-
PDF
- Split View
-
Views
-
Cite
Cite
Espen Moen Eilertsen, Line C Gjerde, Ted Reichborn-Kjennerud, Ragnhild E Ørstavik, Gun Peggy Knudsen, Camilla Stoltenberg, Nikolai Czajkowski, Espen Røysamb, Kenneth S Kendler, Eivind Ystrom, Maternal alcohol use during pregnancy and offspring attention-deficit hyperactivity disorder (ADHD): a prospective sibling control study, International Journal of Epidemiology, Volume 46, Issue 5, October 2017, Pages 1633–1640, https://doi.org/10.1093/ije/dyx067
Close - Share Icon Share
Abstract
Maternal alcohol use during pregnancy has repeatedly been associated with development of attention-deficit hyperactivity disorder (ADHD) in the offspring. It is, however not known whether this reflects a direct casual intra-uterine effect or a non-causal relationship due to confounding. We used three different approaches to control for measured and unmeasured confounding: statistical adjustment for covariates, negative control comparison against maternal pre-pregnancy alcohol use, and comparison among differentially exposed siblings.
The sample comprised 114 247 children (34 283 siblings) from 94 907 mothers, recruited to the Norwegian Mother and Child Birth Cohort Study between 1999 and 2008. Self-reported measurements of alcohol use were obtained in week 30 during the pregnancy. Mothers rated offspring ADHD symptoms at 5 years on two measures. Clinical ADHD diagnoses were obtained from the Norwegian Patient Registry.
We found an overall positive association between maternal alcohol use during pregnancy and offspring ADHD symptoms, which was only marginally attenuated after inclusion of measured covariates. Both the negative control and the sibling comparison analysis further attenuated the estimated association, but it remained greater than zero [ = 0.017, 95% confidence interval (CI) = 0.005–0.030). No association was found between maternal alcohol use during pregnancy and offspring ADHD diagnosis.
For offspring ADHD symptoms we found a weak, but possibly causal association with maternal alcohol use during pregnancy, but no such effect was observed for clinical ADHD diagnosis.
Introduction
Attention-deficit hyperactivity disorder (ADHD), characterized by inattention and/or hyperactivity-impulsivity, is the most common behavioural disorder in childhood, manifests at an early age and affects approximately 5% of children worldwide.1,2
Some women continue to drink after becoming pregnant,3 and ADHD is highly comorbid with fetal alcohol spectrum disorders.4 A positive association between maternal alcohol use during pregnancy and symptoms of ADHD in children has been reported in several studies,5,6 including a meta-analysis.7
Due to the large timespan between pregnancy and the development of child ADHD symptoms, prospective data collection is essential to ensure validity of measurements. In a prospective Danish birth cohort sample comprising 1628 mothers and children, no association between attention deficits in children was found when mothers abstaining from alcohol were compared with mothers with low to moderate consumption. However, when abstaining mothers were compared with mothers consuming more than eight drinks a week, a weak association was found.8 In a population-based cohort of 604 8-year-old children assessed for ADHD by both clinical diagnosis and ratings scales,9 an association between maternal alcohol use during pregnancy and ADHD was found but disappeared after adjustment for measured covariates.
These studies are limited with respect to inferences regarding causal effects. An observed association might be spurious, caused by unmeasured familial factors common to both mothers and children.10 For instance, twin and family studies report high heritability estimates for both ADHD11–13 and alcohol use disorders14,15 and that the genetic risk factors are partly overlapping.16–18 Familial factors are therefore likely to confound estimates of the association, and must be addressed in order to make valid causal inferences. Additionally, the inconsistencies in results across studies might indicate that the effect of alcohol on ADHD is small, requiring large samples to be reliably identified.
The aims of this study are 2-fold. First, we investigate whether maternal alcohol use during pregnancy is related to offspring ADHD; and second, we investigate if such an association is likely to reflect a causal effect. Integration of several methods and consensus of results would provide stronger support for causal inference. We apply three different approaches in order to control for confounding variables. First, we statistically adjust for measured covariates previously shown to be related to ADHD. Second, we use maternal alcohol use before pregnancy as a negative control comparison. Third, we use a quasi-experimental sibling comparison design, controlling for unmeasured family-varying variables.
Methods
Sample
The study is based on data from the Norwegian Mother and Child Cohort Study (MoBa), a large prospective population-based pregnancy cohort study conducted by the Norwegian Institute of Public Health.19 Participants were recruited from 1999 to 2008. The women consented to participation in 41% of the pregnancies. The cohort includes approximately 114 500 children and 95 200 mothers. Version 9 of the quality-assured MoBa data files, released for research in December 2015, was used for the analyses. Written informed consent was obtained from all participants upon recruitment. The MoBa study has been granted a licence from the Norwegian Data Inspectorate, and the current study was approved by the Regional Committee for Medical Research Ethics. Clinical ADHD diagnoses from the Norwegian Patient Registry (NPR) were linked with the MoBa cohort. Since 2008, NPR has collected data on diagnosis from all hospitals and outpatient clinics in Norway.
Measurements of offspring ADHD
Symptoms of ADHD at 5 years of age were assessed by maternal report, using the revised Conner’s Parent Rating Scale (CPRS-R)20 included in the MoBa questionnaire. The scale consists of 12 four-point items which were summed to a score ranging from 12 to 48 (see Table 2 for description of key variables). ADHD symptoms were also assessed using the Diagnostic and Statistical Manual of Mental Disorders (DSM)-oriented ADHD scale of the Child Behaviour Checklist (CBCL).21 This included six three-point items summed to a score ranging from 6 to 18. Both measures were standardized to zero mean and unit variance prior to analyses.
Children diagnosed with an International Classification of Diseases 10 (ICD-10) diagnosis of hyperkinetic disorder (HKD; F90.0, F90.1, F90.8 or F90.9) in the NPR between 2008 and 2014 were identified as having ADHD, corresponding to 220 cases. HKD requires the combination of inattentive and hyperactive symptoms, and may therefore be regarded as a subtype of DSM-5 ADHD.22 In comparison with ADHD, HKD is characterized more by neurodevelopmental alterations in language and motor development.23 Age of onset for diagnosis ranged from 5 to 15 years. The prevalence of HKD in the sample was approximately 2%, with an estimated lifetime prevalence of 4% at age 13 years.
Measurements of maternal alcohol use
In MoBa, alcohol use was measured using the Alcohol Use Disorder Identification Test Consumption (AUDIT-C)24 designed to identify harmful patterns of usage. The scale consists of three five-point items measuring drinking frequency, quantity and binge drinking, summed to a score ranging from 0 to 12. The questionnaire was administered around week 30 in pregnancy, and mothers reported on their use pre-pregnancy and during the first trimester. Both measurements were standardized to zero mean and unit variance prior to analysis.
Additional covariates
Variables previously shown to be related to ADHD were adjusted for in the analysis9,25 and included parental education and income, maternal smoking during pregnancy, children’s birth order and children’s gender.
Statistical analysis
To facilitate comparisons across analyses of ADHD symptoms, we restricted the sample to mothers with two or more birth records in MoBa, comprising 16 407 mothers and 34 283 children. Symptoms were modelled using linear multilevel regression models with random intercepts for mothers, allowing for residual correlations (familial dependence) in responses of siblings. The two outcome measures were analysed separately. All parameter estimates are reported as standardized regression coefficients () with 95% confidence intervals (CI).
First, a crude estimate of the association with the outcome measures was obtained by including maternal alcohol use during pregnancy as a fixed effect in the models. In the next step, we adjusted for measured covariates. We then used maternal alcohol use before the pregnancy as a negative control comparison. Maternal alcohol use before pregnancy is likely to be influenced by many of the same unmeasured variables as maternal alcohol use during pregnancy, but is not expected to have any direct causal influence on offspring ADHD. Differences in these estimates can then, at least partially, be attributed to causal effects of maternal alcohol use during pregnancy.26 We estimated the difference after fitting regression models including both variables, in addition to the control variables. Last, our most stringent control for confounding was obtained by comparing siblings differentially exposed to alcohol during pregnancy. By decomposing the effect of maternal alcohol use during pregnancy into a within-mother effect and a between-mother effect, all mother-specific covariates are held constant, thereby implicitly controlling the within-effect for confounding variables at the mother level.27 Estimates were obtained by adjusting alcohol use during pregnancy for each mother’s average alcohol use (sample means) across all pregnancies, in addition to the control variables.
We tested whether effect sizes associated with the two ADHD symptom measures were different, and if effect sizes associated with the negative control and sibling comparisons were different from those only adjusted for the measured covariates. Estimates were obtained fitting a main-effects model using weighted least squares, weighting the effect sizes by their precision.
We investigated the relationship between maternal alcohol use during pregnancy and ADHD diagnosis in the complete sample, using a Cox proportional hazards model with children’s age in months as the time metric. Results are presented as hazard ratios (HR) with 95% CI. We used a robust variance estimator to account for correlations in responses of siblings. First, we estimated the crude association between maternal alcohol use during pregnancy and ADHD diagnosis, and next we adjusted for measured covariates.
For all analyses, partially missing observations were imputed using a multiple imputation procedure based on 20 independent chained equations. Diagnosis and symptoms data were imputed separately. To accommodate clustering of responses within families, family means of the outcome variables were included in the imputation models. All analyses were conducted using Stata 14.28
Results
Descriptives
Distributions of characteristics for the full sample and the restricted sibling sample are presented in Table 1. Summary statistics for key variables are presented in Table 2. There was no indication of discrepancies between the samples for either of the statistics. Acceptable reliability estimates were found for all scales, although AUDIT-C before pregnancy was slightly lower.
Distributions of child and parent characteristics for the selected sibling sample and the full sample
| . | Sibling sample . | Full sample . | ||
|---|---|---|---|---|
| Male children | 52% | 51% | ||
| Highest education mother | ||||
| Below high school | 5% | 8% | ||
| High school | 24% | 28% | ||
| Above high school | 71% | 64% | ||
| Highest education father | ||||
| Below high school | 8% | 11% | ||
| High school | 36% | 38% | ||
| Above high school | 55% | 51% | ||
| Income mother | ||||
| < 150 NOK | 16% | 18% | ||
| 150–300 NOK | 46% | 45% | ||
| 300–500 NOK | 32% | 32% | ||
| > 500-NOK | 5% | 5% | ||
| Income father | ||||
| < 150 NOK | 6% | 7% | ||
| 150–300 NOK | 26% | 27% | ||
| 300–500 NOK | 50% | 49% | ||
| > 500 NOK | 17% | 17% | ||
| Parity | ||||
| 1 | 37% | 44% | ||
| 2 | 45% | 36% | ||
| 3 | 15% | 16% | ||
| 4 | 3% | 3% | ||
| 5 | 1% | 1% | ||
| Maternal smoking in pregnancy | ||||
| Never | 94% | 91% | ||
| Sometimes | 2% | 3% | ||
| Daily | 4% | 6% | ||
| . | Sibling sample . | Full sample . | ||
|---|---|---|---|---|
| Male children | 52% | 51% | ||
| Highest education mother | ||||
| Below high school | 5% | 8% | ||
| High school | 24% | 28% | ||
| Above high school | 71% | 64% | ||
| Highest education father | ||||
| Below high school | 8% | 11% | ||
| High school | 36% | 38% | ||
| Above high school | 55% | 51% | ||
| Income mother | ||||
| < 150 NOK | 16% | 18% | ||
| 150–300 NOK | 46% | 45% | ||
| 300–500 NOK | 32% | 32% | ||
| > 500-NOK | 5% | 5% | ||
| Income father | ||||
| < 150 NOK | 6% | 7% | ||
| 150–300 NOK | 26% | 27% | ||
| 300–500 NOK | 50% | 49% | ||
| > 500 NOK | 17% | 17% | ||
| Parity | ||||
| 1 | 37% | 44% | ||
| 2 | 45% | 36% | ||
| 3 | 15% | 16% | ||
| 4 | 3% | 3% | ||
| 5 | 1% | 1% | ||
| Maternal smoking in pregnancy | ||||
| Never | 94% | 91% | ||
| Sometimes | 2% | 3% | ||
| Daily | 4% | 6% | ||
Distributions of child and parent characteristics for the selected sibling sample and the full sample
| . | Sibling sample . | Full sample . | ||
|---|---|---|---|---|
| Male children | 52% | 51% | ||
| Highest education mother | ||||
| Below high school | 5% | 8% | ||
| High school | 24% | 28% | ||
| Above high school | 71% | 64% | ||
| Highest education father | ||||
| Below high school | 8% | 11% | ||
| High school | 36% | 38% | ||
| Above high school | 55% | 51% | ||
| Income mother | ||||
| < 150 NOK | 16% | 18% | ||
| 150–300 NOK | 46% | 45% | ||
| 300–500 NOK | 32% | 32% | ||
| > 500-NOK | 5% | 5% | ||
| Income father | ||||
| < 150 NOK | 6% | 7% | ||
| 150–300 NOK | 26% | 27% | ||
| 300–500 NOK | 50% | 49% | ||
| > 500 NOK | 17% | 17% | ||
| Parity | ||||
| 1 | 37% | 44% | ||
| 2 | 45% | 36% | ||
| 3 | 15% | 16% | ||
| 4 | 3% | 3% | ||
| 5 | 1% | 1% | ||
| Maternal smoking in pregnancy | ||||
| Never | 94% | 91% | ||
| Sometimes | 2% | 3% | ||
| Daily | 4% | 6% | ||
| . | Sibling sample . | Full sample . | ||
|---|---|---|---|---|
| Male children | 52% | 51% | ||
| Highest education mother | ||||
| Below high school | 5% | 8% | ||
| High school | 24% | 28% | ||
| Above high school | 71% | 64% | ||
| Highest education father | ||||
| Below high school | 8% | 11% | ||
| High school | 36% | 38% | ||
| Above high school | 55% | 51% | ||
| Income mother | ||||
| < 150 NOK | 16% | 18% | ||
| 150–300 NOK | 46% | 45% | ||
| 300–500 NOK | 32% | 32% | ||
| > 500-NOK | 5% | 5% | ||
| Income father | ||||
| < 150 NOK | 6% | 7% | ||
| 150–300 NOK | 26% | 27% | ||
| 300–500 NOK | 50% | 49% | ||
| > 500 NOK | 17% | 17% | ||
| Parity | ||||
| 1 | 37% | 44% | ||
| 2 | 45% | 36% | ||
| 3 | 15% | 16% | ||
| 4 | 3% | 3% | ||
| 5 | 1% | 1% | ||
| Maternal smoking in pregnancy | ||||
| Never | 94% | 91% | ||
| Sometimes | 2% | 3% | ||
| Daily | 4% | 6% | ||
Summary statistics of ADHD symptoms and maternal alcohol use from the selected sibling sample and the full sample (parentheses)
| Measurement . | M . | SD . | Alpha . | r . |
|---|---|---|---|---|
| ADHD symptoms | ||||
| CPRS-R | 16.0 (16.4) | 4.3 (4.6) | 0.87 (.88) | |
| CBCL | 8.3 (8.6) | 2.1 (2.2) | 0.73 (.74) | 0.64 (0.65) |
| Maternal alcohol use | ||||
| AUDIT-C before pregnancy | 3.2 (3.4) | 2.1 (2.1) | 0.66 (.66) | |
| AUDIT-C during pregnancy | 0.63 (0.70) | 1.3 (1.4) | 0.73 (0.76) | 0.38 (0.38) |
| Measurement . | M . | SD . | Alpha . | r . |
|---|---|---|---|---|
| ADHD symptoms | ||||
| CPRS-R | 16.0 (16.4) | 4.3 (4.6) | 0.87 (.88) | |
| CBCL | 8.3 (8.6) | 2.1 (2.2) | 0.73 (.74) | 0.64 (0.65) |
| Maternal alcohol use | ||||
| AUDIT-C before pregnancy | 3.2 (3.4) | 2.1 (2.1) | 0.66 (.66) | |
| AUDIT-C during pregnancy | 0.63 (0.70) | 1.3 (1.4) | 0.73 (0.76) | 0.38 (0.38) |
M, mean; SD, standard deviation; Alpha, Cronbach’s alpha; r, Pearson’s correlation coefficient between ADHD scales and between measures of alcohol usage.
Summary statistics of ADHD symptoms and maternal alcohol use from the selected sibling sample and the full sample (parentheses)
| Measurement . | M . | SD . | Alpha . | r . |
|---|---|---|---|---|
| ADHD symptoms | ||||
| CPRS-R | 16.0 (16.4) | 4.3 (4.6) | 0.87 (.88) | |
| CBCL | 8.3 (8.6) | 2.1 (2.2) | 0.73 (.74) | 0.64 (0.65) |
| Maternal alcohol use | ||||
| AUDIT-C before pregnancy | 3.2 (3.4) | 2.1 (2.1) | 0.66 (.66) | |
| AUDIT-C during pregnancy | 0.63 (0.70) | 1.3 (1.4) | 0.73 (0.76) | 0.38 (0.38) |
| Measurement . | M . | SD . | Alpha . | r . |
|---|---|---|---|---|
| ADHD symptoms | ||||
| CPRS-R | 16.0 (16.4) | 4.3 (4.6) | 0.87 (.88) | |
| CBCL | 8.3 (8.6) | 2.1 (2.2) | 0.73 (.74) | 0.64 (0.65) |
| Maternal alcohol use | ||||
| AUDIT-C before pregnancy | 3.2 (3.4) | 2.1 (2.1) | 0.66 (.66) | |
| AUDIT-C during pregnancy | 0.63 (0.70) | 1.3 (1.4) | 0.73 (0.76) | 0.38 (0.38) |
M, mean; SD, standard deviation; Alpha, Cronbach’s alpha; r, Pearson’s correlation coefficient between ADHD scales and between measures of alcohol usage.
ADHD symptoms
Without adjusting for any of the covariates (Model 1), we found maternal alcohol use during pregnancy to be associated with offspring symptoms of ADHD assessed via both CBCL ( = 0.051, 95% CI = 0.035–0.067) and CPRS-R ( = 0.065, 95% CI = 0.051–0.079).
After inclusion of measured covariates (Model 2), parameter estimates were attenuated, but still significantly different from zero for both CBCL ( = 0.040, 95% CI = 0.024–0.055) and CPRS-R ( = 0.054, 95% CI = 0.039–0.068).
Adjusted for covariates, maternal alcohol use before pregnancy was associated with ADHD symptoms assessed by the CBCL ( = 0.046, 95% CI = 0.029–0.063) and CPRS-R ( = 0.037, 95% CI = 0.022–0.051). For CBCL, the effect was further reduced after subtracting the effect of maternal alcohol use before pregnancy (Model 3) and was no longer statistically different from zero ( = −0.005, 95% CI = −0.031–0.020). The estimate for CPRS-R was further attenuated ( = 0.029, 95% CI = 0.006–0.052), but remained greater than zero.
Last, we estimated the sibling comparison models, adjusting for the covariates (Model 4). Parameter estimates were reduced for both CBCL ( = 0.011, 95% CI = −0.002–0.024) and CPRS-R ( = 0.017, 95% CI = 0.005–0.030). Maternal alcohol use during pregnancy was still associated with symptoms of ADHD as measured by CPRS-R, but not as measured by CBCL.
Averaged over the models, effect sizes associated with the CPRS-R scale were significantly larger than those associated with the CBCL scale. Furthermore, the effect sizes estimated under Models 3 and 4 were significantly lower than those estimated under Model 2, averaged over the outcome measures (see Table 3).
Results from comparing effect sizes associated with ADHD symptoms across models
| Contrast . | Estimate . | SE . | Z . | P-value . |
|---|---|---|---|---|
| Constant | 0.040 | 0.006 | 6.335 | 0.000 |
| CPRS-R > CBCL | 0.013 | 0.007 | 2.046 | 0.041 |
| Model 3 > Model 2 | −0.034 | 0.010 | −3.315 | 0.001 |
| Model 4 > Model 2 | −0.033 | 0.007 | −4.667 | 0.000 |
| Contrast . | Estimate . | SE . | Z . | P-value . |
|---|---|---|---|---|
| Constant | 0.040 | 0.006 | 6.335 | 0.000 |
| CPRS-R > CBCL | 0.013 | 0.007 | 2.046 | 0.041 |
| Model 3 > Model 2 | −0.034 | 0.010 | −3.315 | 0.001 |
| Model 4 > Model 2 | −0.033 | 0.007 | −4.667 | 0.000 |
Regression coefficients are listed under the column titled estimate. The inequality symbol > describes the estimated contrast. CPRS-R > CBCL means that the estimate represents how much larger the effect size associated with CPRS-R is compared with CBCL. Actual effect sizes are presented in the result section.
SE, standard error.
Results from comparing effect sizes associated with ADHD symptoms across models
| Contrast . | Estimate . | SE . | Z . | P-value . |
|---|---|---|---|---|
| Constant | 0.040 | 0.006 | 6.335 | 0.000 |
| CPRS-R > CBCL | 0.013 | 0.007 | 2.046 | 0.041 |
| Model 3 > Model 2 | −0.034 | 0.010 | −3.315 | 0.001 |
| Model 4 > Model 2 | −0.033 | 0.007 | −4.667 | 0.000 |
| Contrast . | Estimate . | SE . | Z . | P-value . |
|---|---|---|---|---|
| Constant | 0.040 | 0.006 | 6.335 | 0.000 |
| CPRS-R > CBCL | 0.013 | 0.007 | 2.046 | 0.041 |
| Model 3 > Model 2 | −0.034 | 0.010 | −3.315 | 0.001 |
| Model 4 > Model 2 | −0.033 | 0.007 | −4.667 | 0.000 |
Regression coefficients are listed under the column titled estimate. The inequality symbol > describes the estimated contrast. CPRS-R > CBCL means that the estimate represents how much larger the effect size associated with CPRS-R is compared with CBCL. Actual effect sizes are presented in the result section.
SE, standard error.
ADHD diagnosis
We found no relationship between maternal alcohol consumption during pregnancy and offspring ADHD diagnosis, based on the crude model without any covariates (HR = 0.98, 95% CI = 0.93–1.03). Adjustment for the measured covariates did not substantially change parameter estimates (HR = 0.97, 95% CI = 0.93–1.01).
Discussion
We aimed to investigate the causal relationship between maternal alcohol use during pregnancy and offspring ADHD, using different non-experimental methodological approaches to control for confounding. Our analyses revealed four main findings. First, ADHD symptoms measured by the CPRS-R scale were associated with maternal alcohol use during pregnancy even under our most stringent control, indicating a possible causal effect. Second, the effect sizes were systematically different across the two symptom measures and the clinical diagnoses. Third, unmeasured variables appear to confound the association. Fourth, the estimated effect sizes were small. We discuss these findings in turn.
Causal effect
We found a positive association between maternal alcohol use during pregnancy and offspring ADHD symptoms, which remained after controlling for the covariates. This pattern replicated across both ADHD symptom measures. Although the results conform to others findings5,6 and suggest that maternal alcohol use during pregnancy is related to ADHD, the approach is not very informative about the potential causal nature of the association. Because it is not clear which covariates are critical to adjust for, it is likely that this approach will omit important variables from the analysis.
Comparing maternal alcohol use pre-pregnancy and during pregnancy, we observed stronger associations for alcohol use during pregnancy for CPRS-R but not for CBCL. It seems that, at least for CPRS-R, there is an association between maternal alcohol use during pregnancy and ADHD symptoms which cannot be attributed to factors influencing alcohol use both pre-pregnancy and during pregnancy.
The sibling comparison analysis showed a similar pattern of results; we found an effect only for CPRS-R. This design is powerful in that it allowed us to estimate the associations free from unmeasured familial risk factors that could bias the associations and distort inferences. Because siblings share many important aspects of social and household characteristics, the design excludes a wide set of potential confounding variables. Importantly, maternal genetic liability towards alcohol use is also constant across pregnancies, consequently excluding genetic confounding as a source of bias.29
These results are consistent with maternal alcohol consumption during pregnancy exerting a causal effect on offspring ADHD symptoms. Convergence of results from the negative control and sibling comparison strengthens our inferences.
Different measures of ADHD
Effect sizes were consistently larger when ADHD symptoms were assessed by the CPRS-R scale rather than the CBCL scale (Figure 1). Because the two scales include different items, with the CPRS-R scale comprising more items related to inattention, they may capture somewhat different aspects of ADHD symptomatology. However, as the CPRS-R includes twice as many items as the CBCL scale, and thereby may be measured with higher reliability, the differences in effect sizes may also be explained by differential influences of measurement error.
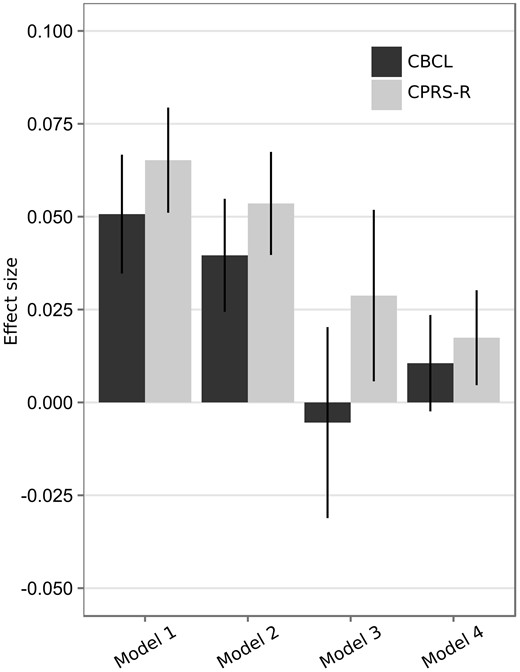
Association between maternal alcohol use during pregnancy and the two measures of ADHD symptoms. Bars represent standardized regression coefficients with 95% CI. Model 1, crude association; Model 2, association adjusted for measured covariates; Model 3, difference between maternal alcohol use during pregnancy and before pregnancy, adjusted for measured covariates; Model 4, association estimated from sibling comparison, adjusted for measured covariates.
Unlike for offspring ADHD symptoms, we found no association between maternal alcohol use during pregnancy and offspring ADHD diagnosis. As the number of children with an ADHD diagnosis by necessity will be smaller than children showing symptoms of ADHD, the statistical power and the possibility of detecting weak effects will be reduced. In the present study, the problem may be exaggerated, as close to 90% of the children were 11 years or younger, and the lifetime diagnosis of ADHD may be underestimated. It will therefore be important to repeat the study as children grow older.
Unmeasured confounding
Our results suggest that the measured covariates do not adequately adjust for confounding and that unobserved familial factors contribute to the association. This is supported by the significant drop in effect size from Model 2 to Model 3 and Model 4 in the summary analysis. In line with our results, D’Onofrio et al.,30 using the CBCL, found that familial factors influenced the association between prenatal alcohol exposure and attention/impulsivity problems. These results highlight the importance of methodological designs that control for confounding beyond traditional statistical adjustment.
The genetic resemblance between mothers and offspring is a factor that is controlled in the sibling comparison design. Results from two previous studies18,31 suggest that shared genes play a major role in determining the co-occurrence between maternal alcohol use during pregnancy and offspring ADHD. These studies therefore offer an explanation for the reduced associations we observed in the sibling comparison analysis.
Effect size
Our estimated effect sizes are small, even without adjustments. For example, a standard deviation increase on AUDIT-C is associated with an average of 8% of a standard deviation increase in CPRS-R across pregnancies. Given the nature of this association, we expect its magnitude to be small. If maternal alcohol use during pregnancy was a key determinant in explaining why some children develop ADHD, it would probably be well established and there would be less inconsistent findings in the literature. A plausible explanation is that several studies are under-powered for studying effects of this magnitude.32 For instance, a meta-analysis found that the odds of children prenatally exposed to alcohol having ADHD were 2.3 times greater than those of children not exposed.7 However, the 95% CI ranged from 1.2 to 4.6, reflecting large uncertainty. For comparison, we dichotomized the CPRS-R variable at the 95th percentile, roughly corresponding to the population rate of ADHD in children,2 and the AUDIT-C measure during pregnancy at the suggested cut-off,24 and obtained an estimated odds ratio of 1.4. Given the large sample investigated in the current study, estimates are more likely to reflect the magnitude of true population effects. Consequently, our results suggest that maternal alcohol use during pregnancy is involved in development of ADHD symptoms, but that it only plays a minor role among several other risk factors.
Strengths and limitations
There are several strengths in this study: First, validity of measurement is enhanced due to prospective data collection; second, we use several methodological approaches to control for confounding variables; third, our large sample size provides sufficient statistical power to identify small associations; and fourth, comparison of associations across different measures enhances the validity of our conclusions.
There are also limitations in our design. First, siblings are not identical. They experience different environmental exposures beyond the stable family environment. We statistically adjusted for several potential intra-familial confounding variables, including maternal smoking during pregnancy, which repeatedly have been shown to be associated with ADHD,9,25 but we have probably not been able to include all variables of relevance. Second, we have investigated average effects across families. There might be important moderators that determine the effect of maternal alcohol consumption, such as genetic factors (i.e. gene-environment interactions). Third, our analyses of symptoms were restricted to ADHD levels at 5 years of age, hence symptoms may have not yet manifested owing to later age of onset or later parental recognition. Although this would lead to an underestimation of the association, this problem is reduced by considering ADHD symptom levels on a continuum. Even if children at this age have not yet reached clinical thresholds for a diagnosis, they are still likely to exhibit increased symptom levels. Fourth, given the social stigma associated with alcohol use during pregnancy it is possible, but unclear whether or to what extent, that this is under-reported. Under-reporting would be problematic if it led to ‘floor effects’ which might bias estimates of associations. However, AUDIT-C has been shown to perform as well in a sample of pregnant women as in samples from the general population.33 We did not have sufficient information to evaluate this in the current study.
Implications and conclusions
We found a positive association between maternal alcohol use during pregnancy and offspring ADHD symptoms. When studying probability of ADHD diagnosis, we were not able to demonstrate a comparable effect. Taken together, the association between maternal alcohol use during pregnancy and ADHD symptoms appears to be confounded by familial factors that bias the association upwards if not accounted for. Maternal alcohol use during pregnancy represents a small and potentially causal risk of developing ADHD symptoms, and may thereby be of importance for understanding the aetiology of ADHD. Given the weak associations found in the present study as well as in other studies of the same phenomena, the effectiveness of preventive efforts, aimed at reducing ADHD on an individual level, is debatable. However, for large-scale public health interventions even weak causal effects may be of great importance–for global health as well as for social economic parameters.
Funding
The work was supported by a grant from the Medicine, Health Sciences and Biology Program at the Norwegian Research Council (grant number 231105). The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and Care Services and the Ministry of Education and Research, NIH/NIEHS (contract no. N01-ES-75558), NIH/NINDS (grant no. 1, UO1 NS 047537‐01 and grant no. 2, UO1 NS 047537‐06A1).
This study investigated the potential causal relationship between maternal alcohol use during pregnancy and offspring ADHD.
Controlling for confounding using observed covariates, a negative control and sibling comparison, we found an association for ADHD symptoms. No association was found for clinical diagnoses.
The results are consistent with a weak, causal effect of maternal alcohol use during pregnancy on offspring ADHD.
Acknowledgements
We are grateful to all the participating families in Norway who take part in this ongoing cohort study.
Conflict of interest: None declared.
References



