-
PDF
- Split View
-
Views
-
Cite
Cite
Sachith Munasinghe, Irina Geiculescu, Adam Greene, Sana Syed, Jason Matthews, S Kugathasan, MODULATION OF INTESTINAL EPITHELIAL IMMUNE RESPONSES BY HMG-COA PATHWAYS, Inflammatory Bowel Diseases, Volume 31, Issue Supplement_1, February 2025, Page S49, https://doi.org/10.1093/ibd/izae282.116
Close - Share Icon Share
Abstract
While the involvement of the immune system has been established in sustaining Crohn’s disease (CD), growing evidence suggests that dysfunction in epithelial metabolic pathways might be linked with CD pathogenesis. Preliminary bioinformatic analysis of ileal CD organoid transcriptomes suggests that imbalances in the mevalonate pathway are associated with CD. To this end, we investigated the effect of inhibiting a key mediator in the pathway, HMG-CoA reductase, with atorvastatin, on inflammatory signaling in patient-derived organoids.
Organoids from patients consented at Children’s Healthcare of Atlanta were established from their ileal and rectal mucosal biopsies. Organoids grown in Intesticult Human Growth Media were stimulated alone with IFN-γ (1 ng/ml), TNF-α (25 ng/ml), and IL-13 (10 ng/ml), or in combination (cytomix) for 48 hrs. mRNA levels of CXCL8, CXCL9 and ICAM1 were measured by real time PCR. Phosphorylated STAT1 (Y701 and S727), STAT3 (Y705 and S727), STAT6 (Y641), and ERK1/2 (T202/Y204) levels were determined via Western Blot. Cell survival was determined by cleaved caspase 3 (via western blot) and CellTiter-Glo® 3D Cell Viability Assay. Atorvastatin was used at 10 µM to the culture media with the other cytokines simultaneously to test its ability to mitigate inflammatory signals. Conditioned media from these organoid cultures were collected for ELISA at 48 hours to assess changes in secretomes.
Atorvastatin 10 μM co-administration with IFN-γ blocked phosphorylation of STAT1 at S727; however, the induced STAT1 (Y701) phosphorylation and CXCL9 and ICAM1 transcript levels were relatively unaffected. Atorvastatin did not block IL-13-induced phosphorylation of STAT6 (Y641), but did lower endogenous levels of pERK1/2 (T202/Y204). Stimulation with TNF-α significantly increased CXCL8 mRNA in organoids but Atorvastin co-treatment had minimal effect on this increase. When combined as a cytomix, ileal organoids showed increased levels of pSTAT1 (S727), pSTAT1 (Y701), pSTAT6 (Y641) and pERK1/2 (T202/Y204) after 48 hrs (n=4) (Figure 1). When co-administered with atorvastatin, the cytomix-dependent increases in levels of pSTAT1 (S727) and pERK 1/2 (T202/Y204) were mitigated but phosphorylation of STAT1 (Y701), STAT3 (Y705 and S727) and STAT6 (Y461), as well as the levels of CXCL9, ICAM1, and CXCL8, were not affected (Figure 1 and 2).
HMG-CoA regulates aspects of the JAK/STAT and ERK pathways during inflammatory signaling mediated by IFN-γ and IL-13 alone or in combination with TNF-α, but did not appear to affect CXCL8, CXCL9 and ICAM1 expression levels. Ongoing studies will examine the impact of HMG-CoA inhibition on the secretome with focus on functional consequences of these differences with effects on the epithelium.

Figure 1: Changes in phosphorylation of pSTAT1, pSTAT 6 and pERK 1/2 with atorvastatin treatment in ileal organoids stimulated with IFN-λ, TNF-α and IL13.
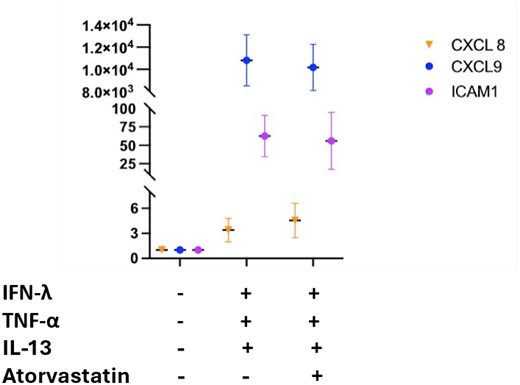
Figure 2: CXCL8, ICAM1 and CXCL9 relative abundance after treatment with atorvastatin in the cytokine mix.
- atorvastatin
- cytokine
- polymerase chain reaction
- tumor necrosis factors
- signal transduction
- western blotting
- enzyme-linked immunosorbent assay
- biopsy
- immune response
- crohn's disease
- epithelium
- cell survival
- child
- coenzyme a
- culture media
- culture media, conditioned
- hydroxymethylglutaryl-coa reductases
- immune system
- intercellular adhesion molecule 1
- interleukin-13
- intestines
- organoids
- phosphorylation
- rna, messenger
- ileum
- mucous membrane
- stat3 protein
- stat1 gene
- mevalonate
- caspase-3
- bioinformatics
- secretome



