-
PDF
- Split View
-
Views
-
Cite
Cite
Elizabeth A. Mitchell, Darren P. Berman, Patrick I. McConnell, Jonathan Buber, Aortic root thrombosis with coronary embolization following neo-aortic reconstruction in a child with hypoplastic left heart syndrome, Interactive CardioVascular and Thoracic Surgery, Volume 21, Issue 2, August 2015, Pages 249–251, https://doi.org/10.1093/icvts/ivv123
Close - Share Icon Share
Abstract
In the recent era, the diagnosis, treatment options, postoperative management and outcomes of infants born with hypoplastic left heart syndrome (HLHS) have undergone dramatic changes. As is the case with many other novel treatment modalities used for congenital heart diseases, data concerning the long-term outcomes and complications of the various strategies become gradually more available as the numbers of survivors grow. In general, complications of the three-stage surgical palliation used for HLHS tend to occur most commonly following the first-stage surgery. Post-stage 2 complications are substantially less common, and centre on the procedure itself and the unique physiology of the cavopulmonary connection. In the following case report, we describe a relatively rare adverse outcome that occurred following a stage 2 surgery in the form of native aortic root thrombosis extending to the coronary arteries. The selected methods of treatment used in the catheterization laboratory and later in the operating theatre, as well as its outcomes are described.
CASE REPORT
The patient was a 13-month old female born with hypoplastic left heart syndrome (HLHS, severe mitral stenosis and a nearly-atretic aortic valve with a z-score of −5.8 and minimal antegrade flow), who initially underwent a hybrid stage 1 procedure (patent ductus arteriosus stent, branch pulmonary artery (PA) banding and balloon atrial septostomy), followed by comprehensive stage 2 procedure (PA band removal, Damus–Kaye–Stansel (DKS) type aortic reconstruction, Glenn anastomosis and atrial septectomy) at 4 months of age. Following the DKS anastomosis, the length of the native ascending aorta was 1.7 cm. The diameter at the sinuses was 7.5 mm and at the sino-tubular junction 5 mm, both yielding a z-score of −3.5. Per institutional protocol, she was treated for 6 weeks with low-molecular-weight heparin for thromboprophylaxis following her stage 2 procedure.
Video 1: Non-selective coronary angiography, straight lateral projection. During contrast injection performed with the catheter placed high in the native aortic root, a thrombus is dislodging from the aortic sinus down the right coronary artery and immediately dissolves. A small coronary fistula to the pulmonary artery is also observed.
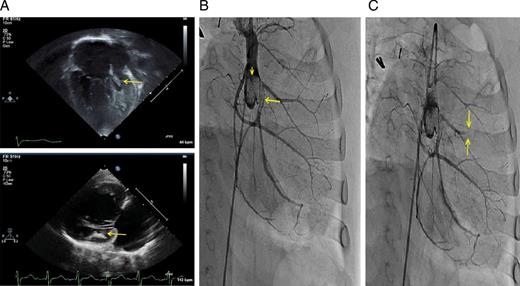
(A) Transthoracic echocardiography at presentation. Apical (top, performed in complete heart block) and parasternal-long (bottom, performed in normal sinus rhythm) views showing an enlarged right ventricle and a hypoplastic left ventricle, with an intracavitary thrombus measuring 3.5 × 9 mm (arrows). (B) Contrast injection above the native aortic root, performed in right anterior oblique angulation with caudal tilt. Large thrombotic mass is present in the aortic sinuses (arrowhead). Large thrombus is also observed in the proximal portion of the left circumflex artery (arrow). (C) Following bolus administration of thrombolytic therapy, a new cut-off of a diagonal artery was observed (arrows), likely due to an embolic event.
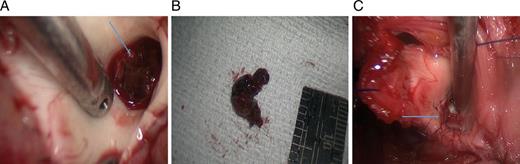
In situ (A, arrow) and post-removal (B) aortic root thrombus as observed at surgery. (C) Following thrombus removal, the aortic valve was over-sawn (arrow).
DISCUSSION
Following neo-aortic reconstruction in children born with HLHS, the short, often diminutive residual root segment of the native aorta remains a conduit for retrograde blood flow to the coronary vessels arising from it. Although the occurrence of native root thrombosis has been rarely reported, one post-mortem study reported that among 122 patients who had undergone the Norwood procedure, the incidence of coronary insufficiency was as high as 27%, making it the most important cause of death in this series [1]. Based on several prior reports [2–4], common manifestations of root thrombosis in single ventricle patients include conduction disturbances, congestive heart failure and thromboembolism, including to the cerebral vasculature. In the absence of large cohort data, hypothesized risk factors include anatomic native aortic valve abnormality prompting stasis, prolonged hospitalizations, absence of anticoagulant/anti-aggregant therapies and surgery-related issues, such as the length of the remnant native root (longer remnant may increase the risk for thrombus formation) and the amount of sutures placed [2–4]. In addition, several small cohort studies demonstrated that some patients may obtain the tendency for hypercoagulability [5], likely as a result of the altered haemodynamic state following this procedures, but possibly also of congenital coagulopathies. Data on the risks and benefits of possible therapeutic interventions is absent, leaving intervention-related decisions to be made on a case-by-case basis and based on the specific clinical scenario. Options include anticoagulation alone, systemic thrombolysis, directed thrombolysis injected to the aortic root and surgical thromboembolectomy.
Meticulous clinical and imaging-based follow-up is recommended for the single-ventricle post stage 2 patients. The optimal period for anticoagulation administration and whether certain sub-populations can benefit from longer periods are issues that remain to be addressed in larger studies. In addition, data from large patient cohorts on the long-term outcomes and adverse events after the stage 2 procedure would be an important contribution to the paediatric cardiology literature.
Acknowledgements
The authors are grateful to Kan Hor and Corey Stiver for their contribution to this report.
Conflict of interest: none declared.
REFERENCES




