-
PDF
- Split View
-
Views
-
Cite
Cite
Paolo Vanelli, Massimo Lemma, Carlo Antona, Right mini-thoracotomy for left maze with transesophageal echo guidance, Interactive CardioVascular and Thoracic Surgery, Volume 10, Issue 6, June 2010, Pages 843–846, https://doi.org/10.1510/icvts.2009.227462
Close - Share Icon Share
Abstract
Minimally invasive surgery (MIS) is widening with the development of new specialized instrumentation, allied with improved surgical experience and techniques, some of which have shown to be effective for the ablation of atrial fibrillation (AF). These developments enable us to achieve a so-called ‘ideal procedure’, epicardially on beating hearts, with less operative risk, high cure rates and rapid patient recovery. Epicor (St Jude Medical, Sunnyvale, CA, USA) low profile (LP) system is a device using high intensity focused ultrasound (HIFU). We describe the use of this technology for ablation of AF through MIS approach using transesophageal echocardiography (TEE) to pilot the ablation on mitral isthmus. Ten patients underwent monolateral small thoracotomy, through the 4th intercostal space. HIFU was carried out in all cases to create an epicardial box lesion of the pulmonary veins (PVs) and mitral isthmus. TEE was employed to guide the positioning of the ablation device on mitral isthmus, in all patients. There were no mortalities or major complications, including pacemaker implantation. One patient had postoperative atrial tachycardia and was cardioverted before hospital discharge. Three patients had a postoperative AF and were scheduled for cardioversion after three months, and one patient spontaneously revealed a normal sinus rhythm (SR). During the follow-up period, all patients recorded a normal SR. We consider Epicor LP system safe and effective for AF ablation through a single right minimal invasive approach.
1. Introduction
The original Cox Maze III is a milestone for the treatment of atrial fibrillation (AF) [1]. This ‘cut and sew’ technique shows the best results after short- and long-term follow-up [2, 3]. Catheter ablation was introduced to facilitate the AF treatment and reduce complications [4]. The development of new ablation devices with various energy sources enables the surgeon to widen the indications for the treatment of AF. The Epicor Medical Ablation System (St Jude Medical Sunnyvale, CA, USA), was designed to deliver high intensity focused ultrasound (HIFU), which allows the creation of pulmonary vein isolation (PVI) and the mitral isthmus, epicardially without extracorporeal circulation. Different authors have reported good results for concomitant AF [5, 6]. Recently, the Epicor system was updated with the introduction of a new device with a low profile (LP) and which is more flexible, for minimally invasive surgery (MIS) use.
We have used this new system for AF ablation in 10 patients, managing the device positioning on mitral isthmus with transesophageal echocardiography (TEE).
2. Material and methods
2.1. Patient population
From November 2008 to December 2009 10 consecutive patients, five male and five female, were admitted to the Luigi Sacco, University and General Hospital, for elective mitral valve surgery, with concomitant AF. These patients were selected according the EHRS/EHRA/ECAS recommendations [8]. Exclusion criterions were a presence of atrial or left appendage thrombi, ‘giant’ left atrium (LA) (diameter >65 mm), coronary artery disease, and previous pulmonary or cardiac surgery. Individual informed consent for this technique was obtained from the patients. Table 1 shows the preoperative characteristics of the patients. Eight patients had permanent AF and two persistent AF according the EHRS/EHRA/ECAS definitions [8].
| Patient | Age | Associated | AF | CVA | DCC | LA | LA | NYHA |
| (M/F) | (years) | disease | type | diameter | area | class | ||
| (mm) | (cmq) | |||||||
| F | 66 | MR | Pt | 58 | 25 | 3 | ||
| M | 70 | MS | Pt | Yes | 1 | 53 | 25 | 3 |
| F | 55 | MR | Ps | 55 | 36 | 4 | ||
| F | 60 | MS | Pt | 2 | 55 | 47 | 1 | |
| M | 74 | MR | Ps | 53 | 30 | 2 | ||
| M | 73 | MR | Pt | 52 | 30 | 3 | ||
| F | 72 | MS | Pt | 1 | 62 | 30 | 3 | |
| M | 68 | MR | Pt | 55 | 29 | 2 | ||
| M | 60 | MR | Pt | 42 | 21 | 1 | ||
| F | 69 | MR-TR | Pt | 55 | 32 | 2 |
| Patient | Age | Associated | AF | CVA | DCC | LA | LA | NYHA |
| (M/F) | (years) | disease | type | diameter | area | class | ||
| (mm) | (cmq) | |||||||
| F | 66 | MR | Pt | 58 | 25 | 3 | ||
| M | 70 | MS | Pt | Yes | 1 | 53 | 25 | 3 |
| F | 55 | MR | Ps | 55 | 36 | 4 | ||
| F | 60 | MS | Pt | 2 | 55 | 47 | 1 | |
| M | 74 | MR | Ps | 53 | 30 | 2 | ||
| M | 73 | MR | Pt | 52 | 30 | 3 | ||
| F | 72 | MS | Pt | 1 | 62 | 30 | 3 | |
| M | 68 | MR | Pt | 55 | 29 | 2 | ||
| M | 60 | MR | Pt | 42 | 21 | 1 | ||
| F | 69 | MR-TR | Pt | 55 | 32 | 2 |
M, male; F, female; AF, atrial fibrillation; CVA, cerebrovascular accident; DCC, direct current cardioversion; LA, left atrium; NYHA, New York Heart Association; MR, mitral regurgitation; Pt, permanent; MS, mitral stenosis; Ps, persistent; TR, tricuspid regurgitation.
| Patient | Age | Associated | AF | CVA | DCC | LA | LA | NYHA |
| (M/F) | (years) | disease | type | diameter | area | class | ||
| (mm) | (cmq) | |||||||
| F | 66 | MR | Pt | 58 | 25 | 3 | ||
| M | 70 | MS | Pt | Yes | 1 | 53 | 25 | 3 |
| F | 55 | MR | Ps | 55 | 36 | 4 | ||
| F | 60 | MS | Pt | 2 | 55 | 47 | 1 | |
| M | 74 | MR | Ps | 53 | 30 | 2 | ||
| M | 73 | MR | Pt | 52 | 30 | 3 | ||
| F | 72 | MS | Pt | 1 | 62 | 30 | 3 | |
| M | 68 | MR | Pt | 55 | 29 | 2 | ||
| M | 60 | MR | Pt | 42 | 21 | 1 | ||
| F | 69 | MR-TR | Pt | 55 | 32 | 2 |
| Patient | Age | Associated | AF | CVA | DCC | LA | LA | NYHA |
| (M/F) | (years) | disease | type | diameter | area | class | ||
| (mm) | (cmq) | |||||||
| F | 66 | MR | Pt | 58 | 25 | 3 | ||
| M | 70 | MS | Pt | Yes | 1 | 53 | 25 | 3 |
| F | 55 | MR | Ps | 55 | 36 | 4 | ||
| F | 60 | MS | Pt | 2 | 55 | 47 | 1 | |
| M | 74 | MR | Ps | 53 | 30 | 2 | ||
| M | 73 | MR | Pt | 52 | 30 | 3 | ||
| F | 72 | MS | Pt | 1 | 62 | 30 | 3 | |
| M | 68 | MR | Pt | 55 | 29 | 2 | ||
| M | 60 | MR | Pt | 42 | 21 | 1 | ||
| F | 69 | MR-TR | Pt | 55 | 32 | 2 |
M, male; F, female; AF, atrial fibrillation; CVA, cerebrovascular accident; DCC, direct current cardioversion; LA, left atrium; NYHA, New York Heart Association; MR, mitral regurgitation; Pt, permanent; MS, mitral stenosis; Ps, persistent; TR, tricuspid regurgitation.
2.2. Surgical technique
The right-sided access consists of a small thoracotomy (7 cm), in the 4th intercostal space, a 10-mm port in the 6th intercostal space, at the mid-axillary line, and a 5-mm camera port, in the 4th intercostal space anterior axillary line. The pericardium is divided 2 cm anterior to the right phrenic nerve, from ascending aorta to the diaphragm. The entire oblique and transverse sinuses are widely opened with blunt and sharp dissection. Similarly, dissection of the entire transverse sinus is extended across the entire dome of the LA and the tip of the left atrial appendage. If the cardiopulmonary bypass (CPB) is contemplated for concomitant procedures, the right femoral artery and femoral vein are exposed through a 3-cm longitudinal groin incision. CPB could facilitate the dissection and the positioning of the device around the pulmonary veins (PVs) but, sizing and HIFU ablation is always performed off CPB. A flexible introducer-sizer (EpicorTM PAS, St. Jude Medical, Sunnyvale, CA, USA) is pushed behind the superior vena cava, into the transverse sinus and directed toward both the inferior and posterior LA. Reaching the oblique sinus, the introducer is passed beneath the inferior vena cava, completely encircling all four PVs. After sizing of the circumference of the LA, an appropriate ablation device (EpicorTM UltraCinch LP, St. Jude Medical, Sunnyvale, CA, USA) is selected and anchored to the introducer to lead it around the LA. The PVs box lesion is completed in about 10 min. An additional lesion can be created epicardially, with a handheld ablation device (EpicorTM UltraWand LP, St Jude Medical, Sunnyvale, CA, USA). To obtain the mitral line ablation, extending from the lesion of PVs down to the mitral valve annulus, the handheld ablation device was used (Fig. 1 ) then placed through the oblique sinus, sliding from the inferior right PV towards the left. This linear ablation was completed in about 90 s. The electrical isolation of the PVs was not tested. Left appendage obliteration was performed in all patients during the concomitant mitral surgery .
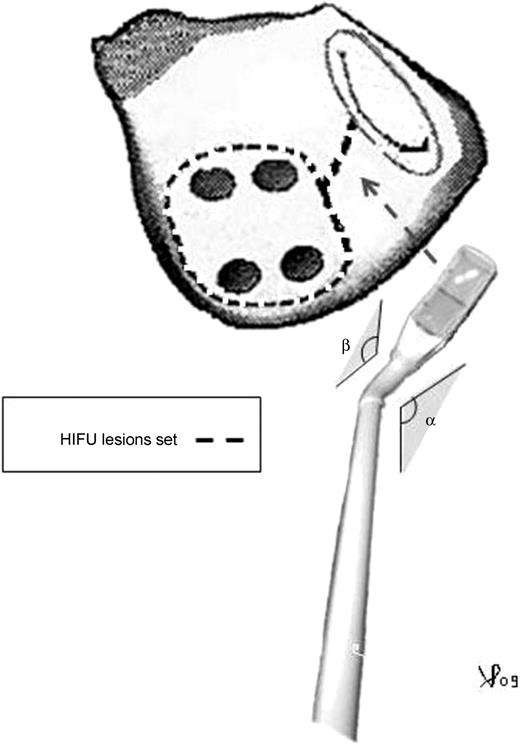
Illustration of the extracardiac left maze lesion pattern; α and β represent the angle of the ablation probe pre-formed before chest positioning. HIFU, high intensity focused ultrasound.
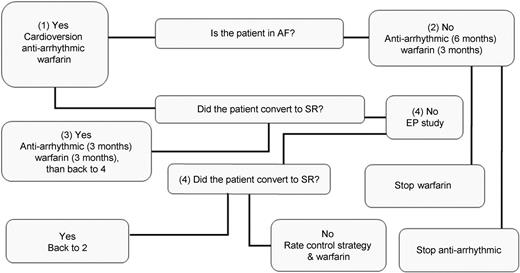
Postoperative management algorithm after three months and follow-up. EP, electrophysiologist; AF, atrial fibrillation; SR, sinus rhythm.
2.3. Echocardiographic technique
A multiplane TEE was used to localize and confirm the correct placement of the UltraWand on mitral isthmus. According to the standard projections for mitral valve analysis suggested by the International Society of Echocardiography, we selected two projections that allowed us to show the target area for mitral isthmus ablation. Upper and mid TEE four chambers, from 0° to 60° projections, allowed us to show the movement of the posterior atrial wall through a gently beating of the UltraWand's tip from the epicardium (Video 1 ). This movement, in the area between the P2 and P3 (according to the Carpentier classification), represents our target area for mitral isthmus ablation. This linear ablation, going from the bottom of the PVs encircling lesion across the coronary sinus left atrial isthmus, can be displayed during the ablation. In particular, we checked that the ablation probe did not obstruct the flow coming from the inferior left PV, because of an overstated pressure. This was obtained by color Doppler TEE imaging.
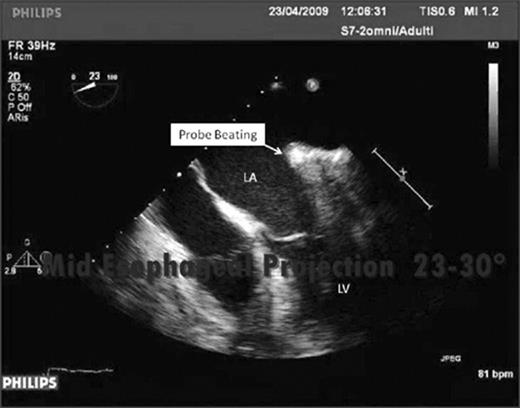
Positioning of the ablation probe on the mitral line verified by transesophageal echocardiography.
2.4. Postoperative management
Early postoperative care, including anticoagulant management, was similar to that given for routine cardiac surgery. Cardiac rhythm was continuously monitored and recorded after surgery until discharge. Anti-arrhythmic drug (AAD) was given from the 1st postoperative day with oral amiodarone, 400 mg, or sotalol, 80–120 mg was given daily. Warfarin was administered postoperatively with INR target of 2.5.
2.5. Follow-up
All patients were followed-up according the EHRS/EHRA/ECAS guidelines [7] usually for a period of one year. During this period, data were collected and sent to the International Atrial Fibrillation Surgery Registry of EACTS. Upon discharge, all patients who showed AF were scheduled for a direct current cardioversion (DCC) after three months. A 24-h Holter monitoring was considered at three months, six months and then annually, or earlier if deemed necessary. The presence of atrial contraction was documented by transthoracic echocardiography, performed at three and six months after surgery. After three months, anticoagulant drugs were discontinued if two following 24 h Holter recording showed a sinus rhythm (SR) or patient had a low thromboembolic risk (CHADs score <2). AADs were tapered gradually after six months post cardiac surgery, if the rhythm was considered stable. After one year and for as long as possible, patient status was determined by screening records of outpatient visits and correspondence with referring physicians (Fig. 2).
3. Results
Table 2 shows the postoperative data. The mean follow-up was 224.9±125.3 days. The operative time for AF ablation decreased significantly after the first two cases (mean 27.2±9.1 min). In these cases, we used two flexible introducers in the transverse and oblique sinus. A single introducer were used in all cases excepted the first two where we used the original technique with two introducers. The mitral line positioning and ablations were performed in <5 min. In four patients, the TEE images of the Ultrawand were more detailed for the device. In all cases TEE detected the movements of the target area using the ablation device.
| Surgery | LA | Hospital | DCC | FU | FU NYHA |
| area | rhythm | rhythm | class | ||
| MVP | 18 | SR | SR | 1 | |
| MVR | 17 | AF | 1 | SR | 1 |
| MVR | 34 | SR | SR | 1 | |
| MVR | 23 | AF | SR | 1 | |
| MVP | 30 | SR | SR | 1 | |
| MVP | 24 | SR | SR | 1 | |
| MVP | 26 | AT | 1 | SR | 1 |
| MVP | 29 | SR | SR | 1 | |
| MVP | 18 | SR | SR | 1 | |
| MVP-TP | 25 | AF | 1 | SR | 2 |
| Surgery | LA | Hospital | DCC | FU | FU NYHA |
| area | rhythm | rhythm | class | ||
| MVP | 18 | SR | SR | 1 | |
| MVR | 17 | AF | 1 | SR | 1 |
| MVR | 34 | SR | SR | 1 | |
| MVR | 23 | AF | SR | 1 | |
| MVP | 30 | SR | SR | 1 | |
| MVP | 24 | SR | SR | 1 | |
| MVP | 26 | AT | 1 | SR | 1 |
| MVP | 29 | SR | SR | 1 | |
| MVP | 18 | SR | SR | 1 | |
| MVP-TP | 25 | AF | 1 | SR | 2 |
LA, left atrium; DCC, direct current cardioversion; FU, follow-up; NYHA, New York Heart Association; MVP, mitral valve plasty; SR, sinus rhythm; MVR, mitral valve replacement; AF, atrial fibrillation; AT, atrial tachycardia; TP, tricuspid plasty.
| Surgery | LA | Hospital | DCC | FU | FU NYHA |
| area | rhythm | rhythm | class | ||
| MVP | 18 | SR | SR | 1 | |
| MVR | 17 | AF | 1 | SR | 1 |
| MVR | 34 | SR | SR | 1 | |
| MVR | 23 | AF | SR | 1 | |
| MVP | 30 | SR | SR | 1 | |
| MVP | 24 | SR | SR | 1 | |
| MVP | 26 | AT | 1 | SR | 1 |
| MVP | 29 | SR | SR | 1 | |
| MVP | 18 | SR | SR | 1 | |
| MVP-TP | 25 | AF | 1 | SR | 2 |
| Surgery | LA | Hospital | DCC | FU | FU NYHA |
| area | rhythm | rhythm | class | ||
| MVP | 18 | SR | SR | 1 | |
| MVR | 17 | AF | 1 | SR | 1 |
| MVR | 34 | SR | SR | 1 | |
| MVR | 23 | AF | SR | 1 | |
| MVP | 30 | SR | SR | 1 | |
| MVP | 24 | SR | SR | 1 | |
| MVP | 26 | AT | 1 | SR | 1 |
| MVP | 29 | SR | SR | 1 | |
| MVP | 18 | SR | SR | 1 | |
| MVP-TP | 25 | AF | 1 | SR | 2 |
LA, left atrium; DCC, direct current cardioversion; FU, follow-up; NYHA, New York Heart Association; MVP, mitral valve plasty; SR, sinus rhythm; MVR, mitral valve replacement; AF, atrial fibrillation; AT, atrial tachycardia; TP, tricuspid plasty.
There were no major complications related with the procedure except for two patients who showed temporary phrenic nerve damage with a right diaphragmatic elevation on chest X-ray. One patient had a postoperative atrial tachycardia (AT) and was cardioverted during hospitalization. At discharge, three patients were in AF and a cardioversion was scheduled for three months later as described in methods section. In one case, a spontaneous SR was recorded after two months and no longer required cardioversion. Three months postoperation, the Holter recordings revealed a stable SR in all patients.
4. Discussion
MIS operations offer easy methods of treating AF even if, they have less impact on invasiveness, AF ablation is mainly focused on PVI [8, 9]. The mitral isthmus, if anatomically feasible from the epicardium and on the closed LA, is performed less often on beating hearts, due to the immediate proximity of the circumflex coronary artery. The inability to safely create this lesion has led most surgeons to eliminate it from the epicardial lesion set [10, 11]. This approach, even if debated, does not address the lesion set to macro re-entry, resulting in impaired efficacy against advanced AF [12]. Ablation with the HIFU device was previously used to perform both PVI and mitral line during beating heart surgery. Some authors reported 76–80% of freedom from recurrence of AF, in the permanent AF group [5, 6]. Damage of the circumflex artery or PVs stenosis was not described. A new release of the Epicor system was improved, mainly for flexibility of the Ultracinch, enabling its use in MIS applications. We had no major complications but the first two cases improved understanding and optimized the skill used in reducing the time for PVI. In particular, the surgical strategy used to position the sizer around the PVs changed, allowing a significant reduction of the operative time to be obtained, from 47 to 21 min (mean 27±8 min). Direct control of the Ultrawand on the mitral isthmus is always preferred, but this operation is limited through the small right thoracotomy. Our proposal was to search for a method to safely pilot the Ultrawand into the target area for ablation. We found that a TEE mid-esophageal four-chamber projection permitted the localization of the transducer placed on posterior mitral annulus. In particular, we observed the movement of the atrial wall and mitral sectors caused by the compression of the ablation device. We could also observe the blood flow in the left inferior PV, which should not be influenced by the force of the operator. The conductance test of the PVs was not performed according to our previous clinical experience and reports [5, 6]. In fact, the presence of immediate electrical isolation after the catheter and surgical ablation does not always correlate with the histological transmurality, and it is not a certain predictor of freedom from late AF [13, 14]. Different mechanisms and structural changes (dilatation of the LA cavity, hypertrophy, fibrosis formation, myocardial fibers), provide an electrophysiological substrate for AF [15]. All factors should influence the selection criteria for a patient's selection.
In conclusion, defining the best treatment for AF is a challenge. This surgical technique supports the concept of the left Maze and brings us closer to a full Maze procedure, as Ad and Cox described [8], with an additional lesion set.
We believe this procedure is effective for permanent AF, and safe with a minimal learning curve. If our results continue to sustain this technique, then this approach will represent a viable therapeutic option for the appropriate group of patients.
Disclosures and freedom of investigation: All equipment was purchased at cost by the host institution. Patient selection, operative technique, and study design were determined solely at the discretion of the authors. The authors disclose that they have a financial relationship with St Jude Medical Italia Spa.
The authors thank Michael Lawrence for editing the manuscript.
References
- atrial fibrillation
- echocardiography
- transesophageal echocardiography
- electric countershock
- atrial tachycardia
- surgical procedures, minimally invasive
- cardiac pacemaker implantation
- ventricular tachycardia, induced
- operative risk
- heart valve bioprosthesis stenosis
- follow-up
- patient discharge
- pulmonary veins
- surgical procedures, operative
- thoracotomy
- heart
- mortality
- minithoracotomy
- sinus rhythm
- high-intensity focused ultrasound
- medical devices
- ablation
- intercostal space




