-
PDF
- Split View
-
Views
-
Cite
Cite
Efstratios I. Charitos, Paul D. Ziegler, Ulrich Stierle, Bernhard Graf, Hans-Hinrich Sievers, Thorsten Hanke, Long-term outcomes after surgical ablation for atrial fibrillation in patients with continuous heart rhythm monitoring devices, Interactive CardioVascular and Thoracic Surgery, Volume 21, Issue 6, December 2015, Pages 712–721, https://doi.org/10.1093/icvts/ivv248
Close - Share Icon Share
Abstract
Surgical ablation for atrial fibrillation (AF) is an established therapy for the treatment of concomitant AF in cardiac surgery patients. We aim to present our prospective experience with 99 continuously monitored patients and investigate whether enhanced monitoring can identify patterns and factors influencing AF recurrence after surgical AF ablation.
Ninety-nine patients (73 males; age: 68.0 ± 9.2 years) with documented preoperative AF (paroxysmal: 29; persistent: 18; long-lasting persistent: 52, mean preoperative duration: 46 ± 53 months) underwent concomitant biatrial surgical ablation (Cox Maze III: 29), full set left atrial cryoablation (n = 22), high-intensity focused ultrasound (HIFU) box lesion (n = 46) or right-sided ablation (n = 2). Postoperative rhythm disclosure was provided via an implantable device. Scheduled follow-up was performed quarterly (mean ± standard deviation: 1.75 ± 1.16 years, 173.7 patient-years).
The mean postoperative AF burden during the follow-up was 7 ± 19% (median: 0.2%). Seventy-one and 82 patients had AF burden <1% and <5%, respectively. The preoperative AF duration, preoperative ejection fraction, mitral valve surgery and HIFU in patients with more persistent AF were associated with statistically significant higher postoperative AF burdens. The pattern of AF recurrence during the 3-month blanking period was associated with the amount of later AF recurrence.
Continuous rhythm disclosure reveals that very small amounts of AF burden after surgical ablation are common. The preoperative duration of AF and the use of a box lesion only in patients with longer AF persistence history were independently associated with higher postoperative AF burden recurrence. The temporal AF pattern during the blanking period after ablation should be considered for further patient management and might serve as a prognostic factor.
INTRODUCTION
Surgical ablation for atrial fibrillation (AF) is an established therapeutic modality for the treatment of concomitant AF in patients undergoing cardiac surgery [1], and can be performed with reproducible results, minimal or even negligible additional risk and without great prolongation of the procedure [2]. Several large and adequately powered studies have shown that surgical ablation is an effective treatment resulting in higher prevalence of sinus rhythm postoperatively, without a significant increase in mortality, need for pacemaker implantation or neurological events [2]. Smaller trials and reports indicate that additional extended surgical ablative procedures result in lower rates of rehospitalization due to heart failure and mortality [3]. In a propensity score matched analysis, Lee et al. [4] were able to show that patients undergoing surgical treatment of AF had survival similar to that of patients without a history of AF and that successful sinus restoration improved survival compared with those who were treated but remained in AF.
Recently, the use of implantable, miniaturized, lead-less, continuous monitoring, heart rhythm recorders (CM) [5–9] has been proposed for more accurate detection of AF recurrence after ablation procedures or novel pharmacological strategies with considerable accuracy, reliability, sensitivity and specificity [6–10]. An apparent benefit from the complete rhythm disclosure provided by these devices is that the patients' rhythm status can be investigated with certainty over the complete observation time and AF recurrence can be reliably detected and measured. Therefore, inferences on the effect of interventions or medical therapies on the successful restoration of sinus rhythm can be reliably obtained. In contrast, when the patients' rhythm status is interrogated intermittently using discontinuous monitoring of variable frequencies and durations, randomness and chance can severely influence and distort the result and evaluations of the efficacy of therapeutic interventions for AF [5, 11, 12].
AF recurrence is a dynamic phenomenon with significant qualitative, quantitative and temporal aspects. A second but perhaps less apparent benefit from continuous rhythm monitoring is that this technology provides the physician and the researcher with the opportunity to evaluate and study AF recurrence while respecting and taking into consideration the quantitative, qualitative and temporal nature of AF recurrence.
The aim of this work is two-fold: firstly, to present our prospective single-centre experience with 99 patients who underwent surgical AF ablation and were followed up with continuous rhythm disclosure using implantable lead-less CM recorders, and evaluate the efficacy and efficiency of this therapy utilizing information from the CM devices with emphasis on AF burden development as this seems to be the true indicator of AF ablation success [13, 14]. Secondly, we sought to identify whether the enhanced monitoring capabilities of CM devices can provide insights on the patterns and the factors influencing AF recurrence after surgical AF ablation.
METHODS
Surgical techniques and ablation lines
The operations were performed in mild hypothermia as required by the primary operation. Details on the surgical procedure have been presented previously [5]. The full Cox Maze III cryoprocedure (CMIII group, n = 29) was performed with a biatrial lesion set according to the published techniques [15]. Left atrial (LA) ablation was performed using either high-intensity focused ultrasound (HIFU group, n = 46) epicardially for the creation of a box lesion ablation line (Epicor Cardiac Ablation System, St Jude Medical) or utilizing cryothermia (ATS CryoMaze Surgical Ablation System, ATS Medical, Inc.; CryoLA group, n = 22) for establishing a full LA lesion set consisting of a box lesion for pulmonary vein and LA antrum isolation, an atrial isthmus line, a LA appendage line and isolation of the coronary sinus by epicardial ablation. The ablation lines in these patients are described in detail elsewhere [16] and are depicted in Fig. 1. Two patients underwent right-sided only concomitant ablation for recurrent AF flutter and have been excluded from inferential and subgroup analyses. All procedures were performed by three surgeons.

Graphical depiction of the left-sided (left panel) and right-sided (right panel) ablation lines in the three ablation groups from the surgeon's point of view. Cryo LA: left atrial cryoablation; HIFU: high-intensity focused ultrasound; AF: atrial fibrillation; LAA: left atrial appendage; MV: mitral valve; SVC: superior vena cava; IVC: inferior vena cava; RAA: right atrial appendage; TV: tricuspid valve; RUPV: right upper pulmonary vein; RLPV: right lower pulmonary vein; LUPV: left upper pulmonary vein; LLPV: left lower pulmonary veins.
In all patients, the CM device (Reveal XT, Medtronic, Minneapolis, MN, USA) was implanted subcutaneously in the left pectoral region during chest closure or prior to discharge. The study was approved by the ethics committee of the University of Lübeck.
Population characteristics
Patient demographics and operative characteristics are presented in Table 1. The cross-tabulation of the ablation modalities employed according to the type and duration of AF is presented in Table 2. The choice of ablation modality was left to the operating surgeon; however, factors such as the type of AF, its preoperative duration, as well as the type of concomitant cardiac surgical procedure influenced the choice of ablation type. Generally, patients with longer preoperative AF durations and patients classified as having long-lasting persistent AF underwent a modified version of the Cox Maze III procedure, whereas patients with less persistent forms of AF or patients with shorter preoperative AF durations were more likely to undergo a left-sided ablation only (Cryo LA, HIFU). Patients requiring coronary artery bypass grafting (CABG) only were more likely to undergo HIFU, thus avoiding the need for atriotomy. Patients with concomitant mitral valve surgery were more likely to undergo left-sided cryoablation (Table 2) than HIFU.
Patient demographics, preoperative and operative characteristics of the patient population
| Data . | Total . |
|---|---|
| n | 99 |
| Age | 66.8 ± 9.2 (37–82) |
| Male gender | 73 |
| AF characteristics | |
| Paroxysmal | 29 |
| Persistent | 18 |
| Long-lasting persistent | 52 |
| AF duration (median; IQR in months) | 27 (10–55) |
| EHRA | |
| Class I | 57 |
| Class II | 26 |
| Class III | 15 |
| Class IV | 1 |
| History of TIA | 10 |
| History of PRIND | 0 |
| History of stroke | 10 |
| History of AF ablation | 11 |
| History of AF surgery | 2 |
| History of AF intervention | 9 |
| Comorbidities | |
| NYHA | |
| Class I | 19 |
| Class II | 40 |
| Class III | 36 |
| Class IV | 4 |
| Systemic hypertension | 79 |
| Renal insufficiency | 17 |
| Diabetes | 24 |
| COPD | 9 |
| Preoperative medication | |
| Aspirin | 36 |
| ACE inhibitor | 63 |
| Loop diuretics | 53 |
| Class I antiarrhythmics | 9 |
| Sotalol | 1 |
| Coumadin | 43 |
| β-Blocker | 77 |
| AT1 receptor blocker | 14 |
| Amiodarone | 10 |
| Digitalis | 33 |
| Statin | 51 |
| Dronedarone | 5 |
| Preoperative characteristics | |
| LVEF | 55.5 ± 12.6 (21–90) |
| LA dimensions (parasternal) | 4.8 ± 0.8 (3.4–5.1) |
| Mitral regurgitation | |
| None | 34 |
| Grade I | 23 |
| Grade II | 18 |
| Grade III | 20 |
| Grade IV | 4 |
| Operative characteristics | |
| CABG | 40 |
| MV | 37 |
| AV | 31 |
| TV | 14 |
| Ascending aortic replacement | 12 |
| Off-pump | 4 |
| Data . | Total . |
|---|---|
| n | 99 |
| Age | 66.8 ± 9.2 (37–82) |
| Male gender | 73 |
| AF characteristics | |
| Paroxysmal | 29 |
| Persistent | 18 |
| Long-lasting persistent | 52 |
| AF duration (median; IQR in months) | 27 (10–55) |
| EHRA | |
| Class I | 57 |
| Class II | 26 |
| Class III | 15 |
| Class IV | 1 |
| History of TIA | 10 |
| History of PRIND | 0 |
| History of stroke | 10 |
| History of AF ablation | 11 |
| History of AF surgery | 2 |
| History of AF intervention | 9 |
| Comorbidities | |
| NYHA | |
| Class I | 19 |
| Class II | 40 |
| Class III | 36 |
| Class IV | 4 |
| Systemic hypertension | 79 |
| Renal insufficiency | 17 |
| Diabetes | 24 |
| COPD | 9 |
| Preoperative medication | |
| Aspirin | 36 |
| ACE inhibitor | 63 |
| Loop diuretics | 53 |
| Class I antiarrhythmics | 9 |
| Sotalol | 1 |
| Coumadin | 43 |
| β-Blocker | 77 |
| AT1 receptor blocker | 14 |
| Amiodarone | 10 |
| Digitalis | 33 |
| Statin | 51 |
| Dronedarone | 5 |
| Preoperative characteristics | |
| LVEF | 55.5 ± 12.6 (21–90) |
| LA dimensions (parasternal) | 4.8 ± 0.8 (3.4–5.1) |
| Mitral regurgitation | |
| None | 34 |
| Grade I | 23 |
| Grade II | 18 |
| Grade III | 20 |
| Grade IV | 4 |
| Operative characteristics | |
| CABG | 40 |
| MV | 37 |
| AV | 31 |
| TV | 14 |
| Ascending aortic replacement | 12 |
| Off-pump | 4 |
TIA: transient ischaemic attack; PRIND: prolonged reversible ischaemic neurological deficit; AF: atrial fibrillation; LVEF: left ventricular ejection fraction; LA: left atrium; CABG: coronary artery bypass graft surgery; MV: mitral valve surgery; AV: aortic valve surgery; TV: tricuspid valve surgery; NYHA: New York Heart Association class; COPD: chronic obstructive pulmonary disease; ACE: angiotensin-converting enzyme; IQR: interquartile range.
Patient demographics, preoperative and operative characteristics of the patient population
| Data . | Total . |
|---|---|
| n | 99 |
| Age | 66.8 ± 9.2 (37–82) |
| Male gender | 73 |
| AF characteristics | |
| Paroxysmal | 29 |
| Persistent | 18 |
| Long-lasting persistent | 52 |
| AF duration (median; IQR in months) | 27 (10–55) |
| EHRA | |
| Class I | 57 |
| Class II | 26 |
| Class III | 15 |
| Class IV | 1 |
| History of TIA | 10 |
| History of PRIND | 0 |
| History of stroke | 10 |
| History of AF ablation | 11 |
| History of AF surgery | 2 |
| History of AF intervention | 9 |
| Comorbidities | |
| NYHA | |
| Class I | 19 |
| Class II | 40 |
| Class III | 36 |
| Class IV | 4 |
| Systemic hypertension | 79 |
| Renal insufficiency | 17 |
| Diabetes | 24 |
| COPD | 9 |
| Preoperative medication | |
| Aspirin | 36 |
| ACE inhibitor | 63 |
| Loop diuretics | 53 |
| Class I antiarrhythmics | 9 |
| Sotalol | 1 |
| Coumadin | 43 |
| β-Blocker | 77 |
| AT1 receptor blocker | 14 |
| Amiodarone | 10 |
| Digitalis | 33 |
| Statin | 51 |
| Dronedarone | 5 |
| Preoperative characteristics | |
| LVEF | 55.5 ± 12.6 (21–90) |
| LA dimensions (parasternal) | 4.8 ± 0.8 (3.4–5.1) |
| Mitral regurgitation | |
| None | 34 |
| Grade I | 23 |
| Grade II | 18 |
| Grade III | 20 |
| Grade IV | 4 |
| Operative characteristics | |
| CABG | 40 |
| MV | 37 |
| AV | 31 |
| TV | 14 |
| Ascending aortic replacement | 12 |
| Off-pump | 4 |
| Data . | Total . |
|---|---|
| n | 99 |
| Age | 66.8 ± 9.2 (37–82) |
| Male gender | 73 |
| AF characteristics | |
| Paroxysmal | 29 |
| Persistent | 18 |
| Long-lasting persistent | 52 |
| AF duration (median; IQR in months) | 27 (10–55) |
| EHRA | |
| Class I | 57 |
| Class II | 26 |
| Class III | 15 |
| Class IV | 1 |
| History of TIA | 10 |
| History of PRIND | 0 |
| History of stroke | 10 |
| History of AF ablation | 11 |
| History of AF surgery | 2 |
| History of AF intervention | 9 |
| Comorbidities | |
| NYHA | |
| Class I | 19 |
| Class II | 40 |
| Class III | 36 |
| Class IV | 4 |
| Systemic hypertension | 79 |
| Renal insufficiency | 17 |
| Diabetes | 24 |
| COPD | 9 |
| Preoperative medication | |
| Aspirin | 36 |
| ACE inhibitor | 63 |
| Loop diuretics | 53 |
| Class I antiarrhythmics | 9 |
| Sotalol | 1 |
| Coumadin | 43 |
| β-Blocker | 77 |
| AT1 receptor blocker | 14 |
| Amiodarone | 10 |
| Digitalis | 33 |
| Statin | 51 |
| Dronedarone | 5 |
| Preoperative characteristics | |
| LVEF | 55.5 ± 12.6 (21–90) |
| LA dimensions (parasternal) | 4.8 ± 0.8 (3.4–5.1) |
| Mitral regurgitation | |
| None | 34 |
| Grade I | 23 |
| Grade II | 18 |
| Grade III | 20 |
| Grade IV | 4 |
| Operative characteristics | |
| CABG | 40 |
| MV | 37 |
| AV | 31 |
| TV | 14 |
| Ascending aortic replacement | 12 |
| Off-pump | 4 |
TIA: transient ischaemic attack; PRIND: prolonged reversible ischaemic neurological deficit; AF: atrial fibrillation; LVEF: left ventricular ejection fraction; LA: left atrium; CABG: coronary artery bypass graft surgery; MV: mitral valve surgery; AV: aortic valve surgery; TV: tricuspid valve surgery; NYHA: New York Heart Association class; COPD: chronic obstructive pulmonary disease; ACE: angiotensin-converting enzyme; IQR: interquartile range.
| Data . | Type of Maze procedure . | |||||
|---|---|---|---|---|---|---|
| CMIII . | Cryo LA . | HIFU . | Other . | Total result . | P-value . | |
| n | 29 | 22 | 46 | 2 | 99 | |
| Paroxysmal | 1 | 7 | 20 | 1 | 29 | 0.003 |
| Persistent | 5 | 3 | 10 | 0 | 18 | 0.763 |
| Long-lasting persistent | 23 | 12 | 16 | 1 | 52 | 0.003 |
| Preoperative AF duration | 57.81 ± 56.99 (3–204) | 54.69 ± 47.21 (3–159) | 35.88 ± 53.58 (1–240) | 36.5 ± 50.2 (1–72) | 46.16 ± 53.65 (1–240) | |
| Median | 41 | 43 | 15 | 36.5 | 27 | |
| 1st quartile | 25.5 | 16.5 | 6.0 | 18.8 | 10.5 | |
| 3rd quartile | 55.0 | 79.5 | 40.0 | 54.3 | 55.0 | |
| EHRA | ||||||
| Class I | 11 | 13 | 24 | 48 | 0.36 | |
| Class II | 7 | 5 | 13 | 1 | 26 | |
| Class III | 6 | 2 | 7 | 15 | ||
| Class IV | 0 | 0 | 1 | 1 | ||
| Unknown | 5 | 2 | 1 | 1 | 9 | |
| LA diameter parasternal (median; 1st–3rd quartile, cm) | 5; 3.4–5.4 | 5.1; 3.5–5.6 | 4.6; 4.0–5.2 | 4.7; 4–5.4 | 0.28 | |
| Concomitant procedures | ||||||
| CABG | 8 | 4 | 27 | 1 | 40 | 0.005 |
| MV surgery | 21 | 16 | 0 | 0 | 37 | <0.001 |
| AV surgery | 3 | 10 | 18 | 0 | 31 | 0.017 |
| TV surgery | 10 | 4 | 0 | 0 | 14 | <0.001 |
| Ascending aortic surgery | 3 | 4 | 5 | 0 | 12 | 0.756 |
| Off-pump CABG | 0 | 0 | 4 | 0 | 4 | 0.185 |
| Data . | Type of Maze procedure . | |||||
|---|---|---|---|---|---|---|
| CMIII . | Cryo LA . | HIFU . | Other . | Total result . | P-value . | |
| n | 29 | 22 | 46 | 2 | 99 | |
| Paroxysmal | 1 | 7 | 20 | 1 | 29 | 0.003 |
| Persistent | 5 | 3 | 10 | 0 | 18 | 0.763 |
| Long-lasting persistent | 23 | 12 | 16 | 1 | 52 | 0.003 |
| Preoperative AF duration | 57.81 ± 56.99 (3–204) | 54.69 ± 47.21 (3–159) | 35.88 ± 53.58 (1–240) | 36.5 ± 50.2 (1–72) | 46.16 ± 53.65 (1–240) | |
| Median | 41 | 43 | 15 | 36.5 | 27 | |
| 1st quartile | 25.5 | 16.5 | 6.0 | 18.8 | 10.5 | |
| 3rd quartile | 55.0 | 79.5 | 40.0 | 54.3 | 55.0 | |
| EHRA | ||||||
| Class I | 11 | 13 | 24 | 48 | 0.36 | |
| Class II | 7 | 5 | 13 | 1 | 26 | |
| Class III | 6 | 2 | 7 | 15 | ||
| Class IV | 0 | 0 | 1 | 1 | ||
| Unknown | 5 | 2 | 1 | 1 | 9 | |
| LA diameter parasternal (median; 1st–3rd quartile, cm) | 5; 3.4–5.4 | 5.1; 3.5–5.6 | 4.6; 4.0–5.2 | 4.7; 4–5.4 | 0.28 | |
| Concomitant procedures | ||||||
| CABG | 8 | 4 | 27 | 1 | 40 | 0.005 |
| MV surgery | 21 | 16 | 0 | 0 | 37 | <0.001 |
| AV surgery | 3 | 10 | 18 | 0 | 31 | 0.017 |
| TV surgery | 10 | 4 | 0 | 0 | 14 | <0.001 |
| Ascending aortic surgery | 3 | 4 | 5 | 0 | 12 | 0.756 |
| Off-pump CABG | 0 | 0 | 4 | 0 | 4 | 0.185 |
CABG: coronary artery bypass graft surgery; MV: mitral valve surgery; AV: aortic valve surgery; TV: tricuspid valve surgery; AF: atrial fibrillation; LA: left atrial; CMIII: Cox Maze III; Cryo LA: left atrial cryoablation; HIFU: high-intensity focused ultrasound; EHRA: European Heart Rhythm Association.
| Data . | Type of Maze procedure . | |||||
|---|---|---|---|---|---|---|
| CMIII . | Cryo LA . | HIFU . | Other . | Total result . | P-value . | |
| n | 29 | 22 | 46 | 2 | 99 | |
| Paroxysmal | 1 | 7 | 20 | 1 | 29 | 0.003 |
| Persistent | 5 | 3 | 10 | 0 | 18 | 0.763 |
| Long-lasting persistent | 23 | 12 | 16 | 1 | 52 | 0.003 |
| Preoperative AF duration | 57.81 ± 56.99 (3–204) | 54.69 ± 47.21 (3–159) | 35.88 ± 53.58 (1–240) | 36.5 ± 50.2 (1–72) | 46.16 ± 53.65 (1–240) | |
| Median | 41 | 43 | 15 | 36.5 | 27 | |
| 1st quartile | 25.5 | 16.5 | 6.0 | 18.8 | 10.5 | |
| 3rd quartile | 55.0 | 79.5 | 40.0 | 54.3 | 55.0 | |
| EHRA | ||||||
| Class I | 11 | 13 | 24 | 48 | 0.36 | |
| Class II | 7 | 5 | 13 | 1 | 26 | |
| Class III | 6 | 2 | 7 | 15 | ||
| Class IV | 0 | 0 | 1 | 1 | ||
| Unknown | 5 | 2 | 1 | 1 | 9 | |
| LA diameter parasternal (median; 1st–3rd quartile, cm) | 5; 3.4–5.4 | 5.1; 3.5–5.6 | 4.6; 4.0–5.2 | 4.7; 4–5.4 | 0.28 | |
| Concomitant procedures | ||||||
| CABG | 8 | 4 | 27 | 1 | 40 | 0.005 |
| MV surgery | 21 | 16 | 0 | 0 | 37 | <0.001 |
| AV surgery | 3 | 10 | 18 | 0 | 31 | 0.017 |
| TV surgery | 10 | 4 | 0 | 0 | 14 | <0.001 |
| Ascending aortic surgery | 3 | 4 | 5 | 0 | 12 | 0.756 |
| Off-pump CABG | 0 | 0 | 4 | 0 | 4 | 0.185 |
| Data . | Type of Maze procedure . | |||||
|---|---|---|---|---|---|---|
| CMIII . | Cryo LA . | HIFU . | Other . | Total result . | P-value . | |
| n | 29 | 22 | 46 | 2 | 99 | |
| Paroxysmal | 1 | 7 | 20 | 1 | 29 | 0.003 |
| Persistent | 5 | 3 | 10 | 0 | 18 | 0.763 |
| Long-lasting persistent | 23 | 12 | 16 | 1 | 52 | 0.003 |
| Preoperative AF duration | 57.81 ± 56.99 (3–204) | 54.69 ± 47.21 (3–159) | 35.88 ± 53.58 (1–240) | 36.5 ± 50.2 (1–72) | 46.16 ± 53.65 (1–240) | |
| Median | 41 | 43 | 15 | 36.5 | 27 | |
| 1st quartile | 25.5 | 16.5 | 6.0 | 18.8 | 10.5 | |
| 3rd quartile | 55.0 | 79.5 | 40.0 | 54.3 | 55.0 | |
| EHRA | ||||||
| Class I | 11 | 13 | 24 | 48 | 0.36 | |
| Class II | 7 | 5 | 13 | 1 | 26 | |
| Class III | 6 | 2 | 7 | 15 | ||
| Class IV | 0 | 0 | 1 | 1 | ||
| Unknown | 5 | 2 | 1 | 1 | 9 | |
| LA diameter parasternal (median; 1st–3rd quartile, cm) | 5; 3.4–5.4 | 5.1; 3.5–5.6 | 4.6; 4.0–5.2 | 4.7; 4–5.4 | 0.28 | |
| Concomitant procedures | ||||||
| CABG | 8 | 4 | 27 | 1 | 40 | 0.005 |
| MV surgery | 21 | 16 | 0 | 0 | 37 | <0.001 |
| AV surgery | 3 | 10 | 18 | 0 | 31 | 0.017 |
| TV surgery | 10 | 4 | 0 | 0 | 14 | <0.001 |
| Ascending aortic surgery | 3 | 4 | 5 | 0 | 12 | 0.756 |
| Off-pump CABG | 0 | 0 | 4 | 0 | 4 | 0.185 |
CABG: coronary artery bypass graft surgery; MV: mitral valve surgery; AV: aortic valve surgery; TV: tricuspid valve surgery; AF: atrial fibrillation; LA: left atrial; CMIII: Cox Maze III; Cryo LA: left atrial cryoablation; HIFU: high-intensity focused ultrasound; EHRA: European Heart Rhythm Association.
Postoperative rhythm-related care
Unless contraindicated, patients received a loading dose of amiodarone early postoperatively (300 mg IV and then orally up to a total dose of 8 g). β-Blocker therapy was promptly resumed on the first postoperative day. Amiodarone (200 mg daily) was prescribed in all patients for a maximum of 3 months. In patients with persisting AF after the 3-month blanking period, a change in antiarrhythmic medication was made. In case of symptomatic AF during the blanking period, electrocardioversion was performed.
Heparin IV was initiated 6 h after the operation and then coumadin derivatives were prescribed within the first postoperative week and maintained for at least 3 months with a target INR of 2.5–3.0. Thereafter, the choice of anticoagulation was dependent on the patient's CHA2DS2-VASc score, the AF burden development as documented by the CM device and whether concomitant LA appendage resection was performed.
Evaluation of atrial fibrillation recurrence
Clinical echocardiographic follow-up was performed in all patients on prescheduled 3-month intervals and on an ‘as-needed’ basis. During the follow-up examination, the CM device was interrogated and the documented ECGs of AF episodes were manually validated by two different examiners (Ulrich Stierle, Bernhard Graf). All clinical information was entered in a prospective manner in our in-house AF database. At the time of the database closure, the complete cardiac rhythm of each patient was reconstructed. AF burden was defined as the proportion of observation time that a patient is in AF. AF density, as described previously [11, 17, 18], is a quantitative measure of the temporal aggregation of AF burden and was calculated as an index consisting of values between 0 (AF burden evenly spread over the observation period) and 1 (maximum possible AF burden aggregation, i.e. ‘one continuous episode of AF’). A thorough presentation of the AF density concept has been reported previously [11, 17, 18].
Statistical analysis
Simple statistical tests (such as the t-test, χ2 test and Mann–Whitney U-test, analysis of variance and Kruskal–Willis tests) were employed to identify differences in the demographics of the patient population subgroups.
Univariable linear regression was used to investigate the influence of patient demographics and operative characteristics on the postoperative development of AF burden (not including the 3-month blanking period): age, type of AF (classification), persistence of symptoms [European Heart Rhythm Association (EHRA) class I/II/III/IV denoting none/mild/severe/disabling symptoms, respectively], gender, type of surgery, functional status (NYHA class), known duration of AF, AF burden and AF density developed during the first 90 days (blanking period), left ventricular ejection fraction (LVEF) and LA diameter. Factors exhibiting a significant influence in the univariable linear regression (P < 0.1) entered the multivariable linear regression model. Backward stepwise elimination (utilizing the reduction of Akaike information criterion as an elimination criterion) was utilized to derive the final model. Logarithmic and power transformations of the outcome variable (using the Box–Cox method) were employed to restore regression assumptions, where appropriate. The assumptions of the linear model were checked and validated. In the setting of non-normal, heavy-tailed error distribution, robust regression methods were employed [19]. Extensions of mixed models for the evaluation of ordinal outcomes (EHRA) were employed to investigate the longitudinal time course of the EHRA class in the patient population. The P-values of two-sided tests at a significance level of 0.05 are reported. All statistical analyses were performed with R version 3.0.1 (R Development Core Team 2013) [20].
RESULTS
Clinical outcomes
There was no intraoperative or intra-hospital mortality. During the follow-up {during a median follow-up of 615 days [interquartile range (IQR): 260–1024 days]}, 1 major bleeding event and 2 strokes were observed in 3 patients receiving anticoagulation (coumadin derivatives in 2 patients, low molecular weight heparin in 1 patient) (Table 3). During the follow-up, 8 deaths were observed (4 non-cardiac, 2 cardiac, 2 unknown).
| Data . | CMIII . | Cryo LA . | HIFU . | Other . | Total result . | P-value . |
|---|---|---|---|---|---|---|
| n | 29 | 22 | 46 | 2 | 99 | |
| Follow-up | 1.13 ± 0.82 (0.09, 3.63) | 1.44 ± 1.05 (0.11–4.6) | 1.41 ± 0.95 (0.13–4.07) | 0.53 ± 0.2 (0.28–0.76) | 1.34 ± 0.95 (0.09–4.6) | 0.02 |
| NYHA | ||||||
| Class I | 16 | 10 | 15 | 0 | 41 | 0.14 |
| Class II | 3 | 7 | 15 | 1 | 26 | |
| Class III | 2 | 1 | 5 | 0 | 8 | |
| Class IV | 0 | 0 | 0 | 0 | 0 | |
| Unknown | 8 | 4 | 11 | 1 | 24 | |
| EHRA | ||||||
| EHRA I | 17 | 17 | 29 | 1 | 64 | 0.62 |
| EHRA II | 2 | 0 | 2 | 0 | 4 | |
| EHRA III | 1 | 0 | 1 | 0 | 2 | |
| EHRA IV | 0 | 0 | 0 | 0 | 0 | |
| Unknown | 9 | 5 | 14 | 1 | 29 | |
| AF burden | ||||||
| Mean ± SD | 0.089 ± 0.243 | 0.004 ± 0.009 | 0.087 ± 0.197 | 0.001 ± 0.001 | 0.067 ± 0.190 | 0.025 |
| 1st quartile–median–3rd quartile | 0.001–0.001–0.004 | 0.001–0.001–0.002 | 0.001–0.004–0.048 | 0.001–0.002–0.002 | 0.001–0.002–0.019 | |
| Patients with AF burden <0.01 | 23 (79.3%) | 19 (86.4%) | 27 (58.7%) | 2 (100%) | 71 (71.7%) | |
| Patients with AF burden <0.05 | 24 (82.8%) | 22 (100%) | 34 (73.9%) | 2 (100%) | 82 (82.8%) | |
| LVEF | 56.1 ± 11.2 (28–83) | 57.63 ± 9.78 (40–75) | 55.88 ± 11.19 (18–81) | 70.33 ± 12.1 (61–84) | 56.47 ± 10.96 (18–84) | 0.70 |
| LAD parasternal | 4.81 ± 0.68 (2.77–6.5) | 5.57 ± 5.5 (3.1–53) | 5.31 ± 5.13 (2.9–48) | 4.4 ± 0.9 (3.5–5.3) | 5.21 ± 4.34 (2.77–53) | 0.28 |
| MR | ||||||
| None/trivial | 11 | 13 | 10 | 1 | 35 | 0.24 |
| Grade I | 8 | 5 | 14 | 0 | 27 | |
| Grade II | 0 | 0 | 1 | 0 | 1 | |
| Unknown | 10 | 4 | 21 | 1 | 36 | |
| Cardioversion attempt | ||||||
| Electrical (n; time range in years) | 8 (0.02–2.9) | 1 (0.1) | 19 (0.16–3.3) | 0 | 28 (0.016–3.3) | 0.002 |
| Pharmacological (n; time range in years) | 1 (2.9) | 0 | 6 (0.7–3.9) | 0 | 7 (0.7–3.9) | 0.88 |
| Complications (n of events) | ||||||
| Major bleeding events | 1 | 1 | ||||
| Re-ablation | 0 | 2 | 3 | 5 | ||
| TIA | 0 | 0 | 0 | 0 | 0 | |
| PRIND | 0 | 0 | 0 | 0 | 0 | |
| Stroke | 1 | 0 | 1 | 0 | 2 | |
| Medication | ||||||
| Sotalol | 0 | 0 | 1 | 0 | 1 | |
| β-Blocker | 24 | 20 | 28 | 1 | 73 | |
| Amiodarone | 13 | 9 | 17 | 1 | 40 | |
| Digitalis | 5 | 5 | 4 | 0 | 14 | |
| Dronedarone | 1 | 1 | 1 | 0 | 3 | |
| Warfarin | 27 | 19 | 27 | 0 | 73 | |
| Aspirin | 7 | 7 | 13 | 1 | 28 | |
| Data . | CMIII . | Cryo LA . | HIFU . | Other . | Total result . | P-value . |
|---|---|---|---|---|---|---|
| n | 29 | 22 | 46 | 2 | 99 | |
| Follow-up | 1.13 ± 0.82 (0.09, 3.63) | 1.44 ± 1.05 (0.11–4.6) | 1.41 ± 0.95 (0.13–4.07) | 0.53 ± 0.2 (0.28–0.76) | 1.34 ± 0.95 (0.09–4.6) | 0.02 |
| NYHA | ||||||
| Class I | 16 | 10 | 15 | 0 | 41 | 0.14 |
| Class II | 3 | 7 | 15 | 1 | 26 | |
| Class III | 2 | 1 | 5 | 0 | 8 | |
| Class IV | 0 | 0 | 0 | 0 | 0 | |
| Unknown | 8 | 4 | 11 | 1 | 24 | |
| EHRA | ||||||
| EHRA I | 17 | 17 | 29 | 1 | 64 | 0.62 |
| EHRA II | 2 | 0 | 2 | 0 | 4 | |
| EHRA III | 1 | 0 | 1 | 0 | 2 | |
| EHRA IV | 0 | 0 | 0 | 0 | 0 | |
| Unknown | 9 | 5 | 14 | 1 | 29 | |
| AF burden | ||||||
| Mean ± SD | 0.089 ± 0.243 | 0.004 ± 0.009 | 0.087 ± 0.197 | 0.001 ± 0.001 | 0.067 ± 0.190 | 0.025 |
| 1st quartile–median–3rd quartile | 0.001–0.001–0.004 | 0.001–0.001–0.002 | 0.001–0.004–0.048 | 0.001–0.002–0.002 | 0.001–0.002–0.019 | |
| Patients with AF burden <0.01 | 23 (79.3%) | 19 (86.4%) | 27 (58.7%) | 2 (100%) | 71 (71.7%) | |
| Patients with AF burden <0.05 | 24 (82.8%) | 22 (100%) | 34 (73.9%) | 2 (100%) | 82 (82.8%) | |
| LVEF | 56.1 ± 11.2 (28–83) | 57.63 ± 9.78 (40–75) | 55.88 ± 11.19 (18–81) | 70.33 ± 12.1 (61–84) | 56.47 ± 10.96 (18–84) | 0.70 |
| LAD parasternal | 4.81 ± 0.68 (2.77–6.5) | 5.57 ± 5.5 (3.1–53) | 5.31 ± 5.13 (2.9–48) | 4.4 ± 0.9 (3.5–5.3) | 5.21 ± 4.34 (2.77–53) | 0.28 |
| MR | ||||||
| None/trivial | 11 | 13 | 10 | 1 | 35 | 0.24 |
| Grade I | 8 | 5 | 14 | 0 | 27 | |
| Grade II | 0 | 0 | 1 | 0 | 1 | |
| Unknown | 10 | 4 | 21 | 1 | 36 | |
| Cardioversion attempt | ||||||
| Electrical (n; time range in years) | 8 (0.02–2.9) | 1 (0.1) | 19 (0.16–3.3) | 0 | 28 (0.016–3.3) | 0.002 |
| Pharmacological (n; time range in years) | 1 (2.9) | 0 | 6 (0.7–3.9) | 0 | 7 (0.7–3.9) | 0.88 |
| Complications (n of events) | ||||||
| Major bleeding events | 1 | 1 | ||||
| Re-ablation | 0 | 2 | 3 | 5 | ||
| TIA | 0 | 0 | 0 | 0 | 0 | |
| PRIND | 0 | 0 | 0 | 0 | 0 | |
| Stroke | 1 | 0 | 1 | 0 | 2 | |
| Medication | ||||||
| Sotalol | 0 | 0 | 1 | 0 | 1 | |
| β-Blocker | 24 | 20 | 28 | 1 | 73 | |
| Amiodarone | 13 | 9 | 17 | 1 | 40 | |
| Digitalis | 5 | 5 | 4 | 0 | 14 | |
| Dronedarone | 1 | 1 | 1 | 0 | 3 | |
| Warfarin | 27 | 19 | 27 | 0 | 73 | |
| Aspirin | 7 | 7 | 13 | 1 | 28 | |
EHRA and NYHA represent values at latest follow up.
TIA: transient ischaemic attack; PRIND: prolonged reversible ischaemic neurological deficit; AF: atrial fibrillation; LVEF: left ventricular ejection fraction; MR: mitral regurgitation; LAD: left atrial diameter; EHRA: European Heart Rhythm Association class; CMIII: Cox Maze III; Cryo LA: left atrial cryoablation; HIFU: high-intensity focused ultrasound; SD: standard deviation.
| Data . | CMIII . | Cryo LA . | HIFU . | Other . | Total result . | P-value . |
|---|---|---|---|---|---|---|
| n | 29 | 22 | 46 | 2 | 99 | |
| Follow-up | 1.13 ± 0.82 (0.09, 3.63) | 1.44 ± 1.05 (0.11–4.6) | 1.41 ± 0.95 (0.13–4.07) | 0.53 ± 0.2 (0.28–0.76) | 1.34 ± 0.95 (0.09–4.6) | 0.02 |
| NYHA | ||||||
| Class I | 16 | 10 | 15 | 0 | 41 | 0.14 |
| Class II | 3 | 7 | 15 | 1 | 26 | |
| Class III | 2 | 1 | 5 | 0 | 8 | |
| Class IV | 0 | 0 | 0 | 0 | 0 | |
| Unknown | 8 | 4 | 11 | 1 | 24 | |
| EHRA | ||||||
| EHRA I | 17 | 17 | 29 | 1 | 64 | 0.62 |
| EHRA II | 2 | 0 | 2 | 0 | 4 | |
| EHRA III | 1 | 0 | 1 | 0 | 2 | |
| EHRA IV | 0 | 0 | 0 | 0 | 0 | |
| Unknown | 9 | 5 | 14 | 1 | 29 | |
| AF burden | ||||||
| Mean ± SD | 0.089 ± 0.243 | 0.004 ± 0.009 | 0.087 ± 0.197 | 0.001 ± 0.001 | 0.067 ± 0.190 | 0.025 |
| 1st quartile–median–3rd quartile | 0.001–0.001–0.004 | 0.001–0.001–0.002 | 0.001–0.004–0.048 | 0.001–0.002–0.002 | 0.001–0.002–0.019 | |
| Patients with AF burden <0.01 | 23 (79.3%) | 19 (86.4%) | 27 (58.7%) | 2 (100%) | 71 (71.7%) | |
| Patients with AF burden <0.05 | 24 (82.8%) | 22 (100%) | 34 (73.9%) | 2 (100%) | 82 (82.8%) | |
| LVEF | 56.1 ± 11.2 (28–83) | 57.63 ± 9.78 (40–75) | 55.88 ± 11.19 (18–81) | 70.33 ± 12.1 (61–84) | 56.47 ± 10.96 (18–84) | 0.70 |
| LAD parasternal | 4.81 ± 0.68 (2.77–6.5) | 5.57 ± 5.5 (3.1–53) | 5.31 ± 5.13 (2.9–48) | 4.4 ± 0.9 (3.5–5.3) | 5.21 ± 4.34 (2.77–53) | 0.28 |
| MR | ||||||
| None/trivial | 11 | 13 | 10 | 1 | 35 | 0.24 |
| Grade I | 8 | 5 | 14 | 0 | 27 | |
| Grade II | 0 | 0 | 1 | 0 | 1 | |
| Unknown | 10 | 4 | 21 | 1 | 36 | |
| Cardioversion attempt | ||||||
| Electrical (n; time range in years) | 8 (0.02–2.9) | 1 (0.1) | 19 (0.16–3.3) | 0 | 28 (0.016–3.3) | 0.002 |
| Pharmacological (n; time range in years) | 1 (2.9) | 0 | 6 (0.7–3.9) | 0 | 7 (0.7–3.9) | 0.88 |
| Complications (n of events) | ||||||
| Major bleeding events | 1 | 1 | ||||
| Re-ablation | 0 | 2 | 3 | 5 | ||
| TIA | 0 | 0 | 0 | 0 | 0 | |
| PRIND | 0 | 0 | 0 | 0 | 0 | |
| Stroke | 1 | 0 | 1 | 0 | 2 | |
| Medication | ||||||
| Sotalol | 0 | 0 | 1 | 0 | 1 | |
| β-Blocker | 24 | 20 | 28 | 1 | 73 | |
| Amiodarone | 13 | 9 | 17 | 1 | 40 | |
| Digitalis | 5 | 5 | 4 | 0 | 14 | |
| Dronedarone | 1 | 1 | 1 | 0 | 3 | |
| Warfarin | 27 | 19 | 27 | 0 | 73 | |
| Aspirin | 7 | 7 | 13 | 1 | 28 | |
| Data . | CMIII . | Cryo LA . | HIFU . | Other . | Total result . | P-value . |
|---|---|---|---|---|---|---|
| n | 29 | 22 | 46 | 2 | 99 | |
| Follow-up | 1.13 ± 0.82 (0.09, 3.63) | 1.44 ± 1.05 (0.11–4.6) | 1.41 ± 0.95 (0.13–4.07) | 0.53 ± 0.2 (0.28–0.76) | 1.34 ± 0.95 (0.09–4.6) | 0.02 |
| NYHA | ||||||
| Class I | 16 | 10 | 15 | 0 | 41 | 0.14 |
| Class II | 3 | 7 | 15 | 1 | 26 | |
| Class III | 2 | 1 | 5 | 0 | 8 | |
| Class IV | 0 | 0 | 0 | 0 | 0 | |
| Unknown | 8 | 4 | 11 | 1 | 24 | |
| EHRA | ||||||
| EHRA I | 17 | 17 | 29 | 1 | 64 | 0.62 |
| EHRA II | 2 | 0 | 2 | 0 | 4 | |
| EHRA III | 1 | 0 | 1 | 0 | 2 | |
| EHRA IV | 0 | 0 | 0 | 0 | 0 | |
| Unknown | 9 | 5 | 14 | 1 | 29 | |
| AF burden | ||||||
| Mean ± SD | 0.089 ± 0.243 | 0.004 ± 0.009 | 0.087 ± 0.197 | 0.001 ± 0.001 | 0.067 ± 0.190 | 0.025 |
| 1st quartile–median–3rd quartile | 0.001–0.001–0.004 | 0.001–0.001–0.002 | 0.001–0.004–0.048 | 0.001–0.002–0.002 | 0.001–0.002–0.019 | |
| Patients with AF burden <0.01 | 23 (79.3%) | 19 (86.4%) | 27 (58.7%) | 2 (100%) | 71 (71.7%) | |
| Patients with AF burden <0.05 | 24 (82.8%) | 22 (100%) | 34 (73.9%) | 2 (100%) | 82 (82.8%) | |
| LVEF | 56.1 ± 11.2 (28–83) | 57.63 ± 9.78 (40–75) | 55.88 ± 11.19 (18–81) | 70.33 ± 12.1 (61–84) | 56.47 ± 10.96 (18–84) | 0.70 |
| LAD parasternal | 4.81 ± 0.68 (2.77–6.5) | 5.57 ± 5.5 (3.1–53) | 5.31 ± 5.13 (2.9–48) | 4.4 ± 0.9 (3.5–5.3) | 5.21 ± 4.34 (2.77–53) | 0.28 |
| MR | ||||||
| None/trivial | 11 | 13 | 10 | 1 | 35 | 0.24 |
| Grade I | 8 | 5 | 14 | 0 | 27 | |
| Grade II | 0 | 0 | 1 | 0 | 1 | |
| Unknown | 10 | 4 | 21 | 1 | 36 | |
| Cardioversion attempt | ||||||
| Electrical (n; time range in years) | 8 (0.02–2.9) | 1 (0.1) | 19 (0.16–3.3) | 0 | 28 (0.016–3.3) | 0.002 |
| Pharmacological (n; time range in years) | 1 (2.9) | 0 | 6 (0.7–3.9) | 0 | 7 (0.7–3.9) | 0.88 |
| Complications (n of events) | ||||||
| Major bleeding events | 1 | 1 | ||||
| Re-ablation | 0 | 2 | 3 | 5 | ||
| TIA | 0 | 0 | 0 | 0 | 0 | |
| PRIND | 0 | 0 | 0 | 0 | 0 | |
| Stroke | 1 | 0 | 1 | 0 | 2 | |
| Medication | ||||||
| Sotalol | 0 | 0 | 1 | 0 | 1 | |
| β-Blocker | 24 | 20 | 28 | 1 | 73 | |
| Amiodarone | 13 | 9 | 17 | 1 | 40 | |
| Digitalis | 5 | 5 | 4 | 0 | 14 | |
| Dronedarone | 1 | 1 | 1 | 0 | 3 | |
| Warfarin | 27 | 19 | 27 | 0 | 73 | |
| Aspirin | 7 | 7 | 13 | 1 | 28 | |
EHRA and NYHA represent values at latest follow up.
TIA: transient ischaemic attack; PRIND: prolonged reversible ischaemic neurological deficit; AF: atrial fibrillation; LVEF: left ventricular ejection fraction; MR: mitral regurgitation; LAD: left atrial diameter; EHRA: European Heart Rhythm Association class; CMIII: Cox Maze III; Cryo LA: left atrial cryoablation; HIFU: high-intensity focused ultrasound; SD: standard deviation.
Heart rhythm status
All patients experienced some AF recurrence. The median duration of these recurrences was 918 min (IQR: 263–6975 min). This recurrence translated quantitatively into very low AF burdens: median 0.0018 [IQR: 0.0004–0.0189] (Table 3 and Figs 2–4). There was a significantly higher need for cardioversion in the HIFU group (Table 3), which was performed throughout the observation period. The postoperative medication therapy is presented in Table 3. Three patients (2 in the HIFU and 1 in the CryoLA group) underwent five re-ablation procedures due to symptomatic LA flutter. Two of these patients (1 in the HIFU and 1 in the CryoLA group) required two ablation sessions for a successful treatment.
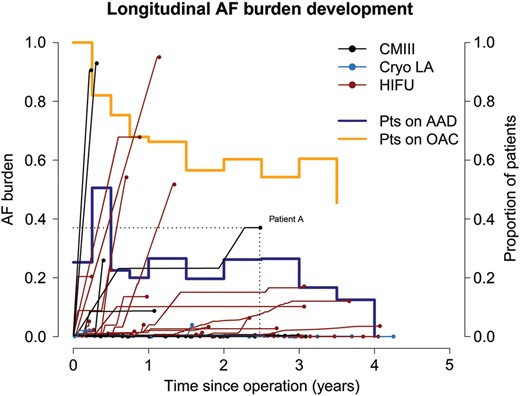
The longitudinal development of each patient's final (cumulative) AF burden. Each patient is represented with a line and a dot. The dots present each patient's final AF burden (over the follow-up) (projection of the dot on the y-axis) and the patient's maximum follow-up (projection of the dot on the x-axis). For example, Patient A developed a total AF burden of 0.4 (40%) during 2.5 years of observation. Horizontal line segments (zero slope) in each patient's longitudinal time course denote AF free periods. Line segments with positive slope indicate quantitative development of AF recurrence. The proportion of patients on OAC and on AAD is depicted with the orange and light blue line, respectively. AF: atrial fibrillation; OAC: oral anticoagulation; AAD: antiarrhythmic medication; HIFU: high-intensity focused ultrasound; CMIII: Cox Maze III.
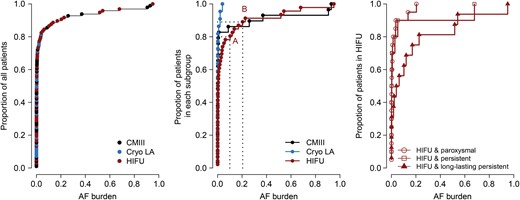
Cumulative distribution functions of the AF burden in the patient cohort. The cumulative distribution functions depict the percentage of patients who have less than a given AF burden. For example, in the middle panel (distribution function of each ablation subgroup), 80% of patients in the HIFU group (red line) have less than 0.1 AF burden (Point A) and 89% of patients have less than 0.2 AF burden. Left: The cumulative distribution function of the total patient cohort. Middle: The cumulative distribution function of each ablation-type subgroup. Right: The cumulative distribution function of the HIFU ablation subgroup. Patients with more persistent forms of AF resulted in higher AF burden postoperatively (P = 0.01). CMIII: Cox Maze III; Cryo LA: left atrial cryoablation; HIFU: high-intensity focused ultrasound; AF: atrial fibrillation.
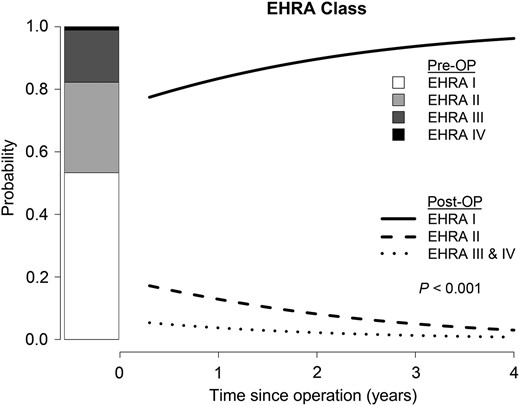
Longitudinal change of EHRA class with time. A statistically significant decrease in EHRA class with time (P < 0.001) could be observed.
Factors influencing postoperative atrial fibrillation burden
In the univariable analysis, the preoperative duration of each patient's AF significantly influenced the amount of postoperative AF observed (P = 0.0002). On the contrary, the type of AF (paroxysmal, persistent, long-lasting persistent) classification did not influence the postoperative burdens. Lower LVEF (P = 0.039) and, interestingly, a higher uncorrected mitral valve regurgitation (P = 0.06) were associated with higher postoperative AF burden developments. The correction of >grade II mitral valve regurgitation was associated with lower AF burden postoperatively (P = 0.03). Patients treated with a box-lesion ablation set (HIFU) had higher unadjusted postoperative AF burdens (P = 0.06). This effect persisted when using the follow-up time as a weighting variable in the regression models. The results of the univariable analysis including estimates, confidence intervals and P-values are presented in detail in Supplementary material, Table S1. The following factors did not exhibit a statistically significant influence on the development of postoperative AF burdens: NYHA class (P = 0.2), EHRA class (P = 0.45) and previous cardiac surgery (P = 0.3), history of AF ablation (P = 0.3), LA diameter (P = 0.3) and type of surgery other than mitral valve surgery for the correction of significant mitral regurgitation (P > 0.4 for each). Within the HIFU group, more persistent forms of the disease such as long-lasting persistent AF were associated with higher postoperative AF burden (P = 0.03) (Supplementary material, Table S2, Fig. 3 right panel). This was not observed within the other ablation-type groups (P > 0.4 for all).
Multivariable linear regression revealed that the preoperative duration of AF (P < 0.001) (Supplementary material, Figs S3 and Supplementary Data) as well as the use of a box-lesion ablation set (HIFU; P = 0.05) was associated with higher postoperative AF burden development (Supplementary material, Table S3).
Information obtained from the 3-month blanking period
The amount of AF recurrence (AF burden) during the 3-month blanking period was significantly associated with the AF burden observed in the later follow-up period. The development of AF burden (P < 0.001) during the first 3 months after surgery was associated with an increased AF burden observed during the rest of the follow-up time. Significant AF burden (>5%) during the follow-up period was associated with later AF recurrence.
Interestingly, the temporal pattern of AF recurrence (AF density) during the 3-month blanking period was also associated with the AF burden during the subsequent period. Higher AF burdens (P < 0.001) and lower AF densities (P = 0.004) during the 3-month blanking period were independently associated with higher AF burdens in the subsequent observation period (Fig. 5 and Supplementary material, Table S4).
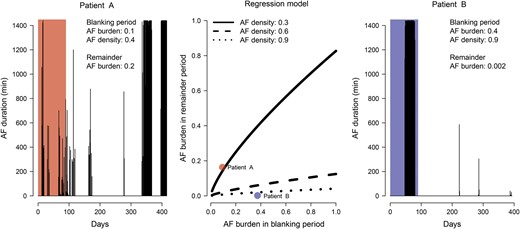
Graphical depiction of the regression model of the temporal AF characteristics during the blanking period on the later AF recurrence (middle panel). The three black lines present regression predictions for AF densities of 0.3, 0.6 and 0.9. The left and right panels present the cardiac rhythm history of 2 exemplary patients (Patient A and Patient B). In the regression model (middle panel) these patients correspond to the red and blue dots, respectively. The AF burden and AF density during the blanking period was significantly and independently associated with the amount of AF recurrence later on. AF: atrial fibrillation.
DISCUSSION
Post-ablation atrial fibrillation burden and implications for the evaluation of ablation procedures
Our results show that surgical AF ablation is an effective modality for the treatment of AF when performed concomitantly with other cardiac surgical procedures and results in very low quantitative AF recurrence (AF burden). Complete absence of AF is rare, when patients are monitored with implantable CM devices over longer time frames. However, the AF recurrence in our patients resulted in very low cumulative AF burdens throughout the entire observation time for up to 4 years. From a quantitative perspective, the total amount of AF recurrence in the vast majority of patients was negligible (83% of patients had a total AF burden over their respective observation time of less than 0.05) (Fig. 3 left panel). A purely qualitative only evaluation of AF recurrence based only on the presence (derived by intermittent rhythm follow-up strategies) and not the amount of AF recurrence may distort the success of ablation procedures and falsely identify patients as non-responders, where, although they experience some AF recurrence, this may be quantitatively negligible (a total median of 15.3 h over a median of 615 days).
Choice of therapy and optimal patient therapy selection
Our choice of ablation procedure was primarily based on the clinical classification of AF based on patient history and intermittent electrocardiographic documentation of the patient's heart rhythm. Patients presenting with paroxysmal AF underwent primarily left-sided ‘box-lesion’ ablation, whereas patients with a more progressive form of the disease (classified as ‘persistent AF’ or ‘long-lasting persistent AF’) underwent primarily biatrial ablation. Early in our experience, patients undergoing solely CABG procedures were more likely to receive box lesion (HIFU) irrespective of the AF classification, thus avoiding atriotomy. At that time, this approach seemed to be supported by the literature [21, 22]. However, when analysing the results with continuous heart rhythm monitoring, the LA box-lesion set (HIFU) in the setting of more chronic AF (long-lasting persistent AF) seems to be less effective and these patients may derive greater benefit from a more extensive ablation procedure, such as the biatrial Cox Maze. When a box-lesion ablation approach is performed in patients with persistent and long-lasting persistent forms of AF, many patients require later repeated catheter ablation interventions [23] or, as in our study population, the need for electrical cardioversion was significantly higher in this group (Table 3).
In the present study, we could reproduce a previously published finding [24] that the duration of AF as derived from the patient's history and not the clinical AF classification was associated with increased AF burden development post-ablation. Although we had hypothesized that patients with ‘persistent’ or ‘long-lasting persistent’ AF would have higher amounts of AF recurrence postoperatively, we were unable to document such an association. Interestingly, only the history of AF duration was strongly associated with the post-ablation amount of AF recurrence.
We have recently shown that the widely used clinical AF classifications poorly reflect the temporal AF recurrence [24, 25] and many patients who are regarded to have only a ‘paroxysmal’ form of AF actually experience high AF burdens. On the other hand, patients classified as having ‘persistent’ forms of AF, based solely on the presence of severe symptomatology, may actually experience low—but highly symptomatic—AF burdens. Similarly, other studies have shown that the amount of fibrosis, as a marker of the severity and persistence of AF, correlates very poorly with the commonly used clinical AF classifications. This fact might partially explain the prevalence of high AF burdens in some patients classified as ‘paroxysmal’ and treated with left-sided ablation procedures. It might well be that these patients had been classified as ‘paroxysmal’ AF when they may actually have a more advanced form of the disease and might have required a biatrial ablation. Similarly, patients classified as ‘persistent AF’ who responded well to biatrial ablation with negligible post-ablation AF burdens may actually have had a more benign form of AF disease which might have responded equally well to left-sided ablation only. Interestingly, the preoperative duration of AF as obtained from the patient history showed a far better association with the success of AF ablation than the AF clinical classification, perhaps denoting that the known duration of AF may more accurately reflect the degree of AF disease or atrial substrate. Simultaneously, there seems to be a disconnection between AF classification and known AF duration (Supplementary material, Fig. S1). Our data point out that patients with known AF over many years respond worse to AF ablation even though these patients—perhaps due to asymptomatic AF recurrence—might be classified as having paroxysmal AF. Whether a more objective pre-ablation evaluation of the AF disease severity—either with CM or using markers of AF disease such as MRI-detected atrial fibrosis—can guide therapy selection better than current AF classifications remains to be seen in future studies.
Role of patient characteristics
Similar to other studies, several patient characteristics were associated with the amount of postoperative AF recurrence. Lower EF, longer preoperative duration of AF and uncorrected mitral valve regurgitation were shown to be associated with higher quantitative amounts of AF recurrence. Perhaps due to the relatively low number of patients, we were unable to investigate or show an influence of other patient-specific factors on the development of AF burden during the follow-up.
In the present study, we were unable to find an association between quantitative AF recurrence and LA diameter (Supplementary material, Fig. S2), although several studies have shown that patients with larger atria may experience more frequent AF recurrence. A significant number of patients had enlarged LA diameters (37% of patients had LA diameter >50 mm and 66% of patients had LA diameter >40 mm); however, even at large LA diameters (>50 mm), the vast majority of patients had negligible AF burdens (Supplementary material, Fig. S2). A possible explanation may be that surgical ablation methods can exclude a larger proportion of posterior wall atrial tissue as well as atrial tissue surrounding the pulmonary veins under direct vision and in the arrested heart, which may mitigate the effect that increased atrial tissue may have in patients with enlarged atria. Our data show that even in patients with large LA diameters, concomitant surgical AF ablation can restore sinus rhythm with negligible AF recurrence up to 4 years postoperatively and that enlarged LA diameters should not be regarded as a contraindication for concomitant surgical ablation of AF.
We observed that the correction of significant mitral valve regurgitation was associated with lower AF burdens during the total observation period. In a similar manner, patients with mild to moderate mitral valve regurgitation who did not undergo mitral valve repair had significantly higher AF burden during the follow-up. Similar conclusions have been obtained after catheter ablation which underscore the influence of coexisting mitral regurgitation on the outcomes of AF ablation procedures. In the case of the surgical patient, correction of mild to moderate mitral regurgitation in patients undergoing concomitant surgical AF ablation leads to lower quantitative AF recurrence and should be strongly considered.
Information obtained during the 3-month blanking period
Although the AF recurrence information obtained within the first 3 months after an ablation procedure is conventionally excluded from the overall evaluation of the therapeutic efficacy [1], several groups have shown that information on AF recurrence within the so-called blanking period may provide prognostic information for the effectiveness of the ablation procedure and predict later AF recurrence [9] for up to the follow-up period of this study. In our study population, AF recurrence (AF burden >0.01) during the 3-month blanking period was significantly associated with quantitatively more AF recurrence later on.
More interestingly, the pattern of AF recurrence as evaluated by both the AF burden and AF density during the blanking period was significantly and independently associated with the amount of AF recurrence later on. High AF burdens and low AF density (Fig. 5 and Supplementary material, Table S4) during the 3-month blanking period were independently associated with greater AF recurrence later in the observation period. This suggests that information obtained during the ‘blanking period’ may have prognostic value and although this information should not be used for the judgement of therapeutic efficacy, it may be valuable for patient management purposes and should not be discarded. In our study population, the temporal AF characteristics during the blanking periods showed good association with the AF burden observed for up to 1 year thereafter (Fig. 5 and Supplementary material, Table S4).
Oral anticoagulation after successful ablation procedures
An important issue in patients undergoing ablation procedures for AF, especially when these patients are continuously monitored with implantable devices, is whether the absence of AF, as documented by the CM device, after the initial 3-month period can justify a modification or even cessation of oral anticoagulation therapy [1]. Although AF is a known risk factor for stroke, it remains unknown if interrupting AF can reduce the risk of stroke in a given patient. Several well-powered studies have failed to show a strong temporal relationship between AF recurrence and cardioembolic complications. These studies imply that there may be other pathophysiological mechanisms responsible for cardioembolic complications such as changes in atrial and endothelial function and/or structure. Therefore, the possible merit of cessation or modification of oral anticoagulation therapy in patients after ablation procedure and documented absence of AF remains unknown. Our policy is to discuss modification of oral anticoagulation after the first 3 months postoperatively only in patients in whom the absence of AF (or AF burden <0.01) has been documented with the CM device and additionally in whom a complete surgical LA appendage resection has been performed.
Limitations
Owing to the relatively small number of patients, multivariable regression methods controlling for several covariates could not be fully utilized. However, complete rhythm disclosure for up to 4 years postoperatively has been obtained and, thus, AF recurrence has been detected with high certainty, which validates the utility of concomitant surgical AF ablation as a therapeutic option for the treatment of AF in patients undergoing cardiac surgical procedures. In the present prospective cohort, all patients have been followed for different amounts of time. This implies that patients with the same AF burden will have inevitably different amounts of absolute AF recurrence (minutes of AF). This might introduce some bias when evaluating and analysing the amount of AF recurrence. To compensate for this, we performed all regression models with and without the follow-up time as a weighting variable in the regression models investigating the factors influencing the total AF burden. Weighting by length of follow-up attaches greater importance on the AF burden developed for longer follow-up durations (thus a greater absolute amount of AF recurrence). The results of these two analyses have similar conclusions. During the observation period, 8 deaths were observed. This may add another level of complexity since these patients provide AF recurrence data for the period up to their death. However, with the exception of 1 death at 0.3 years, all other deaths were observed with at least 1 year of heart rhythm continuous monitoring data (mean: 2.1 years, standard deviation: 1.7 years, IQR: 1.0–2.6 years, range: 0.3–5.3 years), which is sufficient to study the amount of AF recurrence, the pattern of AF recurrence and thus the ablation success. Owing to the limited number of patients, the effect of the antiarrhythmic therapy could not be evaluated. Surgical ablation under direct vision in the arrested heart likely results in greater atrial tissue exclusion than endocardial catheter ablation or other forms of epicardial surgical ablation. Therefore, it is unknown if and to what extent the results and conclusions presented here can be transferred to the catheter ablation population. This remains to be seen in larger catheter-based studies employing continuous heart rhythm monitoring.
CONCLUSION
Surgical ablation is an effective modality for the treatment of AF when performed concomitantly with other cardiac surgical procedures. Surgical complications of the ablation procedure were not observed. Continuous rhythm monitoring reveals that short episodes of AF late after surgical ablation are common; however, for the great majority of patients, this translated to very low AF burden during the follow-up. The preoperative duration of AF and the use of a box-lesion ablation set in patients with higher forms of AF persistence were independently associated with higher postoperative AF recurrence and burden. The temporal AF pattern (AF burden and AF density) during the blanking period following the ablation can be considered a prognostic marker for later AF recurrence and for further patient management.
SUPPLEMENTARY MATERIAL
Supplementary material is available at ICVTS online.
ACKNOWLEDGEMENTS
The authors thank Stefan Klotz for proofreading the manuscript.
Conflict of interest: Paul D. Ziegler is an employee and stockholder of Medtronic, Inc. (>10 000 USD). Thorsten Hanke received moderate lecture fees from Medtronic and SJM (<10 000 USD) and is a consultant for Medtronic and SJM (<10 000 USD).
REFERENCES





Comments
© The Author 2015. Published by Oxford University Press on behalf of the European Association for Cardio-Thoracic Surgery. All rights reserved
Atrial fibrillation (AF) is an independent risk factor for death in patients undergoing cardiac surgery [1]. Atrial fibrillation surgery may improve survival. Charitos et al. reported results of patients undergoing concomitant surgical AF ablation during cardiac surgery. Detection of AF recurrence was done via implantable lead-less, continuous heart rhythm recorders (CM). All patients experienced some AF recurrence but patients who underwent surgical AF ablation with high-intensity focused ultrasound (HIFU) demonstrated a significantly higher need of cardioversion. They concluded that concomitant surgical AF ablation is an effective modality for the treatment of AF and continuous rhythm monitoring reveals short episodes of AF in the majority of patients during long-term follow-up. AliveCor heart monitor (AHM; AliveCor Inc, San Francisco, CA, USA) is a smartphone-dependent device that converts electrical signals gathered from finger tips into ultrasound signals. These signals then get transmitted to smartphone's microphone and an electrocardiographic (ECG) lead I is recorded. It is a promising technology, cheap and accurate. AliveCor follow-up has already been validated for post-AF catheter ablation and QT interval monitoring in patients on Dofetilide [2, 3].
In everyday practice, it is not possible to monitor every surgical AF ablation patient with implantable, continuous heart rhythm recorders (CM). But results of this study clearly show additional benefit of CM in these patients. We conclude that rhythm follow-up when the patient is symptomatic or with pre-set intervals may have clinical benefits. In the light of this study, patients who have undergone AF ablation with HIFU are especially appropriate for AliveCor follow-up.
References
[1] Charitos EI, Ziegler PD, Stierle U, Graf B, Sievers HH and Hanke T. Long-term outcomes after surgical ablation for atrial fibrillation in patients with continuous heart rhythm monitoring devices. Interact CardioVasc Thorac Surg 2015; doi:10.1093/icvts/ivv248.
[2] Tarakji KG, Wazni OM, Callahan T, Kanj M, Hakim AH, Wolski K et al. Using a novel wireless system for monitoring patients after the atrial fibrillation ablation procedure: the iTransmit study. Heart Rhythm 2015;12:554-9.
[3] Muhlestein JB. QTC intervals can be assessed with the AliveCor heart monitor in patients on dofetilide for atrial fibrillation. J Electrocardiol 2015;48:10-1.
Conflict of interest: none declared.