-
PDF
- Split View
-
Views
-
Cite
Cite
Kristofer Skoglund, Peter Eriksson, Gunnar Svensson, Mikael Dellborg, Homograft reconstruction of the right ventricular outflow tract in adults with congenital heart disease: a systematic review, Interactive CardioVascular and Thoracic Surgery, Volume 22, Issue 1, January 2016, Pages 57–62, https://doi.org/10.1093/icvts/ivv264
Close - Share Icon Share
Abstract
Reconstruction of the right ventricular outflow tract with a homograft is an established surgical method in congenital heart disease. Studies from children and adolescents suggest that homograft durability is shorter than the life expectancy of the patient; therefore, durability in adults is addressed in this systematic review. The PubMed database was searched in May 2012 and repeated in May 2015 with the terms ‘homograft AND pulmonary valve’, generating 665 hits. We included only studies involving more than 50 patients with a mean or median age >18 years. Six studies with a cumulative total of 560 patients were included. The long-term mortality rate was 2–8.8% at 8.1–10 years. Reintervention was common during patients' life spans, with a 10-year event-free survival rate of 78–80%. Early postoperative echocardiographic or magnetic resonance imaging defects appear to predict rapid homograft degeneration. Further studies on various malformations and risk markers for degeneration are needed to make qualified and accurate decisions regarding lifetime management.
INTRODUCTION
Improvements in surgical and medical therapy have helped an increasing number of patients born with complex congenital heart disease reach adulthood [1, 2]. Further improvements in the care for these patients call for a treatment strategy that goes beyond initial surgical correction and focuses on repeated surgery, pregnancy, quality of life and other factors related to lifetime management.
The use of an aortic homograft for reconstruction of the right ventricular outflow tract (RVOT) was first described by Ross and Somerville in 1966 [3]. The method was later improved using a pulmonary homograft [4]. For decades, homograft implantation has been an established surgical method for reconstruction of RVOT with good long-term results. Several risk factors for degeneration of homografts, such as age, underlying malformation, size and type of homograft, have been identified; however, available data on the durability of homografts and the risk factors for degeneration are based predominantly on studies of children and young adults [5–8]. This is problematic, given that adults are increasingly common among patients in need of RVOT reconstruction with a conduit; the majority being patients with tetralogy of Fallot (TOF) with previous transannular plasty. There is no consensus on the best tissue valve [9]; however, in many centres and countries, homograft replacement is a common and often preferred method for RVOT reconstruction [4, 5, 10–15]. According to data from the Swedish registry of congenital heart disease in 2013, more than 500 adult patients have a homograft (www.ucr.uu.se/swedcon).
Because the expected durability of the homograft is often shorter than the life expectancy of the patient, reoperation or reintervention appears unavoidable in adults with various underlying congenital heart defects. A lifetime management approach to the use of open surgery and percutaneous approaches is necessary. To make qualified and accurate decisions, data are needed on event-free survival, perioperative mortality and morbidity and risk factors for homograft dysfunction in adult patients.
The aim of our study was to report outcomes after surgical RVOT reconstruction with a homograft in adult patients with congenital heart disease.
MATERIALS AND METHODS
The study population included adult patients with congenital heart disease undergoing RVOT reconstruction with a homograft. The outcome measure was event-free survival, defined as freedom from mortality, reoperations and percutaneous interventions, as stated in each study. Perioperative or short-term mortality (<30 days) and long-term mortality were recorded. Morbidity, length of hospital stay, functional status and health-related quality of life were also included in the analysis, as well as data from echocardiography and magnetic resonance imaging (MRI). All systematic reviews and observational studies with over 50 patients and a mean or median age of at least 18 years at surgical procedure (either first homograft or reoperation) were considered eligible. No limitation regarding publication year was set, and only studies published in English or the Scandinavian languages were included.
A qualified librarian performed a search in PubMed and the Cochrane database from their inception to May 2012. The search term in the PubMed NLM catalog Medical Subject Heading database was ‘homograft OR homografts AND pulmonary valve’. Abstracts from 580 search hits were screened for eligibility by one author (Kristofer Skoglund). Articles on other subjects, animal studies, pure imaging or biomarker studies, tissue engineering (including Synergraft® [CryoLife, Inc., Kennesaw, GA, USA]), experimental studies, immunology or cell studies and studies of specified non-homograft conduits were excluded. Commentaries and editorials were excluded from the review, as well as articles regarding specific surgical techniques, such as size-reduced homografts, bicuspidalized homografts, monocusp valve surgery and peel operations. Articles that included only patients who had undergone the Ross procedure were excluded because these patients have no congenital right-sided cardiac malformation and the anatomical location of the homograft has been found to influence its durability [16]. However, we did include articles with both congenital heart disease patients and Ross patients in the full paper review. Cryopreserved, fresh and irradiated homografts were included, as well as both pulmonary and aortic homografts. Concomitant surgery, such as surgery of the tricuspid valve and suture of residual ventricular septal defect, was included. We also accepted both implantation of the patient's first homograft and reoperation.
After screening the PubMed abstracts according to these criteria, 106 articles were retrieved in full text for full paper review. One author (Kristofer Skoglund) also did a manual search of the reference lists of the retrieved articles and found 23 additional articles to retrieve for the full paper review. The expanded search in May 2015 included the additional search term, ‘allograft’, and revealed 85 more articles of which 4 were retrieved for full paper review and reference list cross check. The Cochrane library was also searched in May 2015 for all articles using the same additional search term, which produced 13 articles. All of these articles were excluded after screening the abstract, as they did not meet the required criteria. When reporting our findings, we used the proposed guidelines from the Meta-analysis of Observational Studies in Epidemiology (MOOSE) group [17].
A total of 133 articles were retrieved for detailed full paper review by two authors (Mikael Dellborg and Kristofer Skoglund). Criteria for exclusion were met in 127 articles, leaving six articles that met the inclusion criteria; these were included in the final systematic review [15, 18–22]. A summary of the selection process is shown in Fig. 1.
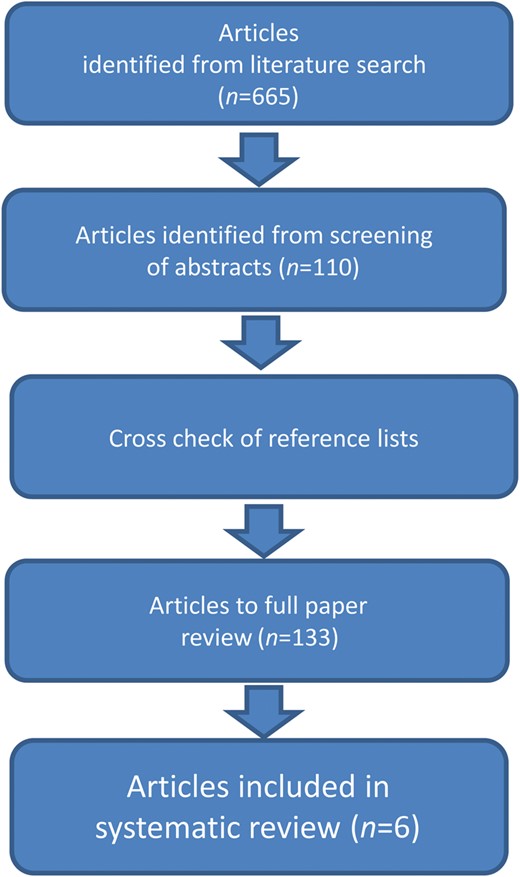
The risk of bias in the included studies was assessed by two authors (Kristofer Skoglund and Mikael Dellborg) and no obvious risk of bias was observed other than the bias inherent in case series.
RESULTS
Of the six articles included, three were case series [18, 20, 21], one was a prospective cohort study [19] and one was a prospective multicentre cohort study [22]. There was also one retrospective study of the CONCOR registry (http://concor.net/en/) [15]. In total, 560 patients were included. Patient diagnosis, age at operation and mean follow-up time are reported in Table 1. One study did not specify patients' ages and only described them as ‘adult’ [19]. Various follow-up methods were used, including hospital charts and telephone [18], registry [15], database [20], hospital charts and visit [19, 22], hospital charts and municipal registries [21].
| Publication author, year . | Diagnosis (n) . | Mean/median follow-up . | Mean age at operation . |
|---|---|---|---|
| Hazekamp, 2001a | TOF (51) | Mean 1.7 ± 1.4 years | 25.7 ± 11.9 years |
| Oosterhof, 2006 | TOF (158) | Median 4.2 years | ‘Adult’ |
| Troost, 2007 | TOF (68) | Median 8.4 years | 24 years |
| Nordmeyer, 2009 | Mixed (60) | Mean 40 ± 10 months | 21 ± 10 years |
| Scherptong, 2010a | TOF (90) | Mean 5.5 ± 3.5 years | 31.4 ± 10.3 years |
| van de Woestijne, 2011 | TOF (133) | Mean 8.1 ± 5.6 years | 28.1 ± 12.2 years |
| Publication author, year . | Diagnosis (n) . | Mean/median follow-up . | Mean age at operation . |
|---|---|---|---|
| Hazekamp, 2001a | TOF (51) | Mean 1.7 ± 1.4 years | 25.7 ± 11.9 years |
| Oosterhof, 2006 | TOF (158) | Median 4.2 years | ‘Adult’ |
| Troost, 2007 | TOF (68) | Median 8.4 years | 24 years |
| Nordmeyer, 2009 | Mixed (60) | Mean 40 ± 10 months | 21 ± 10 years |
| Scherptong, 2010a | TOF (90) | Mean 5.5 ± 3.5 years | 31.4 ± 10.3 years |
| van de Woestijne, 2011 | TOF (133) | Mean 8.1 ± 5.6 years | 28.1 ± 12.2 years |
Mixed refers to various malformations involving the right ventricular outflow tract.
TOF: tetralogy of Fallot.
aHazekamp and Scherptong studied patients with pulmonary regurgitation only.
| Publication author, year . | Diagnosis (n) . | Mean/median follow-up . | Mean age at operation . |
|---|---|---|---|
| Hazekamp, 2001a | TOF (51) | Mean 1.7 ± 1.4 years | 25.7 ± 11.9 years |
| Oosterhof, 2006 | TOF (158) | Median 4.2 years | ‘Adult’ |
| Troost, 2007 | TOF (68) | Median 8.4 years | 24 years |
| Nordmeyer, 2009 | Mixed (60) | Mean 40 ± 10 months | 21 ± 10 years |
| Scherptong, 2010a | TOF (90) | Mean 5.5 ± 3.5 years | 31.4 ± 10.3 years |
| van de Woestijne, 2011 | TOF (133) | Mean 8.1 ± 5.6 years | 28.1 ± 12.2 years |
| Publication author, year . | Diagnosis (n) . | Mean/median follow-up . | Mean age at operation . |
|---|---|---|---|
| Hazekamp, 2001a | TOF (51) | Mean 1.7 ± 1.4 years | 25.7 ± 11.9 years |
| Oosterhof, 2006 | TOF (158) | Median 4.2 years | ‘Adult’ |
| Troost, 2007 | TOF (68) | Median 8.4 years | 24 years |
| Nordmeyer, 2009 | Mixed (60) | Mean 40 ± 10 months | 21 ± 10 years |
| Scherptong, 2010a | TOF (90) | Mean 5.5 ± 3.5 years | 31.4 ± 10.3 years |
| van de Woestijne, 2011 | TOF (133) | Mean 8.1 ± 5.6 years | 28.1 ± 12.2 years |
Mixed refers to various malformations involving the right ventricular outflow tract.
TOF: tetralogy of Fallot.
aHazekamp and Scherptong studied patients with pulmonary regurgitation only.
Five of six studies included patients with TOF only [15, 18, 20–22]. The fifth study included a mixed cohort of congenital right heart malformations [19] and two studies included only patients with reoperation for pulmonary valve regurgitation (PR) [18, 22] (Table 1).
Cryopreserved pulmonary homografts were used most often for RVOT reconstruction in the included articles. Type and mean homograft diameter are reported in Table 2. In the present analysis, no distinction was made regarding whether the homograft was the patient's first or a reoperation. Concomitant surgery, such as surgical closure of residual ventricular septal defect, resection of infundibular musculature, plasty of the pulmonary artery or tricuspid valve repair, was present and specified in three studies [15, 21, 22].
Type of homograft, diameter and method of preservation in the included articles
| Publication author, year . | Type of homograft . | Mean diameter (mm) . | Method of preservation . |
|---|---|---|---|
| Hazekamp, 2001 | 49 Pulm, 2 Ao | 25 ± 1.8 | Cryopreserved |
| Oosterhof, 2006 | 167 Pulm, 8 Ao | 25 ± 2.1 | |
| Troost, 2007 | 65 Pulm, 3 Ao | 24, range 18–29 | Cryopreserved |
| Nordmeyer, 2009 | 22 ± 2 | Cryopreserved | |
| Scherptong, 2010 | Pulm | 25.6 ± 1.6 | Cryopreserved |
| van de Woestijne, 2011 | 24 ± 2, range 14–28 | Cryopreserved 130/133 |
| Publication author, year . | Type of homograft . | Mean diameter (mm) . | Method of preservation . |
|---|---|---|---|
| Hazekamp, 2001 | 49 Pulm, 2 Ao | 25 ± 1.8 | Cryopreserved |
| Oosterhof, 2006 | 167 Pulm, 8 Ao | 25 ± 2.1 | |
| Troost, 2007 | 65 Pulm, 3 Ao | 24, range 18–29 | Cryopreserved |
| Nordmeyer, 2009 | 22 ± 2 | Cryopreserved | |
| Scherptong, 2010 | Pulm | 25.6 ± 1.6 | Cryopreserved |
| van de Woestijne, 2011 | 24 ± 2, range 14–28 | Cryopreserved 130/133 |
Method of preservation was not stated in Oosterhof's study.
Pulm: pulmonary homograft; Ao: aortic homograft.
Type of homograft, diameter and method of preservation in the included articles
| Publication author, year . | Type of homograft . | Mean diameter (mm) . | Method of preservation . |
|---|---|---|---|
| Hazekamp, 2001 | 49 Pulm, 2 Ao | 25 ± 1.8 | Cryopreserved |
| Oosterhof, 2006 | 167 Pulm, 8 Ao | 25 ± 2.1 | |
| Troost, 2007 | 65 Pulm, 3 Ao | 24, range 18–29 | Cryopreserved |
| Nordmeyer, 2009 | 22 ± 2 | Cryopreserved | |
| Scherptong, 2010 | Pulm | 25.6 ± 1.6 | Cryopreserved |
| van de Woestijne, 2011 | 24 ± 2, range 14–28 | Cryopreserved 130/133 |
| Publication author, year . | Type of homograft . | Mean diameter (mm) . | Method of preservation . |
|---|---|---|---|
| Hazekamp, 2001 | 49 Pulm, 2 Ao | 25 ± 1.8 | Cryopreserved |
| Oosterhof, 2006 | 167 Pulm, 8 Ao | 25 ± 2.1 | |
| Troost, 2007 | 65 Pulm, 3 Ao | 24, range 18–29 | Cryopreserved |
| Nordmeyer, 2009 | 22 ± 2 | Cryopreserved | |
| Scherptong, 2010 | Pulm | 25.6 ± 1.6 | Cryopreserved |
| van de Woestijne, 2011 | 24 ± 2, range 14–28 | Cryopreserved 130/133 |
Method of preservation was not stated in Oosterhof's study.
Pulm: pulmonary homograft; Ao: aortic homograft.
The study by Oosterhof et al. [15] included pregnancy data; 4 patients were pregnant after surgery and were found to have no increased risk of homograft failure.
Early mortality, late mortality and event-free survival
In-hospital/30-day mortality was examined in four studies, as presented in Table 3. Analysis of the length of hospital stay was included in two studies: Hazekamp et al. [18] reported 8.7 ± 4.2 days and Nordmeyer et al. [19] reported 7 ± 3 days. Reoperation for bleeding was reported in two studies: 4.5% of the 133 patients in the Hazekamp et al.'s study [18] and 6% of the 51 patients in van de Woestijne et al.'s study[ 21].
| Publication author, year (n) . | Early mortality (%) . | Late mortality . | Event-free survival . |
|---|---|---|---|
| Hazekamp, 2001 (51) | 2 | 4% at 1.7 years | |
| Oosterhof, 2006 (158) | 2% at 5.1 years | 88% at 5 years, 78% at 10 years, 68% at 15 years | |
| Troost, 2007 (68) | 2.9 | 8.8% at 8.4 years | 92% at 5 years, 79% at 10 years, 69% at 15 years |
| Nordmeyer, 2009 (60) | 0 | 0% at 3.3 years | 95% at 3.3 years |
| Scherptong, 2010 (90) | 0 | 2% at 5.5 years | 89% at 5 years, 78% at 10 years |
| van de Woestijne, 2011 (133) | 1.5 | 8% at 8.1 years | 80% at 10 years, 67% at 15 years |
| Publication author, year (n) . | Early mortality (%) . | Late mortality . | Event-free survival . |
|---|---|---|---|
| Hazekamp, 2001 (51) | 2 | 4% at 1.7 years | |
| Oosterhof, 2006 (158) | 2% at 5.1 years | 88% at 5 years, 78% at 10 years, 68% at 15 years | |
| Troost, 2007 (68) | 2.9 | 8.8% at 8.4 years | 92% at 5 years, 79% at 10 years, 69% at 15 years |
| Nordmeyer, 2009 (60) | 0 | 0% at 3.3 years | 95% at 3.3 years |
| Scherptong, 2010 (90) | 0 | 2% at 5.5 years | 89% at 5 years, 78% at 10 years |
| van de Woestijne, 2011 (133) | 1.5 | 8% at 8.1 years | 80% at 10 years, 67% at 15 years |
Early mortality is defined as in-hospital or 30-day mortality. Event-free survival is defined as survival with freedom from reoperation or reintervention, as stated in the articles.
| Publication author, year (n) . | Early mortality (%) . | Late mortality . | Event-free survival . |
|---|---|---|---|
| Hazekamp, 2001 (51) | 2 | 4% at 1.7 years | |
| Oosterhof, 2006 (158) | 2% at 5.1 years | 88% at 5 years, 78% at 10 years, 68% at 15 years | |
| Troost, 2007 (68) | 2.9 | 8.8% at 8.4 years | 92% at 5 years, 79% at 10 years, 69% at 15 years |
| Nordmeyer, 2009 (60) | 0 | 0% at 3.3 years | 95% at 3.3 years |
| Scherptong, 2010 (90) | 0 | 2% at 5.5 years | 89% at 5 years, 78% at 10 years |
| van de Woestijne, 2011 (133) | 1.5 | 8% at 8.1 years | 80% at 10 years, 67% at 15 years |
| Publication author, year (n) . | Early mortality (%) . | Late mortality . | Event-free survival . |
|---|---|---|---|
| Hazekamp, 2001 (51) | 2 | 4% at 1.7 years | |
| Oosterhof, 2006 (158) | 2% at 5.1 years | 88% at 5 years, 78% at 10 years, 68% at 15 years | |
| Troost, 2007 (68) | 2.9 | 8.8% at 8.4 years | 92% at 5 years, 79% at 10 years, 69% at 15 years |
| Nordmeyer, 2009 (60) | 0 | 0% at 3.3 years | 95% at 3.3 years |
| Scherptong, 2010 (90) | 0 | 2% at 5.5 years | 89% at 5 years, 78% at 10 years |
| van de Woestijne, 2011 (133) | 1.5 | 8% at 8.1 years | 80% at 10 years, 67% at 15 years |
Early mortality is defined as in-hospital or 30-day mortality. Event-free survival is defined as survival with freedom from reoperation or reintervention, as stated in the articles.
Event-free survival was examined in five studies [15, 19–22]. Nordmeyer et al. [19] defined event-free survival as freedom from reoperation or percutaneous valve implantation, Oosterhof et al. [15] and Troost et al. [20] defined it as freedom from valve replacement or percutaneous balloon angioplasty and van de Woestijne et al. [21] as freedom from any valve-related event or replacement, including percutaneous valve implantation and balloon angioplasty. This definition in Scherptong et al.'s study [22] was death, replacement, ventricular tachycardia or heart failure. Hazekamp et al. [18] did not assess event-free survival. Data were extracted from text or Kaplan–Meier curves as published in the articles. Nordmeyer et al. [19] presented point estimates at 40 months, van de Woestijne et al. [21] presented Kaplan–Meier curves at 10 and 15 years and the remaining two studies presented Kaplan–Meier curves at 5, 10 and 15 years [15, 20], as presented in Table 3.
The mean event-free survival was 14.6 years (12.9–16.2) in the study by Troost et al. [20]. Point estimates of late mortality were analysed in all studies, with a mean (or median) follow-up in the range of 1.7–8.4 years, as reported in Table 3.
Functional status and QRS duration
Functional status as measured by the New York Heart Association (NYHA) functional classification system was examined in three studies. Hazekamp et al. [18] analysed 43 patients before and after surgery, resulting in statistically significant improvement of the mean NYHA class from 2.3 to 1.4. There was also a statistically significant improvement in Scherptong et al.'s study [22] reporting an improvement in the mean NYHA class from 2.4 to 1.3. The study by van de Woestijne et al. [21] showed that more than 90% of patients were in NYHA class I or II at late follow-up, concluding that those who undergo RVOT reconstruction with a homograft continue to have good functional status, even long term.
Hazekamp et al. [18] also assessed QRS duration and found that it remained unchanged after RVOT reconstruction with a homograft. Postoperative severe QRS prolongation (>180 ms) and absence of reduction in QRS after pulmonary valve replacement (PVR) was extensively evaluated by Scherptong et al. [22] and found to be a major determinant of adverse outcome up to 9 years after homograft surgery.
Echocardiography and magnetic resonance imaging data
Hazekamp et al. [18] examined 51 patients with echocardiography at follow-up after RVOT reconstruction with a homograft because of severe PR. At postoperative follow-up, 96% had trivial or mild remaining PR. Oosterhof et al. [15] reported data from preoperative and postoperative echocardiographic evaluation. At baseline, 48% (56/116) of the patients had severe PR and 15% (19/105) had valvular pulmonary stenosis (PS) with a peak Doppler gradient of more than 40 mmHg. At 5- and 10-year follow-ups, the mean PR grade (0–4) of the homograft was 1.3 and 1.7, respectively, and the mean peak homograft valve Doppler gradient was 26 and 35 mmHg, respectively. At a median follow-up of 4.1 years, significant PS or PR of the homograft in the first year could predict adverse events in a univariate Cox regression analysis [15]. Troost et al. [20] found (in a linear regression model) that high systolic peak gradient in the homograft as early as 1 month postoperatively predicted accelerated homograft degeneration.
Nordmeyer et al. performed serial echocardiographic assessment after surgical reconstruction. At 1 year postoperatively, 19% of the patients showed ‘more than mild’ homograft PR on echocardiography, significantly more than at first postoperative assessment, when 0.5% showed homograft PR (P = 0.00365). These authors found no signs of PS with low gradients (<3 m/s) [19]. They also assessed 13 of their 60 patients with MRI before and 1 year after surgery. Results showed a significant reduction of the mean pulmonary regurgitation fraction (RF) from 48% (range 25–66%) to 4% (range 0–26%), postoperatively (P < 0.001). Right ventricular end-diastolic and end-systolic volumes were also assessed, showing a significant reduction from 292 (±69) to 214 (±56) ml, and 175 (±75) to 133 (±33) ml, respectively (both P < 0.001). The right ventricular ejection fraction showed no significant improvement in this subset of patients, 37% (±8%) to 44% (±13%) [18]. Preoperative MRI data were available in 74 out of 175 surgeries in Oosterhof et al.'s study [15]. However, postoperative data were not available. The preoperative mean indexed right ventricular end-diastolic volume was 173 (±42) ml/m2 and the end-systolic volume was 103 (±36) ml/m2. The mean right ventricular ejection fraction was 42% (±10%) and the mean RF was 45% (±13%). Nordmeyer et al. studied the flow pattern in the homograft using MRI. Eccentric flow pattern and acute distortion angle indicated alterations in the homograft geometry and was associated with the development of valve incompetence. At 1 year, 17% of the patients had an RF above 20%, indicating valve incompetence [19].
In summary, early postoperative high peak systolic gradient or PR in the homograft could predict adverse events and accelerated degeneration. Asymmetrical geometry measured by MRI was associated with the development of valve incompetence. In the small subset of patients assessed with MRI, homograft RVOT reconstruction reduced the RF and right ventricular end-systolic and -diastolic volumes.
DISCUSSION
To improve care for the increasing number of adults with complex congenital heart disease, studies on mortality, outcome and the need for repeat surgery are necessary. This systematic review focused on the growing group of patients who require lifetime management. Surgical reconstruction of RVOT with a homograft in adults with congenital heart disease was evaluated in six studies involving a total of 560 patients. The studies included predominantly patients with TOF, which makes generalization of the results to other malformations problematic, given that paediatric data show different event-free survival rates for different malformations [5]. The predominant reason for excluding articles from this systematic review was that the patients studied were under 18 years of age at the time of the surgical procedure.
The included studies showed a low 30-day/in-hospital mortality rate in the range of 0–2.9%. Long-term mortality at 8.1–10 years ranged 2–8.8%, similar to results from a large meta-analysis of 3118 mixed adult and paediatric patients with TOF and PVR with homograft, bioprosthesis or mechanical valves, where the pooled perioperative/30-day mortality rate was 0.8% and the 5-year mortality rate was 2.2%. [23]. In the same study, the pooled 5-year redo PVR rate was 4.9% in contrast to this review, which showed a 5-year redo PVR rate of ∼10%. The long-term mortality after PVR with a homograft is fairly low, between 2 and 8.8% at 8.1–10 years in the included studies. Given that event-free survival is ∼80% at 10 years, the durability of the homograft is a limiting factor for adults and creates a need for repeated intervention. There are also likely a significant number of dysfunctional homografts not subject to reintervention. Despite the fact that repeated intervention is needed, no dramatic negative impact on mortality was observed. Although perioperative mortality is low, the accumulated perioperative mortality from repeat surgery during a patient's lifetime is yet to be determined. Since reoperation with conduit replacement is common, more data are required on durability for the second or third implanted homograft. In this systematic review, reoperation as well as novel implantation was included.
The ideal timing of PVR is under constant debate and the impact on mortality is yet to be determined [24]. However, the indication for PVR may have changed over time because of improved understanding of the restoration of right ventricular size and function after PVR [25–28]. Good long-term results following percutaneous stent valve placement (Melody, Medtronic) have also expanded the opportunities for percutaneous reintervention [29]. Future research in this field will likely have a great impact on the indications for this surgery.
Data from the Ross registry of 1775 consecutive adult patients (over 16 years), 93% of whom had homografts, showed excellent results, with 95.5% freedom from reintervention at 10 years and 91.4% at 15 years [30]. The better durability of homografts in the Ross procedure suggests that the use of homografts in native RVOT without congenital right heart malformation has better durability in adults. Reliable data on durability are highly dependent on stringent reporting of outcomes and patients lost to follow-up. Therefore, it cannot be concluded from the data in this review alone whether this excellent durability in adults is a result of native anatomical conditions of the RVOT or an outcome bias from low reporting of outcome data. However, a recent single-institution study of 288 consecutive adult patients with right ventricle to pulmonary artery conduits, including homografts, concluded that the native anatomy of TOF has shorter freedom from conduit dysfunction than Ross-operated aortic valve disease [31]. This supports the suggestion that the use of homografts for the Ross procedure is different from that for primary reconstruction of a malformation of the RVOT, and that the underlying cardiac malformation has an impact on homograft durability in adults.
The available data on functional status suggest that the majority of adult patients will improve in NYHA class and remain in Class I or II at long-term follow-up after RVOT reconstruction with a homograft.
The mean homograft diameter in the included studies was 22–25 mm, suggesting that preferred conduit size often exceeds the 22 mm available with the Contegra bioprosthesis conduit (Medtronic, Inc., Minneapolis, MN, USA). Although Contegra has shown promising results in children [32, 33], less is known about its durability compared with homografts in adults. This, together with good results for RVOT reconstruction with a homograft in adults, may contribute to the continued use of homografts in the future. However, one obvious disadvantage of homografts is the limited availability because homograft banks are not present in every country or site. Further studies on handling of homografts are ongoing. Early results indicate that fresh decellularized homografts seem superior to conventional homografts and xenografts [34]. Development of new and improved biological conduits and valves suitable for various settings will likely also have great impact on how we treat these patients in the future.
Oosterhof's and Troost's echocardiographic studies imply that high postoperative systolic gradient and possibly also regurgitation predict rapid homograft degeneration. The fact that early haemodynamic defects are important for homograft durability is also supported by MRI data, indicating that alterations in homograft geometry can predict valve incompetence. Both of these early postoperative haemodynamic defects might be related to surgical technique, anatomy or the quality of the homograft. Further studies of these defects could lead to improved surgical methods and the identification of predictors of rapid degeneration. Such studies could also guide the timing of reoperation or percutaneous reintervention and increase the possibility of optimal outcomes in this group of patients.
The limitations of this review include the fact that the included studies are not directly comparable because of different baseline patient characteristics. There is also a risk of publication bias given that the review consists mainly of cohort studies. We also lack population-based studies and stringent records of patients lost to follow-up. Also, availability, preservation techniques and handling of homografts likely differ between regions and countries, limiting the scope for generalizing to different settings.
In conclusion, in this systematic review, the expected event-free survival rate for an adult patient operated for RVOT reconstruction with a homograft was 78–80% at 10 years and 67–69% at 15 years. The short-term and long-term mortality rates after surgical reconstruction of the RVOT with a homograft in adults are low. However, long-term event-free survival is limited by the durability of the homograft, and reoperation or percutaneous reintervention is common during the patient's life span. Early haemodynamic defects (i.e. stenosis, regurgitation and geometrical asymmetry) may be important for homograft durability. Further studies of homograft durability in adults with different cardiac malformations are needed to identify robust risk markers for rapid degeneration and to facilitate qualified and accurate decisions regarding lifetime management.
Funding
This work is supported by a grant from the Swedish Heart and Lung Foundation and by ALF-LUA grants from the Sahlgrenska University Hospital.
Conflict of interest: none declared.
ACKNOWLEDGEMENTS
We thank Eva-Lotte Daxberg at the Sahlgrenska University Hospital/Östra Medical Library for excellent librarian assistance and database searches.




