-
PDF
- Split View
-
Views
-
Cite
Cite
Tomoaki Suzuki, Tohru Asai, Takeshi Kinoshita, Total arterial off-pump coronary artery bypass grafting was not associated with inferior outcomes for diabetic when compared with non-diabetic patients, Interactive CardioVascular and Thoracic Surgery, Volume 21, Issue 6, December 2015, Pages 705–711, https://doi.org/10.1093/icvts/ivv234
Close - Share Icon Share
Abstract
Diabetes mellitus is a strong risk factor that worsens the clinical outcome of patients undergoing coronary artery bypass grafting (CABG). With the development of the off-pump CABG technique, there is a current trend in revascularization strategies towards in situ all-arterial grafting. We were interested in whether total arterial off-pump CABG could improve the outcome for diabetic patients.
From January 2002 to December 2013, a total of 1064 patients underwent off-pump CABG at our institution, with total arterial reconstruction carried out in 775 cases. Of the 775 patients, 436 had diabetes (DM) and 339 did not (nDM). After propensity score matching, 301 cases from each group were successfully matched.
All procedures were performed via the off-pump technique without conversion to on-pump. Four patients in the DM group and 1 in the nDM group died in hospital. Multivariate analysis revealed that chronic pulmonary disease [odds ratio (OR) 7.04, P = 0.036] and low (<40%) ejection fraction (OR 11.3, P = 0.009) are independent risk factors for hospital death, and advanced age for deep sternal infection (OR 1.07, P = 0.04). Follow-up was completed in 97.7% of the patients to a maximum of 11.9 years. The rate of 10-year freedom from all-cause mortality was 84.2% in the DM and 74.3% in the nDM group (P = 0.45). The corresponding rates for major adverse cardiac event were 93.3 and 86.9% (P = 0.09). Multivariate Cox regression analysis revealed chronic renal failure, older age, cerebrovascular accident, low ejection fraction and urgency as significant predictors of late death. Diabetes was not associated with any long- or short-term outcome.
Total arterial off-pump CABG using skeletonization technique can provide similar clinical outcome for diabetic patients in the long as well as in the short term as that for non-diabetic patients.
INTRODUCTION
Diabetes mellitus is a strong risk factor that worsens the clinical outcome of coronary artery bypass graft (CABG) surgery in the long as well as the short term [1–3]. The reasons are that diabetic patients have more complex, extensive, diffuse, multiple and rapidly progressive coronary vessel disease, and more extra-cardiac comorbidities. It is well known that diabetic patients have a survival benefit with CABG as opposed to percutaneous coronary intervention (PCI) [4]. Off-pump CABG (OPCAB) has now gained acceptance as an advantageous technique that provides particular benefits in high-risk groups including diabetic patients compared with on-pump conventional CABG [5, 6]. Following the development of the OPCAB technique, the current trend in revascularization strategy is towards in situ all-arterial grafting because of the benefits of the aorta non-touch technique and better long-term clinical outcomes. A number of previous studies have shown the excellent effect of arterial conduits, which maintain a high rate of long-term patency and provide improved clinical outcomes [7, 8].
The population of diabetic patients undergoing CABG is steadily increasing worldwide, especially in Japan, and now accounts for up to 40% of all CABG patients. Optimizing the clinical outcome of diabetic patients is a significant challenge in response to which surgeons need to develop the best CABG strategy. The aim of the present study was to investigate whether the clinical outcome for diabetic patients undergoing total arterial OPCAB is different from that of non-diabetic patients.
MATERIALS AND METHODS
Patient population
All patients had previously granted permission and given their informed consent for use of their medical records for research purposes. The Institutional Review Board approved the study. From January 2002 to December 2013, a total of 1064 patients underwent isolated OPCAB at Shiga Medical University Hospital, with total arterial reconstruction carried out in 775 (72.8%). Patients with acute myocardial infarction were included, but patients who had undergone a salvage procedure and patients with single-vessel disease were excluded from the study. We performed OPCAB in all CABG cases with no exclusion criteria; therefore, we only had 4 on-pump cases during this period. Of these 775 patients, 436 had diabetes (DM group) and 339 did not (nDM group). The propensity score was calculated to achieve one-to-one matching pairs with similar clinical characteristics for fair comparison. Logistic regression with backward selection was performed to create the propensity score based on the following 16 characteristics: sex, age, smoking history, body mass index, hypertension, peripheral vascular disease, dyslipidaemia, chronic kidney disease, left main coronary artery disease, chronic obstructive pulmonary disease, previous stroke, previous myocardial infarction, congestive heart failure, previous PCI, low ejection fraction (<40%) and urgent or emergency condition. By comparing the propensity score, 301 pairs were successfully matched in a one-to-one manner. The discriminatory ability of the logistic model as measured by the C-statistic was 0.67 (P < 0.001) and the Hosmer–Lemeshow goodness-of-fit test result was not statistically significant (P = 0.89), indicating, respectively, good discriminative power and acceptable calibration of the model. Clinical results were compared between the propensity-matched groups.
Definitions
Patients with diabetes were identified as those who were previously diagnosed by the physician as requiring treatment with nutritional modification, oral medication and/or insulin at the time of surgery. Patients with no preoperative diagnosis in whom diabetes was discovered (HbA1c ≧6.1%) at the time of surgery were included in the DM group. Postoperative renal failure was defined as the requirement for new temporary haemodialysis. Postoperative stroke was defined as a new neurological event persisting for more than 24 h after onset and was confirmed by computed tomography. Follow-up was achieved by direct communication with the patient, the patient's family or the attending physician. Significant left main coronary artery disease was defined as the left main coronary artery with stenosis greater than 50% assessed visually by the physician performing the coronary angiography.
Surgery details
Our surgical technique has been described previously [9]. All procedures were performed through a median sternotomy. All conduits [one or both internal thoracic arteries (ITAs) and the right gastroepiploic artery (GEA)] were harvested and skeletonized using an ultrasound scalpel (Harmonic Scalpel, Ethicon Endosurgery, Cincinnati, OH, USA). We used bilateral ITAs routinely for two- or three-vessel disease patients who required grafting to the left anterior descending artery and circumflex artery. A common combination for ITA graft placement was in situ grafting of the left ITA to the circumflex area and of the right ITA to the left anterior descending area. We also used the skeletonized GEA proactively to reconstruct the distal right coronary artery as an in situ graft. In this series, the left ITA was used in all (n = 602, 100%), the right ITA in 520 (86.4%) and the right GEA in 422 (70%) patients. We have never used the radial artery. We use a suction-type mechanical stabilizer (Octopus 4.3, Medtronic, Minneapolis, MN, USA) to immobilize the target coronary artery. An intracoronary shunt tube and CO2 blower were used routinely. The distal anastomosis was constructed with a 7-0 polypropylene suture using a standard technique. A red blood cell-saving device was used in all cases.
Statistical analysis methods
Data are presented as the mean ± standard deviation, or the median and interquartile range. Normality was tested using the Shapiro–Wilk test. Categorical variables were analysed using the χ2 or Fisher's exact test, and continuous variables were examined using the t-test or the Mann–Whitney U-test, when appropriate. Univariate and mulitivariate Cox proportional hazard regression analyses were performed for the analysis of late mortality and cardiac events. The multivariate analyses were performed with a stepwise forward regression model in which each variable with a probability value of <0.25 in the univariate analysis was entered in the model. Actuarial survival and event-free survival curves were estimated using the Kaplan–Meier method, comparing differences between groups with the log-rank test. Calculated P-values of <0.05 were considered significant. Data were analysed using SPSS 22.0 (SPSS, Inc., Chicago, IL, USA) for Windows (Microsoft Corp., Redmond, WA, USA).
RESULTS
Short-term results
The preoperative characteristics of unmatched and matched patients are summarized in Table 1. Among 301 matched DM patients, 89 underwent insulin therapy, 140 had oral medication and 72 received no medication (who were discovered at surgery) or diet control. Preoperative patient comorbidities and cardiac characteristics were equally distributed between the two matched groups. Table 2 presents the outcome after surgery for unmatched and matched patients. As might be expected, all cases have undergone total arterial revascularization. In the matched cohort, there was a significant difference in the number of grafts per patient (3.70 ± 1.2 in the DM group vs 3.42 ± 1.1 in the nDM group; P = 0.002). The rates of bilateral ITA (87.4% in the DM vs 85.4% in the nDM, group, P = 0.8) and GEA use (68.4% in the DM vs 71.2% in the nDM group, P = 0.7) were equally distributed in the two groups. The aorta non-touch technique accounted for 99.6% of cases (600/602). There was no significant difference in other morbidities: perioperative myocardial infarction (1 in the DM group vs 2 in the nDM group), reoperation for bleeding (3 vs 3), cerebrovascular accident (5 vs 2), acute renal failure (5 vs 3), prolonged (>24 h) ventilator support for respiratory insufficiency (6 vs 5) and deep sternal infection (5 vs 2). Four patients in the DM group (4/301; 1.3%) died, 1 of low output syndrome, 1 of superior mesenteric artery thrombus, 1 of multisystem organ failure and 1 of rupture of abdominal aortic aneurysm. One patient in the nDM group (1/301; 0.3%) died due to rupture of abdominal aortic aneurysm.
| Characteristics . | Before propensity matching . | After propensity matching . | ||||
|---|---|---|---|---|---|---|
| DM (N = 436) . | nDM (N = 339) . | P-value . | DM (N = 301) . | nDM (N = 301) . | P-value . | |
| Age (median, interquartile) | 67 (61–73) | 67 (59–75) | 0.81 | 67 (61–73) | 67 (59–75) | 0.77 |
| Female gender | 77 (17.6%) | 49 (14.5%) | 0.23 | 52 (17.3%) | 44 (14.6%) | 0.37 |
| Smoking history | 276 (61.9%) | 206 (60.8%) | 0.50 | 192 (63.8%) | 189 (62.8%) | 0.79 |
| Hypertension | 327 (75.0%) | 232 (68.4%) | 0.04 | 221 (73.4%) | 216 (69.8%) | 0.65 |
| Dyslipidaemia | 256 (58.7%) | 183 (54.0%) | 0.19 | 166 (55.1%) | 165 (54.8%) | 0.93 |
| COPD | 85 (19.5%) | 53 (15.6%) | 0.16 | 61 (20.3%) | 47 (15.6%) | 0.14 |
| Peripheral arterial disease | 37 (8.5%) | 28 (8.3%) | 0.91 | 23 (7.6%) | 27 (9.0%) | 0.55 |
| Previous stroke | 53 (12.2%) | 31 (9.1%) | 0.18 | 42 (14.0%) | 30 (10.0%) | 0.13 |
| Chronic renal failure (Cre >1.5) | 55 (12.6%) | 25 (7.4%) | 0.02 | 36 (12.0%) | 25 (8.3%) | 0.14 |
| Congestive heart failure | 147 (33.7%) | 101 (29.8%) | 0.25 | 76 (25.2%) | 89 (30.0%) | 0.23 |
| Previous myocardial infarction | 132 (30.3%) | 100 (29.5%) | 0.81 | 107 (35.5%) | 92 (30.6%) | 0.19 |
| Left main disease | 163 (37.4%) | 137 (40.4%) | 0.05 | 120 (39.9%) | 127 (42.4%) | 0.56 |
| LVEF <40% | 60 (13.8&) | 32 (9.4%) | 0.06 | 42 (14.0%) | 31 (10.3%) | 0.17 |
| Previous PCI | 132 (30.3%) | 104 (30.7%) | 0.90 | 86 (28.6%) | 91 (30.2%) | 0.65 |
| Urgency | 74 (17.0%) | 68 (20.1%) | 0.27 | 58 (19.7%) | 56 (18.6%) | 0.83 |
| BMI (median, interquartile) | 23.7 (21.8–25.5) | 23.7 (21.5–25.8) | 0.59 | 23.8 (21.9–25.5) | 23.8 (2.17–25.8) | 0.70 |
| Characteristics . | Before propensity matching . | After propensity matching . | ||||
|---|---|---|---|---|---|---|
| DM (N = 436) . | nDM (N = 339) . | P-value . | DM (N = 301) . | nDM (N = 301) . | P-value . | |
| Age (median, interquartile) | 67 (61–73) | 67 (59–75) | 0.81 | 67 (61–73) | 67 (59–75) | 0.77 |
| Female gender | 77 (17.6%) | 49 (14.5%) | 0.23 | 52 (17.3%) | 44 (14.6%) | 0.37 |
| Smoking history | 276 (61.9%) | 206 (60.8%) | 0.50 | 192 (63.8%) | 189 (62.8%) | 0.79 |
| Hypertension | 327 (75.0%) | 232 (68.4%) | 0.04 | 221 (73.4%) | 216 (69.8%) | 0.65 |
| Dyslipidaemia | 256 (58.7%) | 183 (54.0%) | 0.19 | 166 (55.1%) | 165 (54.8%) | 0.93 |
| COPD | 85 (19.5%) | 53 (15.6%) | 0.16 | 61 (20.3%) | 47 (15.6%) | 0.14 |
| Peripheral arterial disease | 37 (8.5%) | 28 (8.3%) | 0.91 | 23 (7.6%) | 27 (9.0%) | 0.55 |
| Previous stroke | 53 (12.2%) | 31 (9.1%) | 0.18 | 42 (14.0%) | 30 (10.0%) | 0.13 |
| Chronic renal failure (Cre >1.5) | 55 (12.6%) | 25 (7.4%) | 0.02 | 36 (12.0%) | 25 (8.3%) | 0.14 |
| Congestive heart failure | 147 (33.7%) | 101 (29.8%) | 0.25 | 76 (25.2%) | 89 (30.0%) | 0.23 |
| Previous myocardial infarction | 132 (30.3%) | 100 (29.5%) | 0.81 | 107 (35.5%) | 92 (30.6%) | 0.19 |
| Left main disease | 163 (37.4%) | 137 (40.4%) | 0.05 | 120 (39.9%) | 127 (42.4%) | 0.56 |
| LVEF <40% | 60 (13.8&) | 32 (9.4%) | 0.06 | 42 (14.0%) | 31 (10.3%) | 0.17 |
| Previous PCI | 132 (30.3%) | 104 (30.7%) | 0.90 | 86 (28.6%) | 91 (30.2%) | 0.65 |
| Urgency | 74 (17.0%) | 68 (20.1%) | 0.27 | 58 (19.7%) | 56 (18.6%) | 0.83 |
| BMI (median, interquartile) | 23.7 (21.8–25.5) | 23.7 (21.5–25.8) | 0.59 | 23.8 (21.9–25.5) | 23.8 (2.17–25.8) | 0.70 |
Cre: serum creatinine; COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; PCI: percutaneous coronary intervention; BMI: body mass index; DM: diabetic patients; nDM: non-diabetic patients.
| Characteristics . | Before propensity matching . | After propensity matching . | ||||
|---|---|---|---|---|---|---|
| DM (N = 436) . | nDM (N = 339) . | P-value . | DM (N = 301) . | nDM (N = 301) . | P-value . | |
| Age (median, interquartile) | 67 (61–73) | 67 (59–75) | 0.81 | 67 (61–73) | 67 (59–75) | 0.77 |
| Female gender | 77 (17.6%) | 49 (14.5%) | 0.23 | 52 (17.3%) | 44 (14.6%) | 0.37 |
| Smoking history | 276 (61.9%) | 206 (60.8%) | 0.50 | 192 (63.8%) | 189 (62.8%) | 0.79 |
| Hypertension | 327 (75.0%) | 232 (68.4%) | 0.04 | 221 (73.4%) | 216 (69.8%) | 0.65 |
| Dyslipidaemia | 256 (58.7%) | 183 (54.0%) | 0.19 | 166 (55.1%) | 165 (54.8%) | 0.93 |
| COPD | 85 (19.5%) | 53 (15.6%) | 0.16 | 61 (20.3%) | 47 (15.6%) | 0.14 |
| Peripheral arterial disease | 37 (8.5%) | 28 (8.3%) | 0.91 | 23 (7.6%) | 27 (9.0%) | 0.55 |
| Previous stroke | 53 (12.2%) | 31 (9.1%) | 0.18 | 42 (14.0%) | 30 (10.0%) | 0.13 |
| Chronic renal failure (Cre >1.5) | 55 (12.6%) | 25 (7.4%) | 0.02 | 36 (12.0%) | 25 (8.3%) | 0.14 |
| Congestive heart failure | 147 (33.7%) | 101 (29.8%) | 0.25 | 76 (25.2%) | 89 (30.0%) | 0.23 |
| Previous myocardial infarction | 132 (30.3%) | 100 (29.5%) | 0.81 | 107 (35.5%) | 92 (30.6%) | 0.19 |
| Left main disease | 163 (37.4%) | 137 (40.4%) | 0.05 | 120 (39.9%) | 127 (42.4%) | 0.56 |
| LVEF <40% | 60 (13.8&) | 32 (9.4%) | 0.06 | 42 (14.0%) | 31 (10.3%) | 0.17 |
| Previous PCI | 132 (30.3%) | 104 (30.7%) | 0.90 | 86 (28.6%) | 91 (30.2%) | 0.65 |
| Urgency | 74 (17.0%) | 68 (20.1%) | 0.27 | 58 (19.7%) | 56 (18.6%) | 0.83 |
| BMI (median, interquartile) | 23.7 (21.8–25.5) | 23.7 (21.5–25.8) | 0.59 | 23.8 (21.9–25.5) | 23.8 (2.17–25.8) | 0.70 |
| Characteristics . | Before propensity matching . | After propensity matching . | ||||
|---|---|---|---|---|---|---|
| DM (N = 436) . | nDM (N = 339) . | P-value . | DM (N = 301) . | nDM (N = 301) . | P-value . | |
| Age (median, interquartile) | 67 (61–73) | 67 (59–75) | 0.81 | 67 (61–73) | 67 (59–75) | 0.77 |
| Female gender | 77 (17.6%) | 49 (14.5%) | 0.23 | 52 (17.3%) | 44 (14.6%) | 0.37 |
| Smoking history | 276 (61.9%) | 206 (60.8%) | 0.50 | 192 (63.8%) | 189 (62.8%) | 0.79 |
| Hypertension | 327 (75.0%) | 232 (68.4%) | 0.04 | 221 (73.4%) | 216 (69.8%) | 0.65 |
| Dyslipidaemia | 256 (58.7%) | 183 (54.0%) | 0.19 | 166 (55.1%) | 165 (54.8%) | 0.93 |
| COPD | 85 (19.5%) | 53 (15.6%) | 0.16 | 61 (20.3%) | 47 (15.6%) | 0.14 |
| Peripheral arterial disease | 37 (8.5%) | 28 (8.3%) | 0.91 | 23 (7.6%) | 27 (9.0%) | 0.55 |
| Previous stroke | 53 (12.2%) | 31 (9.1%) | 0.18 | 42 (14.0%) | 30 (10.0%) | 0.13 |
| Chronic renal failure (Cre >1.5) | 55 (12.6%) | 25 (7.4%) | 0.02 | 36 (12.0%) | 25 (8.3%) | 0.14 |
| Congestive heart failure | 147 (33.7%) | 101 (29.8%) | 0.25 | 76 (25.2%) | 89 (30.0%) | 0.23 |
| Previous myocardial infarction | 132 (30.3%) | 100 (29.5%) | 0.81 | 107 (35.5%) | 92 (30.6%) | 0.19 |
| Left main disease | 163 (37.4%) | 137 (40.4%) | 0.05 | 120 (39.9%) | 127 (42.4%) | 0.56 |
| LVEF <40% | 60 (13.8&) | 32 (9.4%) | 0.06 | 42 (14.0%) | 31 (10.3%) | 0.17 |
| Previous PCI | 132 (30.3%) | 104 (30.7%) | 0.90 | 86 (28.6%) | 91 (30.2%) | 0.65 |
| Urgency | 74 (17.0%) | 68 (20.1%) | 0.27 | 58 (19.7%) | 56 (18.6%) | 0.83 |
| BMI (median, interquartile) | 23.7 (21.8–25.5) | 23.7 (21.5–25.8) | 0.59 | 23.8 (21.9–25.5) | 23.8 (2.17–25.8) | 0.70 |
Cre: serum creatinine; COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; PCI: percutaneous coronary intervention; BMI: body mass index; DM: diabetic patients; nDM: non-diabetic patients.
| . | Before propensity matching . | After propensity matching . | ||||
|---|---|---|---|---|---|---|
| DM (N = 436) . | nDM (N = 339) . | P-value . | DM (N = 301) . | nDM (N = 301) . | P-value . | |
| No. of distal anastomoses | 3.63 ± 1.2 | 3.31 ± 1.0 | 0.002 | 3.70 ± 1.2 | 3.41 ± 1.1 | 0.002 |
| Complete revascularization | 430 (98.6%) | 333 (98.2%) | 0.64 | 297 (98.7%) | 297 (98.7%) | 1.0 |
| BITA use | 387 (88.8%) | 294 (86.7%) | 0.39 | 263 (87.4%) | 257 (85.4%) | 0.81 |
| GEA use | 284 (65.1%) | 235 (69.3%) | 0.22 | 206 (68.4%) | 216 (69.8%) | 0.69 |
| All-arterial reconstruction | 436 (100%) | 339 (100%) | 1.0 | 301 (100%) | 301 (100%) | 1.0 |
| Prolonged ventilation (>24 h) | 10 (2.3%) | 7 (2.1%) | 0.83 | 6 (2.0%) | 5 (1.7%) | 0.78 |
| ICU stay (median, h) | 18 (16–20) | 18 (16–20) | 0.69 | 18 (16–20) | 18 (16–20) | 0.91 |
| Reoperation for bleeding | 3 (0.7%) | 3 (0.9%) | 0.52 | 3 (1.0%) | 3 (1.0%) | 1.0 |
| Deep sternal infection | 9 (2.1%) | 2 (0.6%) | 0.08 | 5 (1.7%) | 2 (0.7%) | 0.23 |
| Permanent stroke | 7 (1.6%) | 2 (0.6%) | 0.18 | 5 (1.7%) | 2 (0.7%) | 0.34 |
| Perioperative myocardial infarction | 2 (0.5%) | 2 (0.6%) | 0.58 | 1 (0.3%) | 2 (0.7%) | 0.50 |
| Atrial fibrillation | 51 (11.7%) | 57 (16.8%) | 0.04 | 41 (13.6%) | 53 (17.6%) | 0.11 |
| Renal failure requiring dialysis | 10 (2.3%) | 3 (0.9%) | 0.12 | 5 (1.7%) | 3 (1.0%) | 0.73 |
| Mortality (hospital) | 7 (1.6%) | 1 (0.3%) | 0.02 | 4 (1.3%) | 1 (0.3%) | 0.19 |
| . | Before propensity matching . | After propensity matching . | ||||
|---|---|---|---|---|---|---|
| DM (N = 436) . | nDM (N = 339) . | P-value . | DM (N = 301) . | nDM (N = 301) . | P-value . | |
| No. of distal anastomoses | 3.63 ± 1.2 | 3.31 ± 1.0 | 0.002 | 3.70 ± 1.2 | 3.41 ± 1.1 | 0.002 |
| Complete revascularization | 430 (98.6%) | 333 (98.2%) | 0.64 | 297 (98.7%) | 297 (98.7%) | 1.0 |
| BITA use | 387 (88.8%) | 294 (86.7%) | 0.39 | 263 (87.4%) | 257 (85.4%) | 0.81 |
| GEA use | 284 (65.1%) | 235 (69.3%) | 0.22 | 206 (68.4%) | 216 (69.8%) | 0.69 |
| All-arterial reconstruction | 436 (100%) | 339 (100%) | 1.0 | 301 (100%) | 301 (100%) | 1.0 |
| Prolonged ventilation (>24 h) | 10 (2.3%) | 7 (2.1%) | 0.83 | 6 (2.0%) | 5 (1.7%) | 0.78 |
| ICU stay (median, h) | 18 (16–20) | 18 (16–20) | 0.69 | 18 (16–20) | 18 (16–20) | 0.91 |
| Reoperation for bleeding | 3 (0.7%) | 3 (0.9%) | 0.52 | 3 (1.0%) | 3 (1.0%) | 1.0 |
| Deep sternal infection | 9 (2.1%) | 2 (0.6%) | 0.08 | 5 (1.7%) | 2 (0.7%) | 0.23 |
| Permanent stroke | 7 (1.6%) | 2 (0.6%) | 0.18 | 5 (1.7%) | 2 (0.7%) | 0.34 |
| Perioperative myocardial infarction | 2 (0.5%) | 2 (0.6%) | 0.58 | 1 (0.3%) | 2 (0.7%) | 0.50 |
| Atrial fibrillation | 51 (11.7%) | 57 (16.8%) | 0.04 | 41 (13.6%) | 53 (17.6%) | 0.11 |
| Renal failure requiring dialysis | 10 (2.3%) | 3 (0.9%) | 0.12 | 5 (1.7%) | 3 (1.0%) | 0.73 |
| Mortality (hospital) | 7 (1.6%) | 1 (0.3%) | 0.02 | 4 (1.3%) | 1 (0.3%) | 0.19 |
BITA: bilateral internal thoracic arteries; GEA: gastroepiploic artery; ICU: intensive care unit; DM: diabetic patients; nDM: non-diabetic patients.
| . | Before propensity matching . | After propensity matching . | ||||
|---|---|---|---|---|---|---|
| DM (N = 436) . | nDM (N = 339) . | P-value . | DM (N = 301) . | nDM (N = 301) . | P-value . | |
| No. of distal anastomoses | 3.63 ± 1.2 | 3.31 ± 1.0 | 0.002 | 3.70 ± 1.2 | 3.41 ± 1.1 | 0.002 |
| Complete revascularization | 430 (98.6%) | 333 (98.2%) | 0.64 | 297 (98.7%) | 297 (98.7%) | 1.0 |
| BITA use | 387 (88.8%) | 294 (86.7%) | 0.39 | 263 (87.4%) | 257 (85.4%) | 0.81 |
| GEA use | 284 (65.1%) | 235 (69.3%) | 0.22 | 206 (68.4%) | 216 (69.8%) | 0.69 |
| All-arterial reconstruction | 436 (100%) | 339 (100%) | 1.0 | 301 (100%) | 301 (100%) | 1.0 |
| Prolonged ventilation (>24 h) | 10 (2.3%) | 7 (2.1%) | 0.83 | 6 (2.0%) | 5 (1.7%) | 0.78 |
| ICU stay (median, h) | 18 (16–20) | 18 (16–20) | 0.69 | 18 (16–20) | 18 (16–20) | 0.91 |
| Reoperation for bleeding | 3 (0.7%) | 3 (0.9%) | 0.52 | 3 (1.0%) | 3 (1.0%) | 1.0 |
| Deep sternal infection | 9 (2.1%) | 2 (0.6%) | 0.08 | 5 (1.7%) | 2 (0.7%) | 0.23 |
| Permanent stroke | 7 (1.6%) | 2 (0.6%) | 0.18 | 5 (1.7%) | 2 (0.7%) | 0.34 |
| Perioperative myocardial infarction | 2 (0.5%) | 2 (0.6%) | 0.58 | 1 (0.3%) | 2 (0.7%) | 0.50 |
| Atrial fibrillation | 51 (11.7%) | 57 (16.8%) | 0.04 | 41 (13.6%) | 53 (17.6%) | 0.11 |
| Renal failure requiring dialysis | 10 (2.3%) | 3 (0.9%) | 0.12 | 5 (1.7%) | 3 (1.0%) | 0.73 |
| Mortality (hospital) | 7 (1.6%) | 1 (0.3%) | 0.02 | 4 (1.3%) | 1 (0.3%) | 0.19 |
| . | Before propensity matching . | After propensity matching . | ||||
|---|---|---|---|---|---|---|
| DM (N = 436) . | nDM (N = 339) . | P-value . | DM (N = 301) . | nDM (N = 301) . | P-value . | |
| No. of distal anastomoses | 3.63 ± 1.2 | 3.31 ± 1.0 | 0.002 | 3.70 ± 1.2 | 3.41 ± 1.1 | 0.002 |
| Complete revascularization | 430 (98.6%) | 333 (98.2%) | 0.64 | 297 (98.7%) | 297 (98.7%) | 1.0 |
| BITA use | 387 (88.8%) | 294 (86.7%) | 0.39 | 263 (87.4%) | 257 (85.4%) | 0.81 |
| GEA use | 284 (65.1%) | 235 (69.3%) | 0.22 | 206 (68.4%) | 216 (69.8%) | 0.69 |
| All-arterial reconstruction | 436 (100%) | 339 (100%) | 1.0 | 301 (100%) | 301 (100%) | 1.0 |
| Prolonged ventilation (>24 h) | 10 (2.3%) | 7 (2.1%) | 0.83 | 6 (2.0%) | 5 (1.7%) | 0.78 |
| ICU stay (median, h) | 18 (16–20) | 18 (16–20) | 0.69 | 18 (16–20) | 18 (16–20) | 0.91 |
| Reoperation for bleeding | 3 (0.7%) | 3 (0.9%) | 0.52 | 3 (1.0%) | 3 (1.0%) | 1.0 |
| Deep sternal infection | 9 (2.1%) | 2 (0.6%) | 0.08 | 5 (1.7%) | 2 (0.7%) | 0.23 |
| Permanent stroke | 7 (1.6%) | 2 (0.6%) | 0.18 | 5 (1.7%) | 2 (0.7%) | 0.34 |
| Perioperative myocardial infarction | 2 (0.5%) | 2 (0.6%) | 0.58 | 1 (0.3%) | 2 (0.7%) | 0.50 |
| Atrial fibrillation | 51 (11.7%) | 57 (16.8%) | 0.04 | 41 (13.6%) | 53 (17.6%) | 0.11 |
| Renal failure requiring dialysis | 10 (2.3%) | 3 (0.9%) | 0.12 | 5 (1.7%) | 3 (1.0%) | 0.73 |
| Mortality (hospital) | 7 (1.6%) | 1 (0.3%) | 0.02 | 4 (1.3%) | 1 (0.3%) | 0.19 |
BITA: bilateral internal thoracic arteries; GEA: gastroepiploic artery; ICU: intensive care unit; DM: diabetic patients; nDM: non-diabetic patients.
Multivariate logistic regression analysis revealed that chronic obstructive pulmonary disease [odds ratio (OR) 7.0, 95% CI 1.14–43.4, P = 0.036] and low ejection fraction (OR 11.3, 95% CI 1.8–71.4, P = 0.009) were independent risk factors for early death, and older age for deep sternal infection (OR 1.07, 95% CI 1.001–1.151, P = 0.04). Diabetes mellitus was not an independent risk factor for either early death or deep sternal infection.
Long-term results
In matched patients, follow-up was completed in 97.7% (588/602) of the patients and the mean follow-up duration was 4.4 ± 3.0 years (maximum 11.9 years). In unmatched patients, overall survival rates at 1, 5 and 10 years were significantly different for DM (96.5, 85.7 and 78.9%) and nDM (98.3, 93.3 and 93.3%) patients (P = 0.04). However, after propensity score matching, they were similar between the two groups (96.8, 89.0 and 84.2% vs 98.2, 92.8 and 74.3%, P = 0.45; Fig. 1A and B). The rates of freedom from the combined endpoint of cardiac death, myocardial infarction, angina pectoris, repeat coronary intervention and heart failure requiring admission treatment at 1, 5 and 10 years were 97.9, 88.6 and 88.6% for unmatched DM patients, and 99.3, 92.5 and 84.4% for unmatched nDM patients, respectively, without significant difference (P = 0.59). Similarly, in matched patients, they were 98.5, 93.3 and 93.3% for DM patients and 96.5, 90.3 and 86.9% for nDM patients, respectively (P = 0.09; Fig. 2A and B). Multivariate Cox proportional hazard regression analysis showed that independent predictors of long-term death from all causes were older age [hazard ratio (HR) 1.1, 95% CI 1.05–1.13, P < 0.001], history of cerebrovascular accident (HR 2.1, 95% CI 1.04–4.35, P = 0.04), chronic kidney disease (HR 2.8, 95% CI 1.4–5.7, P = 0.004), lower ejection fraction (HR 3.4, 95% CI 1.8–6.4, P < 0.001) and urgency status (HR 2.0, 95% CI 1.04–4.0, P = 0.04). Otherwise, there was no statistically significant independent predictor of cardiac events (cardiac death, myocardial infarction, angina pectoris, repeat coronary intervention and heart failure) in the multivariate analysis. Diabetes mellitus did not affect any outcome in the long term as well (Tables 3 and 4).
| Variable . | P-value . | Odds ratio . | 95% confidence interval . |
|---|---|---|---|
| Hospital death | |||
| Diabetes mellitus | 0.37 | ||
| Older age | 0.90 | ||
| Female sex | 0.66 | ||
| Smoking | 0.65 | ||
| Hypertension | 0.61 | ||
| Dyslipidaemia | 0.77 | ||
| COPD | 0.03 | 7.0 | 1.13–43.5 |
| Peripheral vascular disease | 0.26 | ||
| Previous stroke | 0.34 | ||
| Chronic renal failure | 0.06 | ||
| Congestive heart failure | 0.82 | ||
| Perioperative myocardial infarction | 0.86 | ||
| LVEF <40% | <0.01 | 11.3 | 1.82–71.4 |
| Left main disease | 0.16 | ||
| Previous PCI | 0.68 | ||
| Urgency | 0.73 | ||
| Body mass index | 0.05 | ||
| Atrial fibrillation | 0.48 | ||
| BITA use | 0.65 | ||
| GEA use | 0.41 | ||
| Deep sternal infection | |||
| Diabetes mellitus | 0.20 | ||
| Older age | 0.02 | 1.1 | 1.01–1.17 |
| Female sex | 0.32 | ||
| Smoking | 0.29 | ||
| Hypertension | 0.96 | ||
| Dyslipidaemia | 0.69 | ||
| COPD | 0.33 | ||
| Peripheral vascular disease | 0.28 | ||
| Previous stroke | 0.13 | ||
| Chronic renal failure | 0.22 | ||
| Congestive heart failure | 0.82 | ||
| Perioperative myocardial infarction | 0.63 | ||
| LVEF <40% | 0.21 | ||
| Left main disease | 0.67 | ||
| Previous PCI | 0.43 | ||
| Urgency | 0.16 | ||
| Body mass index | 0.91 | ||
| Atrial fibrillation | 0.16 | ||
| BITA use | 0.20 | ||
| GEA use | 0.96 | ||
| Variable . | P-value . | Odds ratio . | 95% confidence interval . |
|---|---|---|---|
| Hospital death | |||
| Diabetes mellitus | 0.37 | ||
| Older age | 0.90 | ||
| Female sex | 0.66 | ||
| Smoking | 0.65 | ||
| Hypertension | 0.61 | ||
| Dyslipidaemia | 0.77 | ||
| COPD | 0.03 | 7.0 | 1.13–43.5 |
| Peripheral vascular disease | 0.26 | ||
| Previous stroke | 0.34 | ||
| Chronic renal failure | 0.06 | ||
| Congestive heart failure | 0.82 | ||
| Perioperative myocardial infarction | 0.86 | ||
| LVEF <40% | <0.01 | 11.3 | 1.82–71.4 |
| Left main disease | 0.16 | ||
| Previous PCI | 0.68 | ||
| Urgency | 0.73 | ||
| Body mass index | 0.05 | ||
| Atrial fibrillation | 0.48 | ||
| BITA use | 0.65 | ||
| GEA use | 0.41 | ||
| Deep sternal infection | |||
| Diabetes mellitus | 0.20 | ||
| Older age | 0.02 | 1.1 | 1.01–1.17 |
| Female sex | 0.32 | ||
| Smoking | 0.29 | ||
| Hypertension | 0.96 | ||
| Dyslipidaemia | 0.69 | ||
| COPD | 0.33 | ||
| Peripheral vascular disease | 0.28 | ||
| Previous stroke | 0.13 | ||
| Chronic renal failure | 0.22 | ||
| Congestive heart failure | 0.82 | ||
| Perioperative myocardial infarction | 0.63 | ||
| LVEF <40% | 0.21 | ||
| Left main disease | 0.67 | ||
| Previous PCI | 0.43 | ||
| Urgency | 0.16 | ||
| Body mass index | 0.91 | ||
| Atrial fibrillation | 0.16 | ||
| BITA use | 0.20 | ||
| GEA use | 0.96 | ||
COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; PCI: percutaneous coronary intervention; BITA: bilateral internal thoracic arteries; GEA: gastroepiploic artery.
| Variable . | P-value . | Odds ratio . | 95% confidence interval . |
|---|---|---|---|
| Hospital death | |||
| Diabetes mellitus | 0.37 | ||
| Older age | 0.90 | ||
| Female sex | 0.66 | ||
| Smoking | 0.65 | ||
| Hypertension | 0.61 | ||
| Dyslipidaemia | 0.77 | ||
| COPD | 0.03 | 7.0 | 1.13–43.5 |
| Peripheral vascular disease | 0.26 | ||
| Previous stroke | 0.34 | ||
| Chronic renal failure | 0.06 | ||
| Congestive heart failure | 0.82 | ||
| Perioperative myocardial infarction | 0.86 | ||
| LVEF <40% | <0.01 | 11.3 | 1.82–71.4 |
| Left main disease | 0.16 | ||
| Previous PCI | 0.68 | ||
| Urgency | 0.73 | ||
| Body mass index | 0.05 | ||
| Atrial fibrillation | 0.48 | ||
| BITA use | 0.65 | ||
| GEA use | 0.41 | ||
| Deep sternal infection | |||
| Diabetes mellitus | 0.20 | ||
| Older age | 0.02 | 1.1 | 1.01–1.17 |
| Female sex | 0.32 | ||
| Smoking | 0.29 | ||
| Hypertension | 0.96 | ||
| Dyslipidaemia | 0.69 | ||
| COPD | 0.33 | ||
| Peripheral vascular disease | 0.28 | ||
| Previous stroke | 0.13 | ||
| Chronic renal failure | 0.22 | ||
| Congestive heart failure | 0.82 | ||
| Perioperative myocardial infarction | 0.63 | ||
| LVEF <40% | 0.21 | ||
| Left main disease | 0.67 | ||
| Previous PCI | 0.43 | ||
| Urgency | 0.16 | ||
| Body mass index | 0.91 | ||
| Atrial fibrillation | 0.16 | ||
| BITA use | 0.20 | ||
| GEA use | 0.96 | ||
| Variable . | P-value . | Odds ratio . | 95% confidence interval . |
|---|---|---|---|
| Hospital death | |||
| Diabetes mellitus | 0.37 | ||
| Older age | 0.90 | ||
| Female sex | 0.66 | ||
| Smoking | 0.65 | ||
| Hypertension | 0.61 | ||
| Dyslipidaemia | 0.77 | ||
| COPD | 0.03 | 7.0 | 1.13–43.5 |
| Peripheral vascular disease | 0.26 | ||
| Previous stroke | 0.34 | ||
| Chronic renal failure | 0.06 | ||
| Congestive heart failure | 0.82 | ||
| Perioperative myocardial infarction | 0.86 | ||
| LVEF <40% | <0.01 | 11.3 | 1.82–71.4 |
| Left main disease | 0.16 | ||
| Previous PCI | 0.68 | ||
| Urgency | 0.73 | ||
| Body mass index | 0.05 | ||
| Atrial fibrillation | 0.48 | ||
| BITA use | 0.65 | ||
| GEA use | 0.41 | ||
| Deep sternal infection | |||
| Diabetes mellitus | 0.20 | ||
| Older age | 0.02 | 1.1 | 1.01–1.17 |
| Female sex | 0.32 | ||
| Smoking | 0.29 | ||
| Hypertension | 0.96 | ||
| Dyslipidaemia | 0.69 | ||
| COPD | 0.33 | ||
| Peripheral vascular disease | 0.28 | ||
| Previous stroke | 0.13 | ||
| Chronic renal failure | 0.22 | ||
| Congestive heart failure | 0.82 | ||
| Perioperative myocardial infarction | 0.63 | ||
| LVEF <40% | 0.21 | ||
| Left main disease | 0.67 | ||
| Previous PCI | 0.43 | ||
| Urgency | 0.16 | ||
| Body mass index | 0.91 | ||
| Atrial fibrillation | 0.16 | ||
| BITA use | 0.20 | ||
| GEA use | 0.96 | ||
COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; PCI: percutaneous coronary intervention; BITA: bilateral internal thoracic arteries; GEA: gastroepiploic artery.
Multivariate Cox proportional hazard regression analyses of long-term outcomes
| Variable . | P-value . | Hazard ratio . | 95% Confidence interval . |
|---|---|---|---|
| Death from all causes | |||
| Diabetes mellitus | 0.86 | ||
| Older age | <0.001 | 1.1 | 1.05–1.13 |
| Female sex | 0.84 | ||
| Smoking | 0.15 | ||
| Hypertension | 0.40 | ||
| Dyslipidaemia | 0.28 | ||
| COPD | 0.08 | ||
| Peripheral vascular disease | 0.14 | ||
| Previous stroke | 0.039 | 2.1 | 1.03–4.35 |
| Chronic renal failure | 0.004 | 2.8 | 1.38–5.71 |
| Congestive heart failure | 0.25 | ||
| Perioperative myocardial infarction | 0.86 | ||
| LVEF <40% | <0.001 | 3.4 | 1.78–6.41 |
| Left main disease | 0.03 | 2.1 | 1.10–4.03 |
| Previous PCI | 0.27 | ||
| Urgency | 0.04 | 2.0 | 1.04–3.98 |
| Body mass index | 0.33 | ||
| Atrial fibrillation | 0.77 | ||
| BITA use | 0.85 | ||
| GEA use | 0.07 | ||
| Cardiac event: cardiac death, angina, heart failure, coronary intervention and acute myocardial infarction | |||
| Diabetes mellitus | 0.09 | ||
| Older age | 0.71 | ||
| Female sex | 0.64 | ||
| Smoking | 0.36 | ||
| Hypertension | 0.39 | ||
| Dyslipidaemia | 0.78 | ||
| COPD | 0.08 | ||
| Peripheral vascular disease | 0.24 | ||
| Previous stroke | 0.26 | ||
| Chronic renal failure | 0.44 | ||
| Congestive heart failure | 0.30 | ||
| Perioperative myocardial infarction | 0.46 | ||
| LVEF <40% | 0.51 | ||
| Left main disease | 0.85 | ||
| Previous PCI | 0.70 | ||
| Urgency | 0.62 | ||
| Body mass index | 0.26 | ||
| Atrial fibrillation | 0.48 | ||
| BITA use | 0.48 | ||
| GEA use | 0.10 | ||
| Variable . | P-value . | Hazard ratio . | 95% Confidence interval . |
|---|---|---|---|
| Death from all causes | |||
| Diabetes mellitus | 0.86 | ||
| Older age | <0.001 | 1.1 | 1.05–1.13 |
| Female sex | 0.84 | ||
| Smoking | 0.15 | ||
| Hypertension | 0.40 | ||
| Dyslipidaemia | 0.28 | ||
| COPD | 0.08 | ||
| Peripheral vascular disease | 0.14 | ||
| Previous stroke | 0.039 | 2.1 | 1.03–4.35 |
| Chronic renal failure | 0.004 | 2.8 | 1.38–5.71 |
| Congestive heart failure | 0.25 | ||
| Perioperative myocardial infarction | 0.86 | ||
| LVEF <40% | <0.001 | 3.4 | 1.78–6.41 |
| Left main disease | 0.03 | 2.1 | 1.10–4.03 |
| Previous PCI | 0.27 | ||
| Urgency | 0.04 | 2.0 | 1.04–3.98 |
| Body mass index | 0.33 | ||
| Atrial fibrillation | 0.77 | ||
| BITA use | 0.85 | ||
| GEA use | 0.07 | ||
| Cardiac event: cardiac death, angina, heart failure, coronary intervention and acute myocardial infarction | |||
| Diabetes mellitus | 0.09 | ||
| Older age | 0.71 | ||
| Female sex | 0.64 | ||
| Smoking | 0.36 | ||
| Hypertension | 0.39 | ||
| Dyslipidaemia | 0.78 | ||
| COPD | 0.08 | ||
| Peripheral vascular disease | 0.24 | ||
| Previous stroke | 0.26 | ||
| Chronic renal failure | 0.44 | ||
| Congestive heart failure | 0.30 | ||
| Perioperative myocardial infarction | 0.46 | ||
| LVEF <40% | 0.51 | ||
| Left main disease | 0.85 | ||
| Previous PCI | 0.70 | ||
| Urgency | 0.62 | ||
| Body mass index | 0.26 | ||
| Atrial fibrillation | 0.48 | ||
| BITA use | 0.48 | ||
| GEA use | 0.10 | ||
COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; PCI: percutaneous coronary intervention; BITA: bilateral internal thoracic arteries; GEA: gastroepiploic artery.
Multivariate Cox proportional hazard regression analyses of long-term outcomes
| Variable . | P-value . | Hazard ratio . | 95% Confidence interval . |
|---|---|---|---|
| Death from all causes | |||
| Diabetes mellitus | 0.86 | ||
| Older age | <0.001 | 1.1 | 1.05–1.13 |
| Female sex | 0.84 | ||
| Smoking | 0.15 | ||
| Hypertension | 0.40 | ||
| Dyslipidaemia | 0.28 | ||
| COPD | 0.08 | ||
| Peripheral vascular disease | 0.14 | ||
| Previous stroke | 0.039 | 2.1 | 1.03–4.35 |
| Chronic renal failure | 0.004 | 2.8 | 1.38–5.71 |
| Congestive heart failure | 0.25 | ||
| Perioperative myocardial infarction | 0.86 | ||
| LVEF <40% | <0.001 | 3.4 | 1.78–6.41 |
| Left main disease | 0.03 | 2.1 | 1.10–4.03 |
| Previous PCI | 0.27 | ||
| Urgency | 0.04 | 2.0 | 1.04–3.98 |
| Body mass index | 0.33 | ||
| Atrial fibrillation | 0.77 | ||
| BITA use | 0.85 | ||
| GEA use | 0.07 | ||
| Cardiac event: cardiac death, angina, heart failure, coronary intervention and acute myocardial infarction | |||
| Diabetes mellitus | 0.09 | ||
| Older age | 0.71 | ||
| Female sex | 0.64 | ||
| Smoking | 0.36 | ||
| Hypertension | 0.39 | ||
| Dyslipidaemia | 0.78 | ||
| COPD | 0.08 | ||
| Peripheral vascular disease | 0.24 | ||
| Previous stroke | 0.26 | ||
| Chronic renal failure | 0.44 | ||
| Congestive heart failure | 0.30 | ||
| Perioperative myocardial infarction | 0.46 | ||
| LVEF <40% | 0.51 | ||
| Left main disease | 0.85 | ||
| Previous PCI | 0.70 | ||
| Urgency | 0.62 | ||
| Body mass index | 0.26 | ||
| Atrial fibrillation | 0.48 | ||
| BITA use | 0.48 | ||
| GEA use | 0.10 | ||
| Variable . | P-value . | Hazard ratio . | 95% Confidence interval . |
|---|---|---|---|
| Death from all causes | |||
| Diabetes mellitus | 0.86 | ||
| Older age | <0.001 | 1.1 | 1.05–1.13 |
| Female sex | 0.84 | ||
| Smoking | 0.15 | ||
| Hypertension | 0.40 | ||
| Dyslipidaemia | 0.28 | ||
| COPD | 0.08 | ||
| Peripheral vascular disease | 0.14 | ||
| Previous stroke | 0.039 | 2.1 | 1.03–4.35 |
| Chronic renal failure | 0.004 | 2.8 | 1.38–5.71 |
| Congestive heart failure | 0.25 | ||
| Perioperative myocardial infarction | 0.86 | ||
| LVEF <40% | <0.001 | 3.4 | 1.78–6.41 |
| Left main disease | 0.03 | 2.1 | 1.10–4.03 |
| Previous PCI | 0.27 | ||
| Urgency | 0.04 | 2.0 | 1.04–3.98 |
| Body mass index | 0.33 | ||
| Atrial fibrillation | 0.77 | ||
| BITA use | 0.85 | ||
| GEA use | 0.07 | ||
| Cardiac event: cardiac death, angina, heart failure, coronary intervention and acute myocardial infarction | |||
| Diabetes mellitus | 0.09 | ||
| Older age | 0.71 | ||
| Female sex | 0.64 | ||
| Smoking | 0.36 | ||
| Hypertension | 0.39 | ||
| Dyslipidaemia | 0.78 | ||
| COPD | 0.08 | ||
| Peripheral vascular disease | 0.24 | ||
| Previous stroke | 0.26 | ||
| Chronic renal failure | 0.44 | ||
| Congestive heart failure | 0.30 | ||
| Perioperative myocardial infarction | 0.46 | ||
| LVEF <40% | 0.51 | ||
| Left main disease | 0.85 | ||
| Previous PCI | 0.70 | ||
| Urgency | 0.62 | ||
| Body mass index | 0.26 | ||
| Atrial fibrillation | 0.48 | ||
| BITA use | 0.48 | ||
| GEA use | 0.10 | ||
COPD: chronic obstructive pulmonary disease; LVEF: left ventricular ejection fraction; PCI: percutaneous coronary intervention; BITA: bilateral internal thoracic arteries; GEA: gastroepiploic artery.
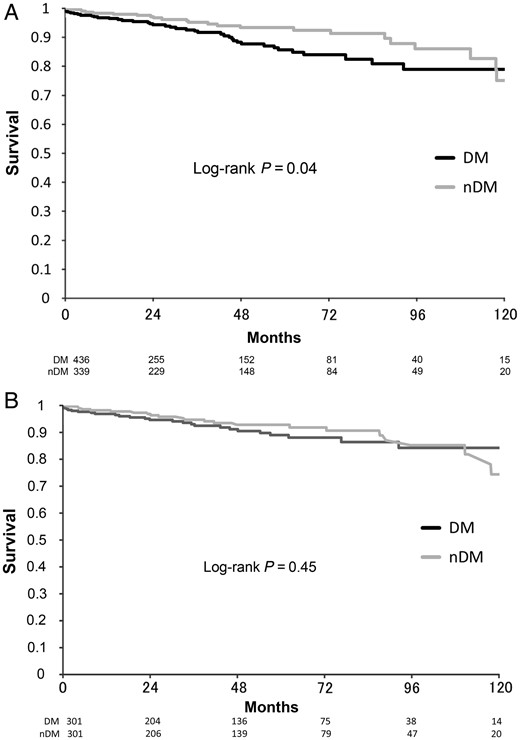
Ten-year actuarial freedom from death of any cause after off-pump coronary surgery according to DM or nDM. (A) Unmatched patients group. (B) Matched patients group. DM: diabetic patients; nDM: non-diabetic patients.
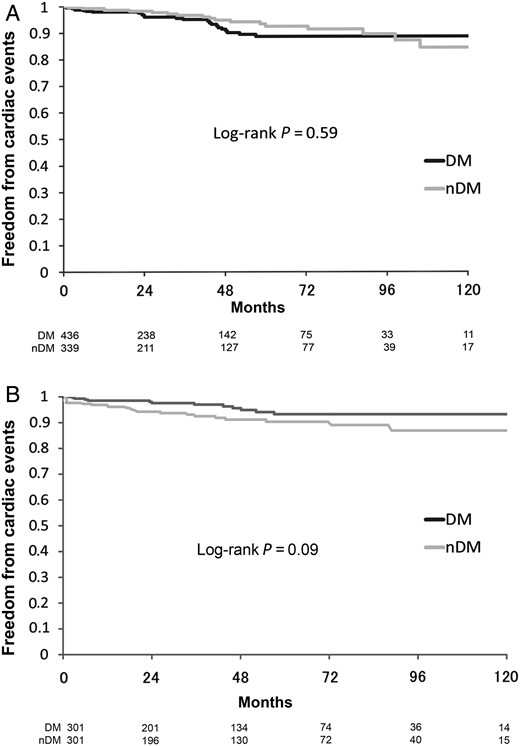
Ten-year actuarial freedom from any cardiac events (cardiac death, myocardial infarction, angina pectoris, coronary reintervention and heart failure) according to DM or nDM. (A) Unmatched patients group. (B) Matched patients group. DM: diabetic patients; nDM: non-diabetic patients.
DISCUSSION
In diabetic patients with severe multivessel disease, CABG has repeatedly been proven to be superior to percutaneous intervention (PCI) in terms of the risk of myocardial infarction, cardiac death and need for repeat revascularization [4]. A growing number of diabetic patients with multivessel coronary artery disease have recently been referred for CABG because of an unfavourable outcome in PCI [4]. However, as historically recognized, diabetes mellitus is a strong risk factor that worsens the clinical outcome of CABG in the long as well as the short term. Many previous studies have demonstrated poor early or late clinical outcomes in patients with DM who underwent CABG compared with those without DM [1, 2]. The reasons are that the diabetic patients have a more morbid anatomical pattern with rapidly progressive disease of the coronary vessels and have more extra-cardiac comorbidities. The lesions of the diabetic patients are more diffuse with smaller luminal diameters in segments adjacent to obstructive lesions [10]. Comorbidities associated with diabetes, including chronic renal failure, peripheral vascular disease and lower ejection fraction, can impact on short- and long-term survival in patients undergoing CABG [11]. The population of diabetic patients undergoing CABG is steadily increasing worldwide, especially in Japan that accounts for up to 40% of all CABG patients. To bring the clinical outcome for diabetic patients up to the same level as for non-diabetic patients, surgeons need to consider the best strategy for CABG.
Cardiopulmonary bypass is reported to be associated with more complications and to induce a greater oxidative stress in diabetic than in non-diabetic patients [12]. Some authors have found reduced surgical morbidity in diabetic patients undergoing OPCAB compared with conventional CABG [5, 6]. Emmert et al. [5] also reported that OPCAB offers lower mortality and superior postoperative outcomes in diabetic patients in a comparison of 540 OPCAB cases and 475 on-pump cases using propensity-adjusted regression analysis. In a very recent study by Renner et al. [6] that compared 355 diabetic patients undergoing OPCAB and 502 on-pump CABG, it was found that OPCAB was associated with a significantly lower rate of 30-day mortality and postoperative complications and with a significantly decreased 6-month and 1-year mortality rate. Srinivasan et al. [13] reported in their propensity score-adjusted study that the incidence of stroke was 6-fold higher and renal failure risk 2.3-fold higher in the conventional group than in the off-pump group. At our institute, all CABG procedures were performed using the off-pump technique without exclusion criteria by two high-volume surgeons who were familiar with the technique. Our data, therefore, are sufficiently reliable due to the lack of technical or surgeon bias.
Following the development of the OPCAB technique, the current trend in revascularization strategy is towards in situ all-arterial grafting because of the benefit of the aorta non-touch technique and better long-term clinical outcomes. A number of previous studies have shown the excellent effect of arterial conduits, which maintain a high rate of long-term patency and provide improved clinical outcomes, even in diabetes [7, 8]. As is generally known, the use of the ITA is associated with low rates of mortality and reintervention. Furthermore, a number of important reports demonstrate that bilateral ITA grafting to the left anterior descending and circumflex coronary arteries offers the best long-term survival and the lowest rates of reintervention [14–18]. In the decade since Lytle et al. [16] and Buxton et al. [17] demonstrated the long-term efficacy of bilateral ITA grafting, it has been gaining acceptance among surgeons. CABG with grafting of the bilateral ITAs to the left coronary system and additionally the GEA to the distal right coronary artery has been reported to provide good long-term outcome [19, 20]. Using the GEA combined with the bilateral ITAs can achieve complete avoidance of manipulation of the aorta. Since the beginning of the present study, we have used the GEA proactively in a skeletonized form and in 2013 reported the excellent long-term patency rate of this artery [21], which has now become a reliable third in situ arterial conduit during total arterial off-pump procedures using a complete aorta non-touch strategy.
Generally, however, bilateral ITA use has been avoided in diabetic patients because of concern for possible deep sternal wound infection. To decrease the risk of sternal infection associated with bilateral ITA harvesting, we adopted the technique of skeletonized dissection using the ultrasonic scalpel and, since the beginning of the present series, have not hesitated to use bilateral ITAs even in DM patients. Skeletonization has many advantages, such as avoidance of early spasm, easy identification of potential bleeding, vessel quality, functionally lengthened and larger graft with maximum flow, ease in performing sequential anastomosis, and preservation of sternal blood flow and venous drainage. Higami et al. [22] first described ultrasonic ITA skeletonization and revealed its technical feasibility and advantage. They showed that the skeletonized ITA was on average 4 cm longer than the pedicled conduit, and that the free flow rate is greater than 100 ml/min, which is at least 20% higher than in the pedicled ITA. In our experience, the ultrasonic scalpel improves technical ease, shortens the harvesting time and increases the effective length and free flow of the ITAs. For high-quality OPCAB, the skeletonization technique is now essential to achieve an arterial graft of optimum condition. A number of previous studies revealed that skeletonization of ITAs lowered the risk of deep sternal wound infection, even in diabetic patients with bilateral ITA use [23, 24]. In the present study, despite the high-frequency use of bilateral ITAs, representing over 85% of all cases, we found no significant difference between the two groups in the occurrence of deep sternal wound infection.
The study demonstrated successfully that OPCAB with total arterial revascularization can improve the clinical outcome of diabetic patients to the same level as in non-diabetic patients in the long as well as the short term. The study found the 10-year survival rate for all causes of death to be 84.2% in the matched DM group and 74.3% in the matched nDM group, showing no significant difference (P = 0.45). Freedom from any cardiac event (cardiac death, myocardial infarction, PCI and cardiac failure) was 93.3% in the matched DM group and 86.9% in the matched nDM group (P = 0.09). We can therefore say that diabetes mellitus is not recognized as a risk factor after total arterial OPCAB in either short-term or long-term outcomes. Hwang et al. [25] also found that diabetes did not affect long-term survival or clinical events in patients with multivessel coronary disease who underwent total arterial off-pump revascularization. We think that the advantageous effect of total arterial OPCAB can reduce the adverse effects of diabetes mellitus to a minimum, resulting in equal outcomes to those of non-diabetic patients.
Limitations
A limitation of the present study is that it is non-randomized and is a retrospective study comparing outcome in patients with and without diabetes mellitus undergoing OPCAB. Our study population was not large, resulting in insufficient statistical power. There were some baseline differences between the two groups which may have affected the outcomes, although multivariate analysis and adjustment of the propensity score were performed to minimize this bias. Because we performed total arterial OPCAB in most of our patients, we focused on patients undergoing this procedure for multivessel disease and did not make a comparison with patients undergoing non-total arterial revascularization. The study results can perhaps not be extrapolated to patients with on-pump CABG or saphenous vein grafting. However, the study does have some strong points: it is a single-centre study covering more than 1000 consecutive cases over a 10-year period; and almost all cases (more than 99% of isolated CABG cases) were performed using the off-pump technique by two high-volume surgeons who are very familiar with the technique.
CONCLUSIONS
We identified the following three important components of a surgical strategy that improve clinical outcome for diabetic patients undergoing CABG: use of the off-pump technique to reduce cardiopulmonary adverse effects; total arterial revascularization with the in situ bilateral ITAs and the GEA using the aorta non-touch technique; and skeletonization to optimize the condition of arterial conduits and prevent sternal wound infection. In conclusion, off-pump CABG with total arterial conduits using the skeletonization technique can provide similar clinical outcome for diabetic patients in the long as well as the short term as that for non-diabetic patients.
Conflict of interest: none declared.




