-
PDF
- Split View
-
Views
-
Cite
Cite
Antonino Loforte, Andrea Montalto, Paola Lilla Della Monica, Francesco Musumeci, Simultaneous temporary CentriMag right ventricular assist device placement in HeartMate II left ventricular assist system recipients at high risk of right ventricular failure, Interactive CardioVascular and Thoracic Surgery, Volume 10, Issue 6, June 2010, Pages 847–850, https://doi.org/10.1510/icvts.2009.230706
Close - Share Icon Share
Abstract
An approach is reported for right ventricle temporary mechanical support in long-term axial left ventricular assist device (LVAD) patients preoperatively judged at high risk of right ventricular (RV) failure. The timing for RV assist device (RVAD) weaning and the technique for its removal through a right mini-thoracotomy are described. This strategy provides a good outcome in LVAD recipients avoiding the risk of immediate postoperative RV failure.
1. Introduction
Implantable left ventricular assist devices (LVADs) in the treatment of end-stage heart failure have recently gained broader application not only as a bridge to heart transplantation but also as a bridge to recovery and permanent support. The Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart (REMATCH) failure trial has demonstrated a statistically significant benefit in survival and quality of life with the long-term use of LVADs in patients with advanced heart failure. More recently, growing evidence indicates that sustained reversal of severe heart failure can be achieved in selected patients with the use of an LVAD and a specific pharmacologic regimen. Despite the clinical benefit of LVAD usage, right ventricular (RV) failure after LVAD implantation continues to be a major postoperative problem and is associated with a high mortality rate. Better preoperative identification of risk factors for RV failure (RVF) should lead to improved selection of the patients who would benefit from biventricular assist devices. Previous studies have demonstrated that female gender, non-ischemic etiology of LV dysfunction, elevated central venous pressare (CVP), low mean pulmonary artery pressare (mPAP), and low RV stroke work index (RVSWI) are independent predictors of RVF or need for an RV assist device (RVAD) support after LVAD implantation [1–5]. Preoperative evaluation of tricuspid incompetence and RV geometry may help to select patients who would benefit from biventricular support even if it is a temporary support [1]. However, the role of echocardiographic parameters in prospective identification of post-LVAD RVF has not been thoroughly investigated. This brief report describes a simple radial RVAD system applied in axial LVAD patients at risk of RVF that we have devised and used successfully and which can be removed through a right mini-thoracotomy without repeat sternotomy.
2. Technique
The axial-flow pump we use is the HeartMate II LVAD (Thoratec Corp, Pleasanton, CA, USA) and is placed in traditional fashion with the inflow cannula in the left ventricle apex and the outflow cannula in the ascending aorta. The RVAD system is comprised of an extracorporeal magnetically levitated radial pump (Levitronix LCC, Waltham, MA, USA) and appropriate cannulae to take blood from the right atrium and return it into the pulmonary artery. Rather than directly cannulating the pulmonary artery, we use a partial occluding clamp to attach an appropriately sized graft (Hemashield; Boston Scientific, Boston, MA, USA) to the vessel. We generally use a 10-mm graft. The arterial catheter (19-French femoral arterial cannula; Medtronic, Minneapolis, MN, USA) is positioned with its tip in the body of the 10-mm graft. The graft is tied tightly around the respective cannula with umbilical tapes inside the chest and secured firmly to the chest wall with multiple heavy sutures (Fig. 1 ). The venous cannula we generally use (2-stage 24-French; Medtronic, Minneapolis, MN, USA) extends through the chest with the tip positioned in the right atrium (Fig. 1). The graft for pulmonary artery associated with the outflow conventional cannula and the inflow cannula for right atrium are tunneled obliquely on the right side through the chest wall and exit inferior to the medial third of the right costal margins (Figs. 2 and 3 ). Institution of RV support facilitates the chest closure. Definitive multilayer sternal closure is performed when feasible. The patient is weaned from mechanical ventilation and extubated as tolerated. After an appropriate interval of RV unloading, attempts are made to wean the patient from support. Pulmonary vasodilators such as nitric oxide (NO) and inotropes such as melrinone and adrenalin may be of value. Systemic heparin is administered to allow progressively lower levels of RVAD support. Once it is determined that RV support is no longer necessary by daily monitorings of hemodynamics via Swann–Ghanz catheter calculations and heart contractility via transesophageal echo evaluations, the patient is re-sedated and is returned again to the operating room. A right lateral mini-thoracotomy, 5 cm in length, is performed in the 4th intercostal space. The incision is placed just below and lateral to the nipple in men, and in the submammary crease in women. A small thoracic and soft tissue retractor is utilized. A video camera is inserted through a 10-mm port in the right 2nd intercostal space if necessary. After adesions debridment an intravenously heparin bolus is achieved and the RVAD is stopped and removed without the aid of cardiopulmonary bypass (CPB). The pump lines are clamped. The tip of inflow atrial cannula is removed first and conventional pursestrings tied. An umbilical tapes is snared at the distal end of outflow graft just close to the pulmonary artery. The graft is then divided and oversewn. The cannulae are totally removed at the end of the procedure. The right thoracotomy and skin incisions are then loosely closed.

Intraoperative view of CentriMag (Levitronix LLC, Waltham, MA, USA) RVAD support cannulae placement in HeartMate II LVAD (Thoratec Corp, Pleasanton, CA, USA) recipients.
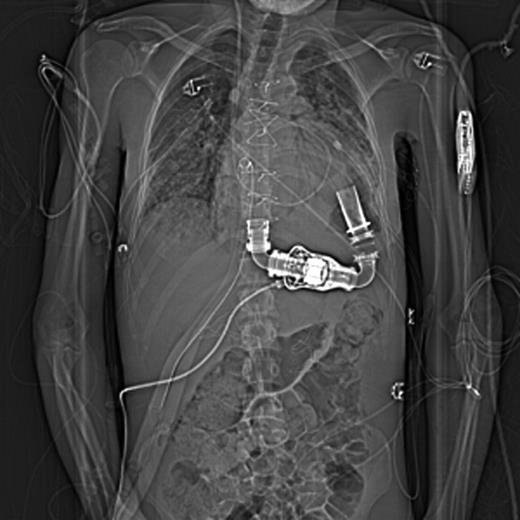
Chest X-ray imaging of position of CentriMag (Levitronix LLC, Waltham, MA, USA) RVAD cannulae on right side through the chest wall and inferior to the medial third of right costal margins just nearby the HeartMate II LVAD (Thoratec Corp, Pleasanton, CA, USA) driveline.
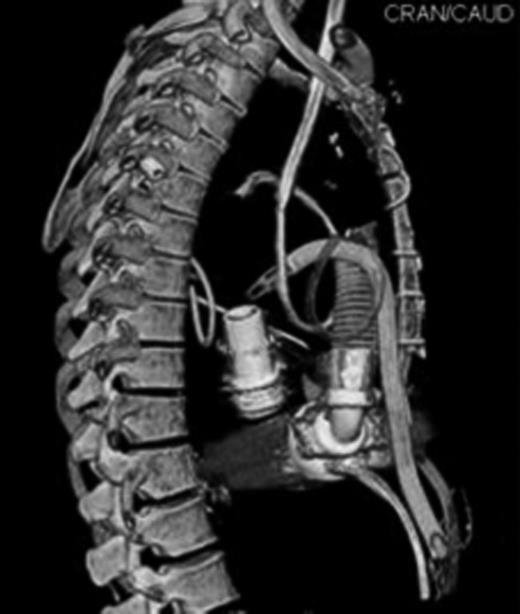
Position of mechanical support devices cannulae in 3-D computed tomography (CT) scan reconstruction.
3. Comment
This technique allows weaning from RVAD support without the confounding hemodynamic challenges of positive pressure ventilation and sternal closure, particularly in subjects preoperatively judged at high risk of RVF after LVAD placement. Potential disadvantages of the temporary retained graft include thrombus liberation and foreign body infection due to chest crossing cannulae. Although the graft may certainly cause thrombosis, thrombus liberation and embolization have not been problematic in our experience of six cases (Table 1 ). The risk of infection should be low if sterile care is strictly adhered to at intensive care unit bedside during support. The risks of a repeat sternotomy are avoided by using a lateral chest approach for RVAD removal even if a really short-term support is provided. All of our patients (Table 1) showed a preoperative moderate RVF according to Berlin algorithm [1] and, after 17.5 days as average time of temporary RV mechanical support, benefited well from this type of approach.
Demographic data, hemodynamic characteristics, echo assessment and end-organ functional status before CentriMag (Levitronix LLC, Waltham, MA, USA) RVAD and HeartMate II LVAD (Thoratec Corp, Pleasanton, CA, USA) support
| Demographics | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 |
| Gender (male) | Yes | Yes | No | Yes | Yes | Yes |
| Age (years) | 31 | 64 | 60 | 61 | 36 | 53 |
| Ischemic DCMP | No | Yes | Yes | Yes | No | Yes |
| Idiopathic DCMP | Yes | No | No | No | Yes | No |
| Re-do surgery | No | No | Yes | No | No | No |
| IABP before surgery | Yes | Yes | Yes | No | Yes | No |
| Preoperative ventilation | No | No | No | No | No | No |
| SAPS II score | 30 | 35 | 32 | 37 | 38 | 30 |
| Inotropic score* | 12 | 13 | 18 | 18 | 20 | 12 |
| Indication for long-term LVAD | BTT | BTT | BTT | Permanent | BTT | BTT |
| support | ||||||
| Status before MCS | ||||||
| CI (l/min/m2) | 1.8 | 1.7 | 1.8 | 1.7 | 1.8 | 1.8 |
| RVSWI (mmHg/ml/m2) | 323 | 335 | 344 | 345 | 300 | 310 |
| mPAP (mmHg) | 40 | 32 | 30 | 35 | 30 | 40 |
| PVR (dyn/s/cm5) | 253 | 210 | 244 | 230 | 200 | 215 |
| CVP (mmHg) | 20 | 20 | 20 | 22 | 22 | 20 |
| PCWP (mmHg) | 23 | 23 | 22 | 24 | 22 | 23 |
| TAM (mm) | 10 | 12 | 10 | 13 | 10 | 10 |
| RVEDD (mm) | 35 | 36 | 35 | 33 | 35 | 35 |
| Moderate TR | Yes | Yes | Yes | Yes | Yes | Yes |
| RVEF (%) | 28 | 28 | 25 | 28 | 25 | 25 |
| LVEF (%) | 20 | 25 | 15 | 25 | 20 | 20 |
| Moderate MR | Yes | Yes | Yes | Yes | Yes | Yes |
| Creatinine (mg/dl) | 1.4 | 1.3 | 1.5 | 1.4 | 1.8 | 1.3 |
| Total bilirubin (mmol/l) | 2.0 | 1.8 | 1.7 | 2.0 | 2.7 | 1.7 |
| AST (U/l) | 30 | 36 | 32 | 32 | 44 | 30 |
| ALT (U/l) | 26 | 30 | 28 | 25 | 38 | 25 |
| INR | 1.64 | 1.72 | 1.58 | 1.62 | 1.92 | 1.56 |
| NT-proBNP (pg/ml) | 13,100 | 15,130 | 14,150 | 13,870 | 17,250 | 15,110 |
| Time of RVAD support (days) | 13 | 17 | 18 | 20 | 18 | 19 |
| Demographics | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 |
| Gender (male) | Yes | Yes | No | Yes | Yes | Yes |
| Age (years) | 31 | 64 | 60 | 61 | 36 | 53 |
| Ischemic DCMP | No | Yes | Yes | Yes | No | Yes |
| Idiopathic DCMP | Yes | No | No | No | Yes | No |
| Re-do surgery | No | No | Yes | No | No | No |
| IABP before surgery | Yes | Yes | Yes | No | Yes | No |
| Preoperative ventilation | No | No | No | No | No | No |
| SAPS II score | 30 | 35 | 32 | 37 | 38 | 30 |
| Inotropic score* | 12 | 13 | 18 | 18 | 20 | 12 |
| Indication for long-term LVAD | BTT | BTT | BTT | Permanent | BTT | BTT |
| support | ||||||
| Status before MCS | ||||||
| CI (l/min/m2) | 1.8 | 1.7 | 1.8 | 1.7 | 1.8 | 1.8 |
| RVSWI (mmHg/ml/m2) | 323 | 335 | 344 | 345 | 300 | 310 |
| mPAP (mmHg) | 40 | 32 | 30 | 35 | 30 | 40 |
| PVR (dyn/s/cm5) | 253 | 210 | 244 | 230 | 200 | 215 |
| CVP (mmHg) | 20 | 20 | 20 | 22 | 22 | 20 |
| PCWP (mmHg) | 23 | 23 | 22 | 24 | 22 | 23 |
| TAM (mm) | 10 | 12 | 10 | 13 | 10 | 10 |
| RVEDD (mm) | 35 | 36 | 35 | 33 | 35 | 35 |
| Moderate TR | Yes | Yes | Yes | Yes | Yes | Yes |
| RVEF (%) | 28 | 28 | 25 | 28 | 25 | 25 |
| LVEF (%) | 20 | 25 | 15 | 25 | 20 | 20 |
| Moderate MR | Yes | Yes | Yes | Yes | Yes | Yes |
| Creatinine (mg/dl) | 1.4 | 1.3 | 1.5 | 1.4 | 1.8 | 1.3 |
| Total bilirubin (mmol/l) | 2.0 | 1.8 | 1.7 | 2.0 | 2.7 | 1.7 |
| AST (U/l) | 30 | 36 | 32 | 32 | 44 | 30 |
| ALT (U/l) | 26 | 30 | 28 | 25 | 38 | 25 |
| INR | 1.64 | 1.72 | 1.58 | 1.62 | 1.92 | 1.56 |
| NT-proBNP (pg/ml) | 13,100 | 15,130 | 14,150 | 13,870 | 17,250 | 15,110 |
| Time of RVAD support (days) | 13 | 17 | 18 | 20 | 18 | 19 |
DCMP, dilative cardiomyopathy; IABP, intra-aortic balloon pump; SAPS, simplified acute physiology score; LVAD, left ventricle assist device; BTT, bridge to transplantation; MCS, mechanical circulatory support; CI, cardiac index; RVSWI, right ventricle stroke work index; mPAP, mean pulmonary arterial pressure; CVP, central venous pressure; PCWP, pulmonary capillare wedge pressure; TAM, tricuspid annular motion; RVEDD, right ventricle end-diastolic dimension; TR, tricuspid regurgitation; RVEF, right ventricle ejection fraction; LVEF, left ventricle ejection fraction; MR, mitral regurgitation; AST, aspartate aminotransferase; ALT, alanine aminotransferase; INR, international normalized ratio; RVAD, right ventricle assist device [1].
*Definition is given by Potapov and Kormos [1].
Demographic data, hemodynamic characteristics, echo assessment and end-organ functional status before CentriMag (Levitronix LLC, Waltham, MA, USA) RVAD and HeartMate II LVAD (Thoratec Corp, Pleasanton, CA, USA) support
| Demographics | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 |
| Gender (male) | Yes | Yes | No | Yes | Yes | Yes |
| Age (years) | 31 | 64 | 60 | 61 | 36 | 53 |
| Ischemic DCMP | No | Yes | Yes | Yes | No | Yes |
| Idiopathic DCMP | Yes | No | No | No | Yes | No |
| Re-do surgery | No | No | Yes | No | No | No |
| IABP before surgery | Yes | Yes | Yes | No | Yes | No |
| Preoperative ventilation | No | No | No | No | No | No |
| SAPS II score | 30 | 35 | 32 | 37 | 38 | 30 |
| Inotropic score* | 12 | 13 | 18 | 18 | 20 | 12 |
| Indication for long-term LVAD | BTT | BTT | BTT | Permanent | BTT | BTT |
| support | ||||||
| Status before MCS | ||||||
| CI (l/min/m2) | 1.8 | 1.7 | 1.8 | 1.7 | 1.8 | 1.8 |
| RVSWI (mmHg/ml/m2) | 323 | 335 | 344 | 345 | 300 | 310 |
| mPAP (mmHg) | 40 | 32 | 30 | 35 | 30 | 40 |
| PVR (dyn/s/cm5) | 253 | 210 | 244 | 230 | 200 | 215 |
| CVP (mmHg) | 20 | 20 | 20 | 22 | 22 | 20 |
| PCWP (mmHg) | 23 | 23 | 22 | 24 | 22 | 23 |
| TAM (mm) | 10 | 12 | 10 | 13 | 10 | 10 |
| RVEDD (mm) | 35 | 36 | 35 | 33 | 35 | 35 |
| Moderate TR | Yes | Yes | Yes | Yes | Yes | Yes |
| RVEF (%) | 28 | 28 | 25 | 28 | 25 | 25 |
| LVEF (%) | 20 | 25 | 15 | 25 | 20 | 20 |
| Moderate MR | Yes | Yes | Yes | Yes | Yes | Yes |
| Creatinine (mg/dl) | 1.4 | 1.3 | 1.5 | 1.4 | 1.8 | 1.3 |
| Total bilirubin (mmol/l) | 2.0 | 1.8 | 1.7 | 2.0 | 2.7 | 1.7 |
| AST (U/l) | 30 | 36 | 32 | 32 | 44 | 30 |
| ALT (U/l) | 26 | 30 | 28 | 25 | 38 | 25 |
| INR | 1.64 | 1.72 | 1.58 | 1.62 | 1.92 | 1.56 |
| NT-proBNP (pg/ml) | 13,100 | 15,130 | 14,150 | 13,870 | 17,250 | 15,110 |
| Time of RVAD support (days) | 13 | 17 | 18 | 20 | 18 | 19 |
| Demographics | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 |
| Gender (male) | Yes | Yes | No | Yes | Yes | Yes |
| Age (years) | 31 | 64 | 60 | 61 | 36 | 53 |
| Ischemic DCMP | No | Yes | Yes | Yes | No | Yes |
| Idiopathic DCMP | Yes | No | No | No | Yes | No |
| Re-do surgery | No | No | Yes | No | No | No |
| IABP before surgery | Yes | Yes | Yes | No | Yes | No |
| Preoperative ventilation | No | No | No | No | No | No |
| SAPS II score | 30 | 35 | 32 | 37 | 38 | 30 |
| Inotropic score* | 12 | 13 | 18 | 18 | 20 | 12 |
| Indication for long-term LVAD | BTT | BTT | BTT | Permanent | BTT | BTT |
| support | ||||||
| Status before MCS | ||||||
| CI (l/min/m2) | 1.8 | 1.7 | 1.8 | 1.7 | 1.8 | 1.8 |
| RVSWI (mmHg/ml/m2) | 323 | 335 | 344 | 345 | 300 | 310 |
| mPAP (mmHg) | 40 | 32 | 30 | 35 | 30 | 40 |
| PVR (dyn/s/cm5) | 253 | 210 | 244 | 230 | 200 | 215 |
| CVP (mmHg) | 20 | 20 | 20 | 22 | 22 | 20 |
| PCWP (mmHg) | 23 | 23 | 22 | 24 | 22 | 23 |
| TAM (mm) | 10 | 12 | 10 | 13 | 10 | 10 |
| RVEDD (mm) | 35 | 36 | 35 | 33 | 35 | 35 |
| Moderate TR | Yes | Yes | Yes | Yes | Yes | Yes |
| RVEF (%) | 28 | 28 | 25 | 28 | 25 | 25 |
| LVEF (%) | 20 | 25 | 15 | 25 | 20 | 20 |
| Moderate MR | Yes | Yes | Yes | Yes | Yes | Yes |
| Creatinine (mg/dl) | 1.4 | 1.3 | 1.5 | 1.4 | 1.8 | 1.3 |
| Total bilirubin (mmol/l) | 2.0 | 1.8 | 1.7 | 2.0 | 2.7 | 1.7 |
| AST (U/l) | 30 | 36 | 32 | 32 | 44 | 30 |
| ALT (U/l) | 26 | 30 | 28 | 25 | 38 | 25 |
| INR | 1.64 | 1.72 | 1.58 | 1.62 | 1.92 | 1.56 |
| NT-proBNP (pg/ml) | 13,100 | 15,130 | 14,150 | 13,870 | 17,250 | 15,110 |
| Time of RVAD support (days) | 13 | 17 | 18 | 20 | 18 | 19 |
DCMP, dilative cardiomyopathy; IABP, intra-aortic balloon pump; SAPS, simplified acute physiology score; LVAD, left ventricle assist device; BTT, bridge to transplantation; MCS, mechanical circulatory support; CI, cardiac index; RVSWI, right ventricle stroke work index; mPAP, mean pulmonary arterial pressure; CVP, central venous pressure; PCWP, pulmonary capillare wedge pressure; TAM, tricuspid annular motion; RVEDD, right ventricle end-diastolic dimension; TR, tricuspid regurgitation; RVEF, right ventricle ejection fraction; LVEF, left ventricle ejection fraction; MR, mitral regurgitation; AST, aspartate aminotransferase; ALT, alanine aminotransferase; INR, international normalized ratio; RVAD, right ventricle assist device [1].
*Definition is given by Potapov and Kormos [1].
In particular, after separation from CPB in patients 1 and 2 RVF occurred: ‘low flow’ of the LVAD, ballooning of the right ventricle, leftwards-shift of the interventricular septum, poor systolic function, and CVP >20 mmHg. The RVAD was immediately implanted without a second attempt at CPB weaning. In patients 3–6 the ‘temporary’ biventricular support was established as primary option without trying to wean off CPB in LVAD support, only due to preoperative alteration of RV function which was similar to that of patients 1–2 (Table 1) who experienced acute RVF at LVAD support initiation instead. In all patients after discontinuation of CPB the LVAD and the RVAD were producing an average flow of 5.4 l/min and 5.0 l/min, respectively, and CVP was maintained at 12 mmHg. NO inhalation at 40 ppm was started together with cathecolamine support (adrenalin 0.15 μg/kg/min). There were no reexplorations for bleeding. Slowly weaning off inhaled NO in stepwise fashion over the first postoperative day (POD) 12–18 h was accomplished. Sildenafil was administered either orally or by nasogastric tube initially (25 mg), and continued orally every 8 h after extubation to facilitate the weaning off NO and help RV function as well [6]. In TEE examinations performed daily, progressive improvement of RV function was documented. After 13.5 PODs, as average, the weaning from CentriMag was carried out by reducing the RVAD flow by 10% every approximately 12 h. After 17.5 PODs, as average, the RVAD was successfully removed. Approximately 7 days after RVAD removal adrenalin was discontinued and all patients were free of cathecolamine support with good systolic funtion of the RV (ejection fraction 38–40%), CVP level of 10–15 mmHg and stable LVAD flow of 5.3 l/min, as average. All patients had an uneventful hospital stay and were successfully discharged.
The preoperative prediction of RV function after LVAD implantation is crucial for device selection and patient outcome but it is still not well established. It is generally accepted that patients who require biventricular assist device support have poorer outcomes than those requiring isolated LVAD support [1–3]. However, it is unknown how the timing of biventricular assist device insertion affects outcomes. The planned biventricular assist device insertion improves survival compared with delayed conversion of LVAD support to biventricular assist device support [1–3]. When patients at high risk for failure of isolated LVAD support are identified, proceeding as a primary option to a biventricular assist device implantation is suggested since it could be hypothesized that the early institution of a ‘temporary’ biventricular support results in improvement of outcome [1–4]. In cases of preoperative alteration of RV function, as assessed by echocardiographic studies, RVAD placement should be performed as a primary option even if in a prophylactical way to avoid the RV ballooning and irreversible cardiomyocyte stretching that occur with a delayed RVAD insertion [4, 5].
The use of a temporary RVAD placement and its further removal could allow patients to be in long-term implantable LVAD support only with better outcome and quality of life as well as longer time of mechanical support if compared to similarly ill patients who have undergone primary long-term paracorporeal biventricular assist device (BVAD) support placement [2, 3]. Therefore, a more accurate assessment of RV function during the follow-up should become mandatory in these patients. However, further studies are necessary to assess the correct strategy in this type of patient population.
This study was carried out within the PhD Program in ‘Transplantation’ awarded by Rome Tor Vergata University, Italy, in cooperation with the Deutsches Herzzentrum Berlin, Germany.
The authors thank E.V. Potapov, Deutsches Herzzentrum Berlin, Germany, for much advice.




