-
PDF
- Split View
-
Views
-
Cite
Cite
Ryo Miyata, Mitsugu Omasa, Ryo Fujimoto, Hiroyuki Ishikawa, Minoru Aoki, Efficacy of Ramelteon for delirium after lung cancer surgery, Interactive CardioVascular and Thoracic Surgery, Volume 24, Issue 1, January 2017, Pages 8–12, https://doi.org/10.1093/icvts/ivw297
Close - Share Icon Share
The aim of the study was to evaluate the feasibility of Ramelteon for the prevention of delirium after lung cancer surgery in elderly patients.
Medical records of patients over 70 years old, who underwent anatomical pulmonary resection for lung cancer at our institution from January 2013 to December 2015, were reviewed. Patients treated in 2013 and 2014 were used as a control group. Ramelteon was administered daily for 7 days after surgery. The incidence of delirium was determined based on the Intensive Care Delirium Screening Checklist (ICDSC). Scores of ≥4 and 1–3 points were used for the diagnoses of delirium and a pre-delirious state, respectively.
There were 24 patients in the Ramelteon group and 58 patients in the control group. ICDSC scores of ≥4 points were found for no patients in the Ramelteon group and 5 (9%) in the control group, whereas 21 (88%) and 49 (85%) patients, in the respective groups, had ICDSC scores of 0 points. The average incidence of events, associated with delirium, showed a trend of being lower in the Ramelteon group (0.25 ± 0.74 vs 1.58 ± 4.93, P = 0.061), and all events in the Ramelteon group occurred on the day of surgery. Thus, only one day was required for complete recovery from delirium in the Ramelteon group, whereas 8 days were needed in the control group. The peak delirious state occurred after 5 days in the control group.
Ramelteon is likely to reduce the incidence and intensity of delirium after surgery for lung cancer in elderly patients.
INTRODUCTION
Delirium is defined as an acute change in cognition with altered consciousness and impaired attention that fluctuates over time [1]. The incidence of postoperative delirium in thoracic surgery is 5–16% [2–4]. Postoperative delirium has proposed risk factors of a history of delirium, age over 70 and pre-existing cognitive impairment [5], and causes serious complications such as falls and self-extubation. Postoperative delirium in patients with chest tube drainage after anatomical pulmonary resection can increase serious tube complications such as tumbling and self-extubation, and thus, a preventive strategy for delirium is required. Ramelteon is a melatonergic agonist with a proven preventive effect on delirium in elderly inpatients [6]. We have used Ramelteon for the prevention of delirium after surgery for lung cancer since 2015. The aim of this study was to evaluate the efficacy in the use of Ramelteon for the prevention of delirium in elderly patients after lung cancer surgery, through comparison with a historical control group that did not receive Ramelteon.
MATERIALS AND METHODS
Patients and data collection
Medical records were reviewed for patients over 70 years old who underwent anatomical pulmonary resection for lung cancer at our institution from January 2013 to December 2015. Perioperative anaesthesic policy in our institution was perivertebral continuous anaesthesia [7] immediately after anaesthetic induction with ropivacaine for 2 days. Paravertebral blocking for thoracoscopic surgery and epidural blocking for thoracotomy were the standard of perivertebral blocking. Oral analgesic agents, loxoprofen and acetaminophen, were administered on the next morning. After the tracheal tube extubation in the operating room, patients with anatomical lung resection stayed in ICU for one night, and patients without serious cardiorespiratory complications were discharged from the ICU the next morning.
Ramelteon was administered to patients over 75 years of age who underwent anatomical pulmonary resection for lung cancer from January 2015. After September 2015, we expanded the age range to over 70 years old. Patients treated in 2013 and 2014 served as a control group. Patient information (age, sex, Brinkman index, body mass index, preoperative pulmonary function test, preoperative partial pressure of carbon dioxide, preoperative comorbidity, history of delirium, pre-existing cognitive impairment, habitual use of hypnotic agents, pathological TNM stage for lung cancer), operative information (surgical approach, surgical procedures, operation time, bleeding amount) and data for postoperative course (ICU stay, complications, drainage time, postoperative hospital stay) were used in the analysis. Medication compliance was evaluated for Ramelteon. The study was approved by the Nishi-Kobe Medical Center Institutional Review Board.
Outcomes and measurements
Ramelteon (8 mg) was administered daily for 7 days after surgery. Day 0 was defined as starting immediately after the operation until 9 pm on postoperative day (POD) 1. Day 1 then started at 9 pm on POD 1. The primary outcome was the incidence of delirium, with this incidence and the number of events determined retrospectively based on the Intensive Care Delirium Screening Checklist (ICDSC) [8] (Table 1), with reference to postoperative medical charts. ICDSC scores of ≥4 and 1–3 points were used for the diagnoses of delirium and a pre-delirious state, respectively. The ICDSC score was used to evaluate the incidence and severity of postoperative delirium. Ramelteon intake was confirmed by a medical chart, recorded by an attending nurse. The data-associated postoperative delirium was collected through POD 9.
| Patient evaluation . | Score (0/1) . |
|---|---|
| 1. Altered level of consciousness: A) No response or B) the need for vigorous stimulation to obtain any response signified a severe alteration in the level of consciousness that precluded evaluation. If there is coma (A) or stupor (B) for most of the time period, then a dash (-) is entered and there is no further evaluation during that period. C) Drowsiness or requirement of mild to moderate stimulation for a response implies an altered level of consciousness (1 point). D) Wakefulness or a sleeping state from which the patient could easily be aroused is considered normal (0 points). E) Hypervigilance is rated as an abnormal level of consciousness (1 point). | |
| 2. Inattention: Difficulty in following a conversation or instructions, easily distracted by external stimuli or difficulty in shifting focus (1 point for any of these items). | |
| 3. Disorientation: Any obvious mistake in time, place or person (1 point). | |
| 4. Hallucination, delusion or psychosis: Unequivocal clinical manifestation of hallucination or of behaviour that is probably due to hallucination (e.g. trying to catch a non-existent object) or delusion. Gross impairment in reality testing (1 point for any of these items). | |
| 5. Psychomotor agitation or retardation: Hyperactivity requiring the use of additional sedative drugs or restraints to control potential danger to oneself or others (e.g., pulling out iv lines, hitting staff). Hypoactivity or clinically noticeable psychomotor slowing (1 point for any of these items). | |
| 6. Inappropriate speech or mood: Inappropriate, disorganized or incoherent speech, or inappropriate display of emotion related to events or situation (1 point for any of these items). | |
| 7. Sleep/wake cycle disturbance: Sleeping <4 h or waking frequently at night, other than waking initiated by medical staff or a loud environment, or sleeping most of the day (1 point for any of these items). | |
| 8. Symptom fluctuation: Fluctuation of the manifestation of any item or symptom over 24 h (e.g. from one shift to another) (1 point). | |
| Total score (0–8 points) |
| Patient evaluation . | Score (0/1) . |
|---|---|
| 1. Altered level of consciousness: A) No response or B) the need for vigorous stimulation to obtain any response signified a severe alteration in the level of consciousness that precluded evaluation. If there is coma (A) or stupor (B) for most of the time period, then a dash (-) is entered and there is no further evaluation during that period. C) Drowsiness or requirement of mild to moderate stimulation for a response implies an altered level of consciousness (1 point). D) Wakefulness or a sleeping state from which the patient could easily be aroused is considered normal (0 points). E) Hypervigilance is rated as an abnormal level of consciousness (1 point). | |
| 2. Inattention: Difficulty in following a conversation or instructions, easily distracted by external stimuli or difficulty in shifting focus (1 point for any of these items). | |
| 3. Disorientation: Any obvious mistake in time, place or person (1 point). | |
| 4. Hallucination, delusion or psychosis: Unequivocal clinical manifestation of hallucination or of behaviour that is probably due to hallucination (e.g. trying to catch a non-existent object) or delusion. Gross impairment in reality testing (1 point for any of these items). | |
| 5. Psychomotor agitation or retardation: Hyperactivity requiring the use of additional sedative drugs or restraints to control potential danger to oneself or others (e.g., pulling out iv lines, hitting staff). Hypoactivity or clinically noticeable psychomotor slowing (1 point for any of these items). | |
| 6. Inappropriate speech or mood: Inappropriate, disorganized or incoherent speech, or inappropriate display of emotion related to events or situation (1 point for any of these items). | |
| 7. Sleep/wake cycle disturbance: Sleeping <4 h or waking frequently at night, other than waking initiated by medical staff or a loud environment, or sleeping most of the day (1 point for any of these items). | |
| 8. Symptom fluctuation: Fluctuation of the manifestation of any item or symptom over 24 h (e.g. from one shift to another) (1 point). | |
| Total score (0–8 points) |
Clear manifestation of an item = 1 point; no manifestation of an item or no assessment possible = 0 points. Scores for Sections 1 to 8 are entered in the corresponding boxes as 0 or 1.
| Patient evaluation . | Score (0/1) . |
|---|---|
| 1. Altered level of consciousness: A) No response or B) the need for vigorous stimulation to obtain any response signified a severe alteration in the level of consciousness that precluded evaluation. If there is coma (A) or stupor (B) for most of the time period, then a dash (-) is entered and there is no further evaluation during that period. C) Drowsiness or requirement of mild to moderate stimulation for a response implies an altered level of consciousness (1 point). D) Wakefulness or a sleeping state from which the patient could easily be aroused is considered normal (0 points). E) Hypervigilance is rated as an abnormal level of consciousness (1 point). | |
| 2. Inattention: Difficulty in following a conversation or instructions, easily distracted by external stimuli or difficulty in shifting focus (1 point for any of these items). | |
| 3. Disorientation: Any obvious mistake in time, place or person (1 point). | |
| 4. Hallucination, delusion or psychosis: Unequivocal clinical manifestation of hallucination or of behaviour that is probably due to hallucination (e.g. trying to catch a non-existent object) or delusion. Gross impairment in reality testing (1 point for any of these items). | |
| 5. Psychomotor agitation or retardation: Hyperactivity requiring the use of additional sedative drugs or restraints to control potential danger to oneself or others (e.g., pulling out iv lines, hitting staff). Hypoactivity or clinically noticeable psychomotor slowing (1 point for any of these items). | |
| 6. Inappropriate speech or mood: Inappropriate, disorganized or incoherent speech, or inappropriate display of emotion related to events or situation (1 point for any of these items). | |
| 7. Sleep/wake cycle disturbance: Sleeping <4 h or waking frequently at night, other than waking initiated by medical staff or a loud environment, or sleeping most of the day (1 point for any of these items). | |
| 8. Symptom fluctuation: Fluctuation of the manifestation of any item or symptom over 24 h (e.g. from one shift to another) (1 point). | |
| Total score (0–8 points) |
| Patient evaluation . | Score (0/1) . |
|---|---|
| 1. Altered level of consciousness: A) No response or B) the need for vigorous stimulation to obtain any response signified a severe alteration in the level of consciousness that precluded evaluation. If there is coma (A) or stupor (B) for most of the time period, then a dash (-) is entered and there is no further evaluation during that period. C) Drowsiness or requirement of mild to moderate stimulation for a response implies an altered level of consciousness (1 point). D) Wakefulness or a sleeping state from which the patient could easily be aroused is considered normal (0 points). E) Hypervigilance is rated as an abnormal level of consciousness (1 point). | |
| 2. Inattention: Difficulty in following a conversation or instructions, easily distracted by external stimuli or difficulty in shifting focus (1 point for any of these items). | |
| 3. Disorientation: Any obvious mistake in time, place or person (1 point). | |
| 4. Hallucination, delusion or psychosis: Unequivocal clinical manifestation of hallucination or of behaviour that is probably due to hallucination (e.g. trying to catch a non-existent object) or delusion. Gross impairment in reality testing (1 point for any of these items). | |
| 5. Psychomotor agitation or retardation: Hyperactivity requiring the use of additional sedative drugs or restraints to control potential danger to oneself or others (e.g., pulling out iv lines, hitting staff). Hypoactivity or clinically noticeable psychomotor slowing (1 point for any of these items). | |
| 6. Inappropriate speech or mood: Inappropriate, disorganized or incoherent speech, or inappropriate display of emotion related to events or situation (1 point for any of these items). | |
| 7. Sleep/wake cycle disturbance: Sleeping <4 h or waking frequently at night, other than waking initiated by medical staff or a loud environment, or sleeping most of the day (1 point for any of these items). | |
| 8. Symptom fluctuation: Fluctuation of the manifestation of any item or symptom over 24 h (e.g. from one shift to another) (1 point). | |
| Total score (0–8 points) |
Clear manifestation of an item = 1 point; no manifestation of an item or no assessment possible = 0 points. Scores for Sections 1 to 8 are entered in the corresponding boxes as 0 or 1.
Objective evaluation of the Intensive Care Delirium Screening Checklist score
The recovery index was used to evaluate recovery from delirium or a pre-delirious state. The recovery index was calculated as the total of ICDSC scores, divided by the number of patients in each group on each day. The intensity index was used to evaluate the daily intensity of delirium and was calculated as the total score for all ICDSC events, divided by the number of scored patients.
Statistical analysis
Categorical variables were compared by χ2 test or Fisher's exact test, normally distributed continuous variables by Student's t-test or Welch t-test, and non-normally distributed continuous variables by Mann–Whitney test, as appropriate, with P < 0.05 considered significant. All analyses were performed with StatMate IV (ATMS Co., Ltd, Tokyo, Japan).
RESULTS
Patient characteristics
| Categories . | Ramelteon group (n = 24) . | Control group (n = 58) . | P-value . |
|---|---|---|---|
| Age (mean [range]) | 79 [70–89] | 76.5 [70–87] | |
| Sex (male/female) | 21/3 | 43/15 | 0.300 |
| Brinkman index | 994 ± 680 | 787 ± 667 | 0.208 |
| Body mass index | 21.7 ± 3.11 | 22.7 ± 3.59 | 0.258 |
| Preoperative PFT | |||
| %VC | 92.3 ± 14.5 | 94.7 ± 15.1 | 0.505 |
| FEV1.0% | 76.1 ± 11.4 | 76.4 ± 9.19 | 0.901 |
| Preoperative PaCO2 | 43.9 ± 4.94 (n = 21) | 42.7 ± 4.49 (n = 57) | 0.306 |
| Preoperative comorbidity | 0.782 | ||
| Diabetes | 4 | 11 | |
| Ischaemic heart disease | 1 | 5 | |
| Cerebrovascular disease | 0 | 2 | |
| Depression | 0 | 0 | |
| History of delirium | 1 | 1 | 0.893 |
| Pre-existing cognitive impairment | 0 | 1 | 0.647 |
| Habitual use of hypnotics | 2 | 13 | 0.235 |
| Approach open/VATS | 1/23 | 1/57 | 0.893 |
| Surgical procedures | 0.813 | ||
| Segmentectomy | 6 | 12 | |
| Lobectomy | 17 | 44 | |
| Bilobectomy | 0 | 1 | |
| Pneumonectomy | 1 | 1 | |
| Operation time (min) | 293 ± 108 | 280 ± 91.4 | 0.612 |
| Bleeding amount (ml) | 175 ± 341 | 228 ± 238 | 0.491 |
| Intensive care unit stay (day) | 1.00 ± 0 | 1.10 ± 0.66 | 0.719 |
| Drainage duration (day) | 4.54 ± 2.23 | 4.97 ± 4.69 | 0.581 |
| Postoperative complication | 10 (41.7%) | 21 (36.2%) | 0.132 |
| Prolonged air leakage | 5 | 4 | |
| Arrhythmia | 0 | 5 | |
| Pneumonia | 1 | 4 | |
| Heart failure | 1 | 0 | |
| Others | 3 | 8 | |
| Pathological stage I/II/III/IV | 17/5/1/1 | 31/12/9/4 | 0.391 |
| Postoperative hospital stay (days) | 13.5 ± 9.62 | 13.6 ± 7.81 | 0.984 |
| Categories . | Ramelteon group (n = 24) . | Control group (n = 58) . | P-value . |
|---|---|---|---|
| Age (mean [range]) | 79 [70–89] | 76.5 [70–87] | |
| Sex (male/female) | 21/3 | 43/15 | 0.300 |
| Brinkman index | 994 ± 680 | 787 ± 667 | 0.208 |
| Body mass index | 21.7 ± 3.11 | 22.7 ± 3.59 | 0.258 |
| Preoperative PFT | |||
| %VC | 92.3 ± 14.5 | 94.7 ± 15.1 | 0.505 |
| FEV1.0% | 76.1 ± 11.4 | 76.4 ± 9.19 | 0.901 |
| Preoperative PaCO2 | 43.9 ± 4.94 (n = 21) | 42.7 ± 4.49 (n = 57) | 0.306 |
| Preoperative comorbidity | 0.782 | ||
| Diabetes | 4 | 11 | |
| Ischaemic heart disease | 1 | 5 | |
| Cerebrovascular disease | 0 | 2 | |
| Depression | 0 | 0 | |
| History of delirium | 1 | 1 | 0.893 |
| Pre-existing cognitive impairment | 0 | 1 | 0.647 |
| Habitual use of hypnotics | 2 | 13 | 0.235 |
| Approach open/VATS | 1/23 | 1/57 | 0.893 |
| Surgical procedures | 0.813 | ||
| Segmentectomy | 6 | 12 | |
| Lobectomy | 17 | 44 | |
| Bilobectomy | 0 | 1 | |
| Pneumonectomy | 1 | 1 | |
| Operation time (min) | 293 ± 108 | 280 ± 91.4 | 0.612 |
| Bleeding amount (ml) | 175 ± 341 | 228 ± 238 | 0.491 |
| Intensive care unit stay (day) | 1.00 ± 0 | 1.10 ± 0.66 | 0.719 |
| Drainage duration (day) | 4.54 ± 2.23 | 4.97 ± 4.69 | 0.581 |
| Postoperative complication | 10 (41.7%) | 21 (36.2%) | 0.132 |
| Prolonged air leakage | 5 | 4 | |
| Arrhythmia | 0 | 5 | |
| Pneumonia | 1 | 4 | |
| Heart failure | 1 | 0 | |
| Others | 3 | 8 | |
| Pathological stage I/II/III/IV | 17/5/1/1 | 31/12/9/4 | 0.391 |
| Postoperative hospital stay (days) | 13.5 ± 9.62 | 13.6 ± 7.81 | 0.984 |
%VC: vital capacity percentage; FEV1.0%: per cent of 1 s forced expiratory volume; VATS: video-assisted thoracoscopic surgery; PaCO2: partial pressure of arterial carbon dioxide.
| Categories . | Ramelteon group (n = 24) . | Control group (n = 58) . | P-value . |
|---|---|---|---|
| Age (mean [range]) | 79 [70–89] | 76.5 [70–87] | |
| Sex (male/female) | 21/3 | 43/15 | 0.300 |
| Brinkman index | 994 ± 680 | 787 ± 667 | 0.208 |
| Body mass index | 21.7 ± 3.11 | 22.7 ± 3.59 | 0.258 |
| Preoperative PFT | |||
| %VC | 92.3 ± 14.5 | 94.7 ± 15.1 | 0.505 |
| FEV1.0% | 76.1 ± 11.4 | 76.4 ± 9.19 | 0.901 |
| Preoperative PaCO2 | 43.9 ± 4.94 (n = 21) | 42.7 ± 4.49 (n = 57) | 0.306 |
| Preoperative comorbidity | 0.782 | ||
| Diabetes | 4 | 11 | |
| Ischaemic heart disease | 1 | 5 | |
| Cerebrovascular disease | 0 | 2 | |
| Depression | 0 | 0 | |
| History of delirium | 1 | 1 | 0.893 |
| Pre-existing cognitive impairment | 0 | 1 | 0.647 |
| Habitual use of hypnotics | 2 | 13 | 0.235 |
| Approach open/VATS | 1/23 | 1/57 | 0.893 |
| Surgical procedures | 0.813 | ||
| Segmentectomy | 6 | 12 | |
| Lobectomy | 17 | 44 | |
| Bilobectomy | 0 | 1 | |
| Pneumonectomy | 1 | 1 | |
| Operation time (min) | 293 ± 108 | 280 ± 91.4 | 0.612 |
| Bleeding amount (ml) | 175 ± 341 | 228 ± 238 | 0.491 |
| Intensive care unit stay (day) | 1.00 ± 0 | 1.10 ± 0.66 | 0.719 |
| Drainage duration (day) | 4.54 ± 2.23 | 4.97 ± 4.69 | 0.581 |
| Postoperative complication | 10 (41.7%) | 21 (36.2%) | 0.132 |
| Prolonged air leakage | 5 | 4 | |
| Arrhythmia | 0 | 5 | |
| Pneumonia | 1 | 4 | |
| Heart failure | 1 | 0 | |
| Others | 3 | 8 | |
| Pathological stage I/II/III/IV | 17/5/1/1 | 31/12/9/4 | 0.391 |
| Postoperative hospital stay (days) | 13.5 ± 9.62 | 13.6 ± 7.81 | 0.984 |
| Categories . | Ramelteon group (n = 24) . | Control group (n = 58) . | P-value . |
|---|---|---|---|
| Age (mean [range]) | 79 [70–89] | 76.5 [70–87] | |
| Sex (male/female) | 21/3 | 43/15 | 0.300 |
| Brinkman index | 994 ± 680 | 787 ± 667 | 0.208 |
| Body mass index | 21.7 ± 3.11 | 22.7 ± 3.59 | 0.258 |
| Preoperative PFT | |||
| %VC | 92.3 ± 14.5 | 94.7 ± 15.1 | 0.505 |
| FEV1.0% | 76.1 ± 11.4 | 76.4 ± 9.19 | 0.901 |
| Preoperative PaCO2 | 43.9 ± 4.94 (n = 21) | 42.7 ± 4.49 (n = 57) | 0.306 |
| Preoperative comorbidity | 0.782 | ||
| Diabetes | 4 | 11 | |
| Ischaemic heart disease | 1 | 5 | |
| Cerebrovascular disease | 0 | 2 | |
| Depression | 0 | 0 | |
| History of delirium | 1 | 1 | 0.893 |
| Pre-existing cognitive impairment | 0 | 1 | 0.647 |
| Habitual use of hypnotics | 2 | 13 | 0.235 |
| Approach open/VATS | 1/23 | 1/57 | 0.893 |
| Surgical procedures | 0.813 | ||
| Segmentectomy | 6 | 12 | |
| Lobectomy | 17 | 44 | |
| Bilobectomy | 0 | 1 | |
| Pneumonectomy | 1 | 1 | |
| Operation time (min) | 293 ± 108 | 280 ± 91.4 | 0.612 |
| Bleeding amount (ml) | 175 ± 341 | 228 ± 238 | 0.491 |
| Intensive care unit stay (day) | 1.00 ± 0 | 1.10 ± 0.66 | 0.719 |
| Drainage duration (day) | 4.54 ± 2.23 | 4.97 ± 4.69 | 0.581 |
| Postoperative complication | 10 (41.7%) | 21 (36.2%) | 0.132 |
| Prolonged air leakage | 5 | 4 | |
| Arrhythmia | 0 | 5 | |
| Pneumonia | 1 | 4 | |
| Heart failure | 1 | 0 | |
| Others | 3 | 8 | |
| Pathological stage I/II/III/IV | 17/5/1/1 | 31/12/9/4 | 0.391 |
| Postoperative hospital stay (days) | 13.5 ± 9.62 | 13.6 ± 7.81 | 0.984 |
%VC: vital capacity percentage; FEV1.0%: per cent of 1 s forced expiratory volume; VATS: video-assisted thoracoscopic surgery; PaCO2: partial pressure of arterial carbon dioxide.
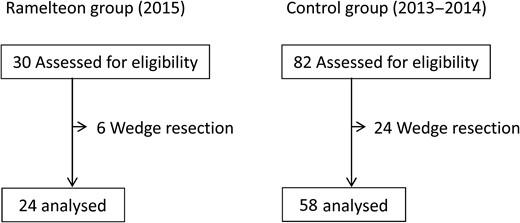
Trial profile. There were 24 patients in the Ramelteon and 58 patients in the control group who met inclusion criteria.
Intensive Care Delirium Screening Checklist scores
| Maximum ICDSC score . | Ramelteon group (n = 24) . | Control group (n = 58) . | P-value . |
|---|---|---|---|
| ≥4 | 0 (0%) | 5 (9%) | 0.328 |
| 3 | 1 (4%) | 0 (0%) | |
| 2 | 1 (4%) | 0 (0%) | |
| 1 | 1 (4%) | 4 (7%) | |
| 0 | 21 (88%) | 49 (85%) | |
| Recovery indexa | 0.25 ± 0.74 | 1.58 ± 4.93 | 0.061 |
| Maximum ICDSC score . | Ramelteon group (n = 24) . | Control group (n = 58) . | P-value . |
|---|---|---|---|
| ≥4 | 0 (0%) | 5 (9%) | 0.328 |
| 3 | 1 (4%) | 0 (0%) | |
| 2 | 1 (4%) | 0 (0%) | |
| 1 | 1 (4%) | 4 (7%) | |
| 0 | 21 (88%) | 49 (85%) | |
| Recovery indexa | 0.25 ± 0.74 | 1.58 ± 4.93 | 0.061 |
ICDSC: Intensive Care Delirium Screening Checklist.
aTotal of ICDSC scores divided by the number of patients in each group.
| Maximum ICDSC score . | Ramelteon group (n = 24) . | Control group (n = 58) . | P-value . |
|---|---|---|---|
| ≥4 | 0 (0%) | 5 (9%) | 0.328 |
| 3 | 1 (4%) | 0 (0%) | |
| 2 | 1 (4%) | 0 (0%) | |
| 1 | 1 (4%) | 4 (7%) | |
| 0 | 21 (88%) | 49 (85%) | |
| Recovery indexa | 0.25 ± 0.74 | 1.58 ± 4.93 | 0.061 |
| Maximum ICDSC score . | Ramelteon group (n = 24) . | Control group (n = 58) . | P-value . |
|---|---|---|---|
| ≥4 | 0 (0%) | 5 (9%) | 0.328 |
| 3 | 1 (4%) | 0 (0%) | |
| 2 | 1 (4%) | 0 (0%) | |
| 1 | 1 (4%) | 4 (7%) | |
| 0 | 21 (88%) | 49 (85%) | |
| Recovery indexa | 0.25 ± 0.74 | 1.58 ± 4.93 | 0.061 |
ICDSC: Intensive Care Delirium Screening Checklist.
aTotal of ICDSC scores divided by the number of patients in each group.
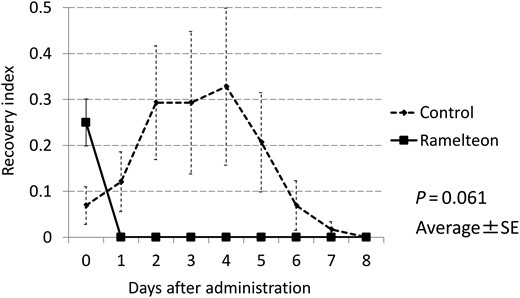
The recovery index (average number of ICDSC events) was calculated as the total of scores on the ICDSC divided by the number of patients in each group. The recovery index in the Ramelteon group was lower than that in the control group (P = 0.061). SE: standard error; ICDSC: Intensive Care Delirium Screening Checklist.
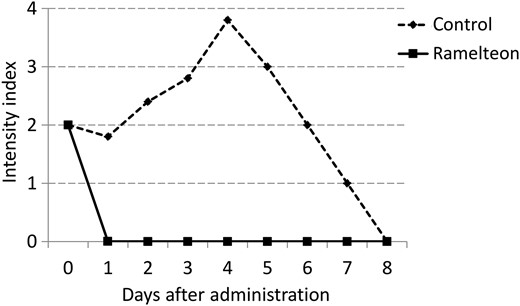
The intensity index was calculated as the total scores for ICDSC events divided by the total number of scored patients. ICDSC: Intensive Care Delirium Screening Checklist.
DISCUSSION
The aim of this study was to evaluate the efficacy of Ramelteon for the prevention of delirium in elderly patients after lung cancer surgery. The results suggest that Ramelteon likely reduces the rate of postoperative delirium, shortens the recovery period and decreases the intensity of delirium.
Ramelteon binds to melatonin (MT1 and MT2) receptors in the central nervous system [9]. MT1 receptors seem to be involved in the sleep-promoting effects of melatonin [10], whereas MT2 receptors appear to play a major role in the resynchronizing activity of melatonin [11, 12]. Ramelteon has a preventive effect on delirium in elderly inpatients [6], but the effects of this drug on postoperative delirium have not been examined. Interventions, including dexmedetomidine sedation and treatment with antipsychotics, have been used to prevent postoperative delirium [13, 14], with the goal of improving the sleep–awake rhythm. In contrast, Ramelteon may directly resynchronize circadian rhythm by the stimulation of MT2 receptors, in addition to a hypnotic effect. Regarding standard drug prices in Japan, Ramelteon has a low cost of 83 Japanese yen (approximately US$0.75).
There are several delirium screening tools for non-experts based on the Diagnostic and Statistical Manual of Mental Disorders (DSM), such as the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU) [15] and the ICDSC. We used the ICDSC as a reliable scale to evaluate delirium retrospectively, because ICDSC is the only scale applied to data collected through medical charts. ICDSC is used for delirium screening based on DSM criteria, and delirium cut-off score of ICDSC was 4 [8]. Because pre-delirium state with ICDSC score of 3 or below can cause serious complications, we defined recovery index and index intensity to evaluate the recovery period and intensity of pre-delirium and delirious state. The sensitivity and specificity for the ICDSC were 74.0–80.1% and 74.6–81.9% and 75.5–81.0% and 95.8–98.0% for the CAM-ICU, respectively [16–18].
Although a history of delirium, age over 70 and pre-existing cognitive impairment were risk factors of postoperative delirium [5], there was no significant difference in the patient background between the two groups. The habitual use of hypnotics (22%) in this study was similar to that for the Japanese population [19], and the incident rate of postoperative delirium in Japanese population did not differ from that in the Westerners [3, 4, 20]. Therefore, we deduced that habitual hypnotic use in Japanese population may not affect the delirium incidence.
Although perioperative anaesthesia can affect the development of postoperative delirium [21], all patients in this study received the similar perioperative anaesthesia technique and analgesic administration under the same perioperative anaesthetic policy.
Postoperative delirium usually peaks between 1 and 3 days [21], and it has been shown that Ramelteon prolongs the time to development of delirium and decreases the frequency of delirium [6]. The shorter recovery period and the lower intensity of delirious state in the Ramelteon group led us to speculate that Ramelteon could reduce the severity of postoperative delirium, although there was no significant difference in the delirium incident rate between the two groups in this study. Furthermore, the high drug compliance (99%) and the low rate of adverse effects (4%) showed an acceptable tolerability of Ramelteon for elderly patients. For these reasons, we believe Ramelteon can be helpful for the prevention of severe postoperative delirium and the following complication.
Limitations
The main limitation of this study is the single-centre retrospective design. The small number of patients in each group from a single centre affects the power of the study. In addition, since the two groups were treated in different years in potentially different settings, even in the same institution, the comparison might include bias. A multi-centre prospective study is needed to confirm the efficacy of Ramelteon.
CONCLUSION
Ramelteon is likely to reduce the incidence and intensity of postoperative delirium in patients treated for lung cancer in elderly patients.
Conflict of interest: none declared.




