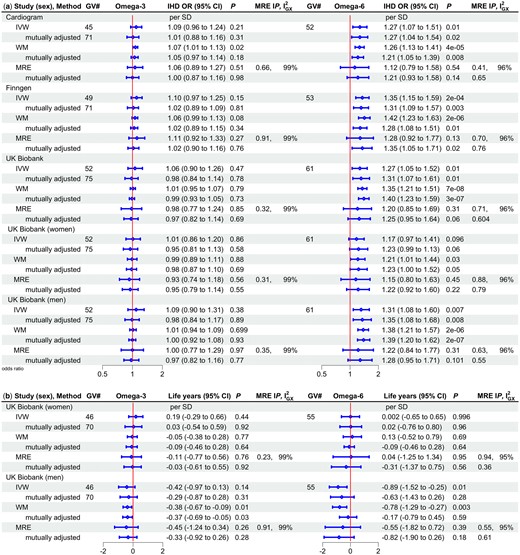
Univariable and multivariable (mutually adjusted) Mendelian randomization estimates for major types of polyunsaturated fatty acids (omega 3 and omega 6) on a) ischaemic heart disease odds ratio by study and sex and b) lifespan (life years*) by sex using different methods. GV#, number of genetic variants; IHD OR, ischaemic heart disease odds ratio; CI, confidence interval; MRE IP, MR-Egger intercept P-value; , measure of heterogeneity in gene-exposure estimates across GVs; IVW, inverse variance weighted; WM, weighted median; MRE, MR Egger; SD, standard deviation. *Life years were obtained as follows: first multiply by 2.5863 and 2.2869 in mothers and fathers, respectively, to account for the genetic associations used here being in children who only share half their genetic endowment with each parent, then years of life were estimated as 10 * log protection ratio, in accordance with a long-standing actuarial rule of thumb recently verified46
Multivariable MR estimates for omega-3 and omega-6 fatty acids
The conditional F statistics were adequate when considering omega-3 and omega-6 fatty acids together (>42). The MR-Egger intercepts did not indicate that the multivariable IVW estimates were invalid. In multivariable MR, omega-6 fatty acids were consistently associated with IHD but omega-3 fatty acids had null associations, with similar estimates from MR-Egger (Figure 2). Estimates for lifespan were in the direction of omega-6 fatty acids reducing lifespan in men. Given the lack of evidence of consistent associations of omega-3 fatty acids with IHD or mortality, only the major subtype of omega-6 fatty acids available, i.e. LA, was considered further.
MR estimates for LA on IHD and lifespan
About 44 genetic variants predicting LA were obtained (Supplementary File 3 Tables G6, available as Supplementary data at IJE online), with mean F statistics greater than 110 and minimum F statistic 29.4. LA was associated with higher IHD risk and shorter lifespan in men based on the IVW estimates (Figure 3). The MR-Egger intercepts were not significant, but the MR-Egger estimates suggested no association, raising the possibility of pleiotropic effects, given the MR-Egger were 95–6%. Genetic variants predicting LA included a FADS1/2 variant (rs174564); repeating the analysis excluding that variant, estimates were similar (Supplementary File 1 Table S3, available as Supplementary data at IJE online).
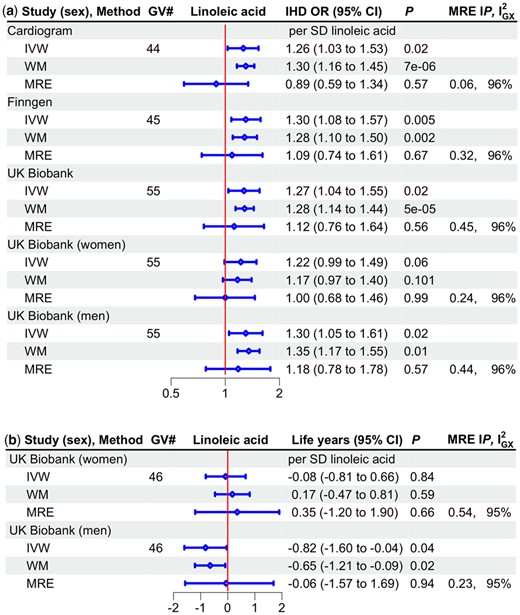
Univariable Mendelian randomization estimates for linoleic acid on a) ischaemic heart disease odds ratio by study and sex and b) lifespan (life years*) by sex using different methods. GV#, number of genetic variants; IHD OR, ischaemic heart disease odds ratio; CI, confidence interval; MRE IP, MR-Egger intercept P-value; , measure of heterogeneity in gene-exposure estimates across GVs; IVW, inverse variance weighted; WM, weighted median; MRE, MR Egger; SD, standard deviation. *Life years were obtained as follows, first multiply by 2.5863 and 2.2869 in mothers and fathers, respectively, to account for the genetic associations used here being in children who only share half their genetic endowment with each parent, then years of life were estimated as 10 * log protection ratio, in accordance with a long-standing actuarial rule of thumb recently verified46
Finally, as an additional means of visualizing these results, plots of instrument on exposure against instrument on outcome are shown in Supplementary File 1 Figure S2, available as Supplementary data at IJE online. They also show PUFAs positively associated with IHD, but different associations with lifespan by sex, with a somewhat similar pattern for omega-6 and possibly linoleic acid, but few clear or consistent association for SFAs, MUFAs or omega-3. The study was powered at 5% alpha and 80% power to detect relatively small associations, of the order of an odds ratio of about 1.18 and differences in lifespan of less than a year per standard deviation of fatty acid.
Discussion
Consistent with RCTs, SFAs5 and omega-3 fatty acids6–9 had little association with IHD or with all-cause mortality, proxied by lifespan. We add by showing that MUFAs may also have little association with IHD or mortality. Our study is consistent with previous MR studies showing omega-6 fatty acids31,33 are associated with higher risk of IHD; we add by showing the same association for PUFAs. We also add by suggesting that PUFA, in particular omega-6 fatty acids, and possibly LA, are associated with shorter lives in specifically men.
Overall, consistent with RCTs, we did not find SFAs, MUFAs or PUFA omega-3 fatty acids protective against IHD or all-cause mortality.6–9,12,13 Potentially harmful effects of PUFAs on lifespan are consistent with trials suggesting PUFA may increase cancer risk.54 However, RCT evidence concerning omega-6 fatty acids on cardiovascular disease or mortality is too limited to draw any conclusion, or to assess sex-specific effects.13 Potential harmful effects of LA are consistent with a resurrected RCT15 but not with the totality of RCT evidence,13,14 which is too limited to be definitive. Nevertheless, we cannot exclude a role of LA via AA, because the instruments for AA are limited (currently no genome-wide significant genetic predictor independent of the FADS1/2 gene region); the extent to which LA contributes to AA in humans is disputed.55
Our findings suggest that dietary PUFA has an effect on lifespan specific to men, possibly acting via omega 6, LA or even AA. PUFA also appeared to be more strongly associated with IHD in men, although the sample size was not large enough to be definitive. Although men have been known to be more vulnerable to cardiovascular diseases than women for many years,56 few factors underlying men’s greater vulnerability have been identified, although androgens are an emerging candidate.23,25 AA is increasingly being implicated in cardiovascular disease,57 when its product thromboxane A2 is modulated by testosterone.58,59 Some indications of sex-specific effects of AA on cardiovascular disease have been found,60 which could contribute possibly via COX-2 inhibiting testosterone glucuronidation.61
Previous MR studies of fatty acids have often been limited by the inevitable use of a small number of genetic instruments and not always considering pleiotropic effects via FADS1/2 variants.29–33 Nevertheless, findings here are consistent with the emerging evidence showing little effect of most fatty acids on health, but some evidence that omega 6 fatty acids are positively associated with IHD.31,33 The associations with lifespan are novel, particularly the possibility of a sex-specific association for PUFAs, which could be relevant to the well-known conundrum of shorter lives in men.
Despite using MR to reduce confounding and a longevity study to reduce selection bias, limitations exist. First, lifespan was based on adult child-reported parental attained age, which could induce measurement error, potentially biasing towards the null. However, self-reports may be less reliable than proxy reports62 and most people know their parents’ age. Estimates could also be biased by non-paternity. However, any such bias is likely to be minor63 and non-differential, so towards the null. Second, the average age of the UK Biobank participants was 57 years; those from the underlying birth cohorts who died before recruitment are missing. However, the genetic instrument for MUFAs, SFAs, PUFA, omega 3 fatty acids, omega 6 fatty acids and LA were not associated with age at recruitment (data not shown), suggesting any bias should be minor. Parents who died early were also excluded, again likely biasing towards the null. Third, the UK Biobank is not representative of the general UK population, which would bias a descriptive study but is not necessarily a source of bias for MR studies.64 Fourth, we did not use sex-specific instruments for fatty acids, because the key question is to mimic the downstream effects of the same dietary exposure (fatty acids). Fifth, MR requires the assumptions of relevance, independence and exclusion. The genetic instruments strongly predicted the exposures, with high F statistics in univariable analysis but not always in multivariable analysis. Random allocation of genetic material at conception means the genetic instruments are unlikely associated with confounders of fatty acids on IHD or lifespan. Use of a longevity study reduces bias from selection on survival.65 Pleiotropic effects of PUFA via adiposity, blood pressure or diabetes are possible. Evidence from trials suggests that PUFA has little effect on body weight,66 blood pressure67 or diabetes incidence.68 Preliminary analysis suggested little effect of PUFA on these factors (data not shown), consistent with the lack of association with lifespan in women. We did not adjust fatty acids for key lipids, such as apolipoprotein B or low density lipoprotein (LDL) cholesterol, because they have strong genetic correlations with these lipids,34 when evidence from meta-analysis of trials suggests that PUFA and omega 6 do not affect LDL-cholesterol.13,66 As such, we cannot exclude the possibility that fatty acids would be protective for IHD and lifespan if any pleiotropic effects via lipids were removed; however, such a benefit would conflict with evidence from trials.13,66 Pleiotropic effects were addressed by using multivariable MR and exclusion of very pleiotropic genetic variants from FADS1/2 which increase AA, in sensitivity analysis where appropriate. One-sample MR, which can be biased towards the confounded estimate, was used for the analysis of fatty acids on lifespan, and some of the MR-Egger were less than optimal, suggesting the possibility of confounded MR-Egger estimates. However, confounding is likely to be similar by sex, so any such bias is unlikely to generate differences by sex. Moreover, estimates for IHD from the UK Biobank using a one-sample study were very similar to the estimates from a two-sample study using Cardiogram and Finngen (Figures 1, 2, 3), suggesting sample overlap is not a major source of bias. Finally, MR estimates relate to lifelong exposures, so may not directly correspond to the effect of an intervention, although some concordance has been shown.69 So triangulation using internally valid real-world evidence would be immensely valuable.
Sex-specific effects of diet have not been extensively investigated in humans, although a study in mice showed different dietary drivers of fertility and lifespan by sex.70 Whether increasing availability of LA and AA in the first half of the 20th century71 underlies the epidemic of heart disease that emerged in specifically men in the first half of the 20th century,56 remains to be determined. In this context, our study draws attention to the importance of explicitly considering whether interventions might have different effects by sex, which could be an important area of research to identify effective dietary recommendations and thereby address substantial differences in lifespan by sex.
Conclusion
Most fatty acids seemed unrelated to IHD or lifespan but PUFAs, likely acting via specific omega 6 fatty acids, appeared to contribute to IHD and in men to shorter lives. Further investigation, of whether more personalized diets could optimize lifespan and begin to explicitly address men’s longevity disadvantage, is warranted.
Ethics approval
This study only uses publicly available genetic summary statistics created from information and materials previously collected with informed consent.
Data availability
This study used publicly available data from the UK Biobank, CARDIoGRAMplusC4D 1000 Genomes and the Finngen consortium (based on a network of existing and new Finnish biobanks matched with national health records [https://www.finngen.fi/en/for_researchers]).
Supplementary data
Supplementary data are available at IJE online.
Author contributions
C.M.S., M.K.K. and J.V.Z. all contributed to the concept and design of the paper. C.M.S. conducted the data analysis, M.K.K. and J.V.Z. contributed to the interpretation. C.M.S. drafted the paper, M.K.K. and J.V.Z. revised it critically for important intellectual content. All authors gave final approval for the version to be published and are accountable for all aspects of the work. J.V.Z. is the guarantor.
Conflict of interest
None declared.



