-
PDF
- Split View
-
Views
-
Cite
Cite
Jong Hun Kim, Tae Youn Kim, Jong Bum Choi, Ja Hong Kuh, Haemodynamic improvement of older, previously replaced mechanical mitral valves by removal of the subvalvular pannus in redo cardiac surgery, Interactive CardioVascular and Thoracic Surgery, Volume 24, Issue 1, January 2017, Pages 148–149, https://doi.org/10.1093/icvts/ivw276
Close - Share Icon Share
Patients requiring redo cardiac surgery for diseased heart valves other than mitral valves may show increased pressure gradients and reduced valve areas of previously placed mechanical mitral valves due to subvalvular pannus formation. We treated four women who had mechanical mitral valves inserted greater than or equal to 20 years earlier and who presented with circular pannus that protruded into the lower margin of the valve ring but did not impede leaflet motion. Pannus removal improved the haemodynamic function of the mitral valve.
INTRODUCTION
After mechanical mitral valve replacement, other diseased heart valves may require surgery. Marked pannus may form along the upper and lower ring planes of previously placed mechanical mitral valves. Although the valve leaflet motion may not be impeded, the pannus may aggravate valve gradients and areas [1]. In redo cardiac surgery for other diseased heart valves, pannus removal from mechanical mitral valves may improve the valve haemodynamic function.
CASE REPORTS
Four women (age, 57–67 years) who had mechanical mitral valve replacement with posterior chordal preservation 20–29 years earlier underwent redo cardiac surgery to treat other diseased heart valves (Table 1). The older mitral valves worked well in all patients without any impaired bileaflet motion.
Patient characteristics and echocardiographic findings before and after redo cardiac surgery
| Patient no. . | Sex/Age (years) . | Previous valve replacement . | Redo valve surgery . | Follow-up (mo) . | Preop or postop . | Max/Mean MVGa (mmHg) . | MVAa (cm2) . | LAD (mm) . | PAP (mmHg) . | Stroke volume (ml) . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mo/Year . | Valve . | Mo/Year . | Valve . | |||||||||
| 1 | F/67 | 2/1987 | MVR (St Jude 27 mm) AVR (St Jude 19 mm) | 8/2014 | Redo AVR (St Jude 19 mm) | 19 | Preop | 23/7 | 3.3 | 60 | 55 | 43 |
| Postop | 16/6 | 3.7 | 60 | 33 | 51 | |||||||
| 2 | F/64 | 6/1994 | MVR (St Jude 29 mm) | 1/2015 | AVR (St Jude 19 mm) | 14 | Preop | 15/5 | 2.5 | 37 | 25 | 45 |
| TAP (MC3 ring 26 mm) | Postop | 8/4 | 2.6 | 43 | 25 | 41 | ||||||
| 3 | F/57 | 5/1991 | MVR (St Jude 27 mm) AVR (St Jude 21 mm) | 8/2015 | TAP (MC3 ring 30 mm) | 8 | Preop | 17/7 | 2.0 | 51 | 36 | 40 |
| Postop | 16/4 | 2.6 | 51 | 27 | 41 | |||||||
| 4 | F/66 | 1/1996 | MVR (Sorin 27 mm) | 1/2016 | AVR (St Jude 19 mm) | 3 | Preop | 10/4 | 2.3 | 58 | 44 | 55 |
| TAP (MC3 30 mm) | Postop | 7/2 | 2.5 | 55 | 35 | 42 | ||||||
| Patient no. . | Sex/Age (years) . | Previous valve replacement . | Redo valve surgery . | Follow-up (mo) . | Preop or postop . | Max/Mean MVGa (mmHg) . | MVAa (cm2) . | LAD (mm) . | PAP (mmHg) . | Stroke volume (ml) . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mo/Year . | Valve . | Mo/Year . | Valve . | |||||||||
| 1 | F/67 | 2/1987 | MVR (St Jude 27 mm) AVR (St Jude 19 mm) | 8/2014 | Redo AVR (St Jude 19 mm) | 19 | Preop | 23/7 | 3.3 | 60 | 55 | 43 |
| Postop | 16/6 | 3.7 | 60 | 33 | 51 | |||||||
| 2 | F/64 | 6/1994 | MVR (St Jude 29 mm) | 1/2015 | AVR (St Jude 19 mm) | 14 | Preop | 15/5 | 2.5 | 37 | 25 | 45 |
| TAP (MC3 ring 26 mm) | Postop | 8/4 | 2.6 | 43 | 25 | 41 | ||||||
| 3 | F/57 | 5/1991 | MVR (St Jude 27 mm) AVR (St Jude 21 mm) | 8/2015 | TAP (MC3 ring 30 mm) | 8 | Preop | 17/7 | 2.0 | 51 | 36 | 40 |
| Postop | 16/4 | 2.6 | 51 | 27 | 41 | |||||||
| 4 | F/66 | 1/1996 | MVR (Sorin 27 mm) | 1/2016 | AVR (St Jude 19 mm) | 3 | Preop | 10/4 | 2.3 | 58 | 44 | 55 |
| TAP (MC3 30 mm) | Postop | 7/2 | 2.5 | 55 | 35 | 42 | ||||||
AVR: aortic valve replacement; LAD: left atrial dimension; MVA: mitral valve area; MVG: mitral valve gradient; MVR: mitral valve replacement; PAP: pulmonary artery pressure; Preop: preoperative; Postop: postoperative; TAP: tricuspid annuloplasty.
aWilcoxon signed-rank test: P = 0.068 and 0.066 for pre- and postoperative MVG and MVA, and P= 0.125 for LAD.
Patient characteristics and echocardiographic findings before and after redo cardiac surgery
| Patient no. . | Sex/Age (years) . | Previous valve replacement . | Redo valve surgery . | Follow-up (mo) . | Preop or postop . | Max/Mean MVGa (mmHg) . | MVAa (cm2) . | LAD (mm) . | PAP (mmHg) . | Stroke volume (ml) . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mo/Year . | Valve . | Mo/Year . | Valve . | |||||||||
| 1 | F/67 | 2/1987 | MVR (St Jude 27 mm) AVR (St Jude 19 mm) | 8/2014 | Redo AVR (St Jude 19 mm) | 19 | Preop | 23/7 | 3.3 | 60 | 55 | 43 |
| Postop | 16/6 | 3.7 | 60 | 33 | 51 | |||||||
| 2 | F/64 | 6/1994 | MVR (St Jude 29 mm) | 1/2015 | AVR (St Jude 19 mm) | 14 | Preop | 15/5 | 2.5 | 37 | 25 | 45 |
| TAP (MC3 ring 26 mm) | Postop | 8/4 | 2.6 | 43 | 25 | 41 | ||||||
| 3 | F/57 | 5/1991 | MVR (St Jude 27 mm) AVR (St Jude 21 mm) | 8/2015 | TAP (MC3 ring 30 mm) | 8 | Preop | 17/7 | 2.0 | 51 | 36 | 40 |
| Postop | 16/4 | 2.6 | 51 | 27 | 41 | |||||||
| 4 | F/66 | 1/1996 | MVR (Sorin 27 mm) | 1/2016 | AVR (St Jude 19 mm) | 3 | Preop | 10/4 | 2.3 | 58 | 44 | 55 |
| TAP (MC3 30 mm) | Postop | 7/2 | 2.5 | 55 | 35 | 42 | ||||||
| Patient no. . | Sex/Age (years) . | Previous valve replacement . | Redo valve surgery . | Follow-up (mo) . | Preop or postop . | Max/Mean MVGa (mmHg) . | MVAa (cm2) . | LAD (mm) . | PAP (mmHg) . | Stroke volume (ml) . | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mo/Year . | Valve . | Mo/Year . | Valve . | |||||||||
| 1 | F/67 | 2/1987 | MVR (St Jude 27 mm) AVR (St Jude 19 mm) | 8/2014 | Redo AVR (St Jude 19 mm) | 19 | Preop | 23/7 | 3.3 | 60 | 55 | 43 |
| Postop | 16/6 | 3.7 | 60 | 33 | 51 | |||||||
| 2 | F/64 | 6/1994 | MVR (St Jude 29 mm) | 1/2015 | AVR (St Jude 19 mm) | 14 | Preop | 15/5 | 2.5 | 37 | 25 | 45 |
| TAP (MC3 ring 26 mm) | Postop | 8/4 | 2.6 | 43 | 25 | 41 | ||||||
| 3 | F/57 | 5/1991 | MVR (St Jude 27 mm) AVR (St Jude 21 mm) | 8/2015 | TAP (MC3 ring 30 mm) | 8 | Preop | 17/7 | 2.0 | 51 | 36 | 40 |
| Postop | 16/4 | 2.6 | 51 | 27 | 41 | |||||||
| 4 | F/66 | 1/1996 | MVR (Sorin 27 mm) | 1/2016 | AVR (St Jude 19 mm) | 3 | Preop | 10/4 | 2.3 | 58 | 44 | 55 |
| TAP (MC3 30 mm) | Postop | 7/2 | 2.5 | 55 | 35 | 42 | ||||||
AVR: aortic valve replacement; LAD: left atrial dimension; MVA: mitral valve area; MVG: mitral valve gradient; MVR: mitral valve replacement; PAP: pulmonary artery pressure; Preop: preoperative; Postop: postoperative; TAP: tricuspid annuloplasty.
aWilcoxon signed-rank test: P = 0.068 and 0.066 for pre- and postoperative MVG and MVA, and P= 0.125 for LAD.
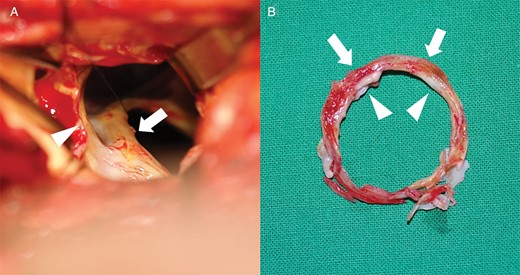
(A) Subvalvular pannus (arrow) around mechanical mitral valve, visible through the aortic valve opening (arrowhead) after aortic valve excision. (B) Subvalvular pannus ring included an inner calcified sharp ring (arrowheads) and an outer hard fibrotic ring (arrows).
DISCUSSION
Prosthetic mechanical valve dysfunction caused by valve thrombosis or pannus formation often requires mitral valve replacement [1, 2]. However, because mechanical valves have not substantially changed over the past two decades, pannus removal instead of valve replacement may be prudent to recover prosthetic mitral valve function [3].
Subvalvular pannus protruding into the valve opening may progressively grow into the valve opening, aggravating valve haemodynamics [4] or limiting leaflet motion [2–4]. For patients requiring redo surgery for valves other than the mitral valve, the older mechanical mitral valves that are hindered by pannus could be replaced. However, in patients requiring continuous anticoagulation, pannus removal alone can improve the valve haemodynamic function, reducing the possibility of future mechanical valve malfunction and the morbidity and mortality associated with redo mitral valve replacement.
Funding
Supported by funding from the Clinical Research of Chonbuk National University and the Biomedical Research Institute of Chonbuk National University Hospital.
Conflict of interest: none declared.




