-
PDF
- Split View
-
Views
-
Cite
Cite
Quentin Fischer, Matthias Kirsch, Eric Brochet, Jean-Michel Juliard, Bailout transcatheter closure of patent foramen ovale for refractory hypoxaemia after left ventricular assist device implantation, Interactive CardioVascular and Thoracic Surgery, Volume 21, Issue 2, August 2015, Pages 246–248, https://doi.org/10.1093/icvts/ivv105
Close - Share Icon Share
Abstract
We describe the interdisciplinary management of a 59-year old man with ischaemic cardiomyopathy on a HeartMate II left ventricular assist device (LVAD) and temporary right extracorporeal membrane oxygenation (ECMO) as a bridge-to-heart transplantation. He suffered refractory hypoxaemia due to massive right-to-left shunting by a patent foramen ovale (PFO), diagnosed after weaning off of temporary right ECMO. Percutaneous closure of the PFO was successfully achieved with an Amplatzer septal occluder device, which allowed the patient's extubation and departure from hospital. The patient received heart transplantation 7 weeks after LVAD implantation and was discharged from the intensive care unit 2 weeks after transplantation.
CASE REPORT
A 59-year old man suffering cardiogenic shock was admitted secondary to ischaemic cardiomyopathy. The patient's condition was stabilized to an INTERMACS level 2 status using an intravenous inotropic agent (dobutamine 10 µg/kg/min), with normalized renal function (creatinine 68 mmol/l) but persisting elevations in liver enzymes (aspartate aminotransferase 104 IU/l; alanine aminotransferase 160 IU/l) and total bilirubin (26 µmol/l). The patient was initially listed for emergency heart transplantation, which grants national priority for 48 h, renewable once. Since no compatible donor graft became available during that period, the patient was scheduled for a HeartMate II left ventricular assist device (LVAD) (Thoratec Corporation, Pleasanton, CA, USA) implantation as a bridge to transplantation.
Intraoperative transoesophageal echocardiography (TOE) performed by the anaesthesiologist did not reveal any abnormal intra-cardiac shunts. The LVAD was implanted through a median sternotomy between the LV apex and the ascending aorta. Because of intraoperative right ventricular (RV) dysfunction, a temporary RV support using an extracorporeal life support perfusion circuit was implanted, as previously described [1, 2]. Briefly, the venous inflow cannula was placed percutaneously into the right atrium through a femoral vein. For the outflow cannula, an 8-mm Dacron graft was anastomosed end-to-side to the main pulmonary artery using a side clamp. The Dacron graft was positioned in front of the LVAD outflow graft and tunnelled to its percutaneous exit site under the right costal margin. The arterial outflow graft was then inserted into the Dacron graft and secured using heavy sutures. This approach allows for easy venous and arterial decannulation without general anaesthesia and reopening of the chest, but at the expense of leaving the distal portion of the Dacron graft within the thorax.
The patient was weaned from mechanical ventilation on postoperative day 4 with ongoing RV support, but required reintubation on postoperative day 6 because of a sudden alteration of consciousness (with unremarkable findings on cerebral computed tomography (CT) and electro-encephalography). Progressive RV recovery allowed weaning from temporary RV support at bedside on postoperative day 8. After weaning, the patient remained haemodynamically stable but became gradually hypoxaemic despite optimized ventilation therapy (FiO2 60%, PaO2 50 mmHg). No abnormalities were noted on chest X-ray and thoracic CT excluded pulmonary embolism. Repeated TOE performed on postoperative day 11 revealed an aneurysm of the interatrial septum with a patent foramen ovale (PFO) associated with a massive right-to-left shunt on colour flow Doppler and bubble contrast study (Fig. 1, Parts 1a–c). After multidisciplinary discussion, it was decided to occlude the PFO via a percutaneous route, to avoid repeated surgery. Thus, on postoperative day 13, an Amplatzer Cribriform (St Jude Medical, Saint Paul, MN, USA) 40/40 septal occluder device was successfully implanted via the left femoral vein under fluoroscopic and TOE guidance (Fig. 1, Parts 2a–c). The duration of the procedure was 30 min. A small residual intraprosthetic shunt across the device was detected on TOE at the end of the procedure, but was no longer detected on postoperative day 17 (Fig. 1, Parts 2a–c).
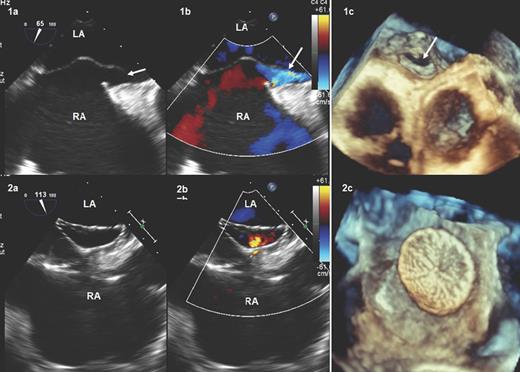
TOE before (1a, two-dimensional; 1b, colour-Doppler; 1c, three-dimensional) and 6 days after (2a, two-dimensional; 2b, colour-Doppler; 2c, three-dimensional) PFO closure with the Cribriform Amplatzer device. The white arrows show the PFO. LA: left atrium; PFO: patent foramen ovale; RA: right atrium; TOE: transoesophageal echocardiography.
The patient's oxygenation improved significantly after the procedure, allowing successful weaning from mechanical ventilation on postoperative day 15. The patient was subsequently discharged from the intensive care unit (postoperative day 21) and went to a rehabilitation centre on postoperative day 28. Seven weeks after LVAD implantation, a compatible donor became available and the patient underwent successful orthotopic heart transplantation (Fig. 2).
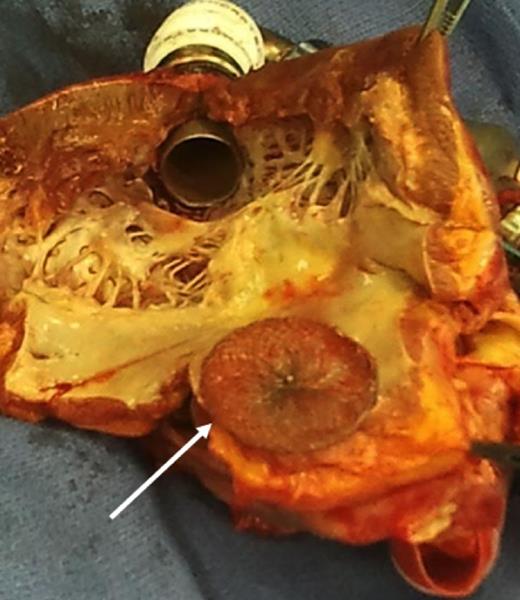
The primitive heart after orthotopic heart transplantation, showing the Amplatzer device.
The presence of a PFO may be masked by some conditions associated with increased left atrial pressure such as advanced LV dysfunction [3]. After initiation of LVAD support, however, LV unloading by the device reduces left atrial pressure and should facilitate diagnosis, after weaning from cardiopulmonary bypass at the latest [4]. In the case of biventricular support using RV assist devices, right and left atrial pressures might be reduced in a symmetric fashion, and the PFO may remain undetected. In the present case, echocardiography was performed preoperatively or intraoperatively while the patient was on cardiopulmonary bypass or temporary RV support, which is probably the reason why the PFO was missed and diagnosed only later, after the weaning of RV support.
To avoid these diagnostic pitfalls, some surgeons advocated systematic intraoperative visual inspection of the interatrial septum by performing a small right atriotomy during LVAD implantation. Considering the high diagnostic sensitivity of echocardiography in the majority of cases, we feel that such a systematic approach is excessive and not always compatible with minimally invasive implant procedures, which pride only limited access to the right atrium or avoid cardiopulmonary bypass [5]. Nevertheless, we use bicaval cannulation in all LVAD patients and our case suggests that visual inspection of the interatrial septum might be advisable in patients who require immediate temporary RV support.
If identified pre- or intraoperatively, PFO should be closed at the time of LVAD implantation. In the event of delayed diagnosis, a percutaneous approach avoids re-operative surgery with its inherent risks of bleeding and infection. The percutaneous implant procedure requires no technical modification in LVAD recipients. The periprocedural risk of bleeding might be higher in LVAD recipients who require anticoagulation with or without antiaggregation. Otherwise, the risk of complications can be expected to be similar to that of conventional patients.
Conflict of interest: Matthias Kirsch is consultant for Thoratec Corporation.
REFERENCES




