-
PDF
- Split View
-
Views
-
Cite
Cite
Bruce E Sands, Silvio Danese, J Casey Chapman, Khushboo Gurjar, Stacy Grieve, Deepika Thakur, Jenny Griffith, Namita Joshi, Kristina Kligys, Axel Dignass, Mucosal and Transmural Healing and Long-term Outcomes in Crohn’s Disease, Inflammatory Bowel Diseases, Volume 31, Issue 3, March 2025, Pages 857–877, https://doi.org/10.1093/ibd/izae159
Close - Share Icon Share
Abstract
Healing in Crohn’s disease is complex and difficult to measure due to incongruencies between clinical symptoms and disease states. Mucosal healing (MH) and transmural healing (TH) are increasingly used to measure clinical improvement in Crohn’s disease, but definitions of MH and TH can vary across studies, and their relationship to long-term outcomes is not clear. To address this knowledge gap, we performed a systematic literature review (SLR) to examine studies measuring MH and TH in Crohn’s disease.
Database records from 2012 to 2022 were searched for real-world evidence and interventional studies that reported the association of MH or TH with clinical, economic, or quality of life outcomes of adult patients with Crohn’s disease.
A total of 46 studies were identified in the systematic literature review, representing a combined patient population of 5530. Outcomes of patients with MH were reported by 39 studies; of these, 14 used validated scales for endoscopic assessment. Thirteen studies reported outcomes of patients with TH. Among studies that examined the outcomes of patients with and without MH or TH, patients with healing generally experienced improved clinical outcomes and reduced healthcare resource utilization, including fewer hospitalizations and surgeries and improved rates of clinical remission. This was especially true for patients with TH.
Mucosal and transmural healing are associated with positive long-term outcomes for adult patients with Crohn’s disease. The adoption of standardized measures and less invasive assessment tools will maximize the benefits of patient monitoring.
Lay Summary
Inflammation of the bowel wall is a key component of Crohn’s disease (CD). A systematic literature review (SLR) showed bowel wall healing was associated with positive long-term outcomes in CD, supporting healing as an indicator of disease control.
Achievement of mucosal healing (MH) and transmural healing (TH) are long-term therapeutic goals despite variations in their definitions and uncertainty in their association with long-term outcomes.
This systematic literature review (SLR) is the first to show that both MH and TH are associated with improved clinical outcomes including increased rates of clinical remission, decreased rates of relapse, and fewer hospitalizations and surgeries among patients with CD.
MH and TH may serve as useful indicators of clinical outcomes and disease control in CD. However, standardization and better education for clinicians will be paramount for the adoption of endoscopic monitoring of healing for CD.
Introduction
Crohn’s disease (CD) is an inflammatory bowel disease characterized by chronic inflammation of the bowel wall (mucosal and transmural inflammation) that causes mucosal injury and intestinal damage, commonly resulting in complications like abscesses, fistulae, stricture, and intestinal obstruction.1–5 Long-term mucosal inflammation is associated with an increased risk of hospitalization, complications that may require surgery, and cancer.2,6 Symptoms of CD include abdominal pain, fatigue, diarrhea, fever, anemia, urgency, and weight loss,7,8 which negatively impact quality of life and social functioning, and slightly more than one-third of patients experience anxiety or depression.9 However, the clinical symptoms of CD are not reliable indicators of mucosal inflammation and provide an incomplete picture of disease control and response to treatment.3,5,10,11 This disconnect between symptoms and mucosal inflammation in CD necessitates monitoring of both for accurate assessment of disease severity.
Until recently, endoscopy has been the primary instrument used to assess mucosal inflammation in CD. Endoscopy is an invasive procedure with barriers to repeatability, including the need for bowel preparation, risk of complications, and high cost.12 Other measures used to assess CD are the biomarkers fecal calprotectin (FC) and C-reactive protein (CRP), although neither is specific to CD.10 While traditional endoscopy is limited in its ability to assess the small intestine due to the length and diameter of the small intestine, small capsule endoscopy and enteroscopy are gaining attention as alternatives to this traditional approach.13,14 Intestinal ultrasound, magnetic resonance imaging (MRI), and computed tomography (CT) are noninvasive options that provide high imaging accuracy, but tools to standardize the measurement of disease severity in CD require further validation.15,16
Adding to the complexity of treating CD, definitions of healing (mucosal or transmural) for the disease have not been clearly defined, and long-term, deep healing has not been taken into consideration until recently.17 The definition of healing in CD has been based on endoscopic or imaging examinations (eg, MRI, CT imaging, or intestinal ultrasound), which are used for diagnosis and treatment monitoring. Endoscopic healing has been recommended by the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) initiative of the International Organization for the Study of Inflammatory Bowel Diseases (IOIBD) as a long-term target for CD and is assessed through ileocolonoscopy, enteroscopy, or video capsule endoscopy for small bowel disease. In STRIDE II, the achievement of endoscopic healing was confirmed as an important therapeutic goal that enabled patients to achieve improved long-term outcomes.10 Historically, mucosal healing (MH) has been synonymous with endoscopic healing, indicating the resolution of mucosal inflammation and damage visible through colonoscopy and other endoscopies (video capsule, enteroscopy with single or double balloon, or spiral, and gastroscopy in the case of upper gastrointestinal [GI] involvement). Mucosal healing has been reported to be significantly associated with long-term clinical remission.18 More recently, definitions of MH increasingly incorporate histologic healing, although this is still not well-defined and lacks agreement among clinicians and experts.19
Increasingly, transmural healing (TH), which represents healing across all layers of the bowel, has been recognized as an important clinical outcome with long-term benefits, since CD involves inflammation throughout the intestinal wall and not just in the mucosa.20–22 In the CALM study, deep remission (defined as “endoscopic remission, clinical remission, and no steroid use”) correlated with TH and was associated with lower rates of disease progression over time.18 The IOIBD’s STRIDE initiative recommended in their 2021 treat-to-target strategies for clinical practice that TH be used to assess the depth of remission but did not recommend it as a formal target since the data were still limited.10
Despite recommendations for MH and TH as treat-to-target goals, they are infrequently implemented in clinical practice, perhaps because of the limited data on their importance for long-term outcomes in CD.18 Both MH and TH may change the natural course of CD by reducing inflammation that would normally lead to structural damage to the bowel and increase the risk of surgery, hospitalization, or long-term complications. However, a clear connection between MH and TH and potential clinical benefits has not been firmly established. The objective of this study was to examine how MH and TH relate to long-term clinical, economic (eg, healthcare resource utilization [HCRU]), and quality of life outcomes among patients with CD.
Methods
A systematic literature review (SLR) was conducted following the principles outlined in the Cochrane Handbook for Systematic Reviews of Interventions,23 Centre for Reviews and Dissemination (CRD)’s Guidance for Undertaking Reviews in Healthcare,24 and Methods for the Development of National Institute for Health and Care Excellence (NICE) Public Health Guidance.25 Cochrane, MEDLINE, EconLit, and EMBASE databases were searched on July 28, 2022, and the CRD database was searched on August 2, 2022, to examine the association of MH or endoscopic outcomes with improved long-term clinical and economic outcomes among adult patients with CD. A detailed search strategy is provided in Supplementary Table 1. Real-world evidence studies and interventional studies (including randomized controlled trials [RCTs]) from within the past 10 years (2012 to July 28, 2022) were included. Systematic literature reviews and meta-analyses were included for the purpose of cross-checking and identifying primary literature sources through a bibliographic search. Publications were reviewed based on their titles and abstracts against the inclusion and exclusion criteria (Supplementary Table 2) by 2 independent researchers, and disagreements were resolved by a third independent reviewer. Two reviewers then screened all citations and full-text articles, and any discrepancies in their decisions were resolved by a third independent reviewer. Data were extracted in a predefined extraction form by one reviewer and independently checked for accuracy and completeness by a second reviewer.
The SLR protocol captured the broadest inclusion of all definitions of MH, TH, and specific outcomes. This review focuses on the studies using validated scales to measure MH, including the Simple Endoscopic Score for Crohn’s Disease (SES-CD),26 Modified Simple Endoscopic Score for Crohn’s Disease (mSES-CD),27 Crohn’s Disease Endoscopic Index of Severity (CDEIS),28 and Lewis score.29 Among the included studies, modalities such as CT enterography (CTE), magnetic resonance enterography (MRE), and MRI were used to monitor TH, with specific measures or scales including bowel wall thickness (BWT) or the Magnetic Resonance Index of Activity (MaRIA) scale.
Results
A total of 5094 records were identified in the SLR. Of these, 484 records were included for full-text review after deduplicating and screening them against the population, intervention, comparator, outcome, and study design (PICOS) criteria (Supplementary Table 2). Forty-six studies met the final inclusion criteria and were selected for extraction. Most of these studies were real-world evidence studies (28 retrospective and 14 prospective), and the 4 remaining studies had other study designs (2 interventional RCTs and 2 post hoc analyses of phase 3 RCTs, Figure 1). Twenty-five studies reported clinical outcomes among patients with MH vs patients without MH. Fourteen studies used validated scales (Table 1; Figure 2).
| Studies Reporting MH Using Validated Measures . | ||||||
|---|---|---|---|---|---|---|
| Study Reference (author, year) | Study Design | Geography | N | Patient Population | Validated Measure Used and Cut-off Score | Interventions |
| Nishikawa, 2020 | Retrospective, single center | Japan | 60 | Patients with CD who underwent capsule endoscopy | Lewis score <270 | Immunomodulator, anti-TNFα agent, prednisolone |
| Hoekman, 2018 | Retrospective, multicenter | Belgium, the Netherlands, Germany | 49 | Adult patients with mild to moderate CD | SES-CD = 0 | Corticosteroids, anti-TNF agents |
| Laterza, 2018 | Prospective, single center | Italy | 57 | Patients with CD | SES-CD ≤2 or Rutgeert’s score i0-i1 (for patients with history of previous surgery) | NR |
| Castiglione, 2019 | Prospective, single center, longitudinal | Italy | 218 | Patients with CD achieving TH following treatment with biologics Patients reaching only MH or no healing | SES-CD ≤2 | Infliximab (IFX) and adalimumab (ADA) |
| Takenaka, 2018 | Prospective, single center | Japan | 116 | Patients with CD in clinical-serological remission | SES-CDa = 0 | Concomitant treatment of steroids, immunomodulator, or anti-TNF inhibitor |
| Sagami, 2019 | Retrospective, single center | Japan | 50 | Patients with CD who underwent ileocolonoscopy and MRE | SES-CD <5 | 5-aminosalicylic acid (5-ASA), glucocorticoid, azathioprine (AZA), 6-mercaptopurine (6-MP), IFX, ADA, elemental diet, antibiotics, none |
| Naganuma, 2016 | Retrospective, single center | Japan | 131 | Patients with CD with clinical remission and who underwent conventional ileocolonoscopy | SES-CD ≤2 | Surgical, nonsurgical |
| Yzet, 2019 | Retrospective, single center | France | 84 | Patients with CD | CDEIS = 0 | IFX, ADA or vedolizumab |
| Sakuraba, 2013 | Retrospective, single center, case review | US | 32 | Patients with CD who underwent ≥1 colonoscopy before or during treatment | SES-CD >70% reduction | Natalizumab |
| Morise, 2015 | Retrospective, single center | Japan | 76 | Patients with CD who underwent transanal double-balloon endoscopy (DBE), included patients with stenosis which hampered passage of the scope and those who underwent DBE with observation for ≥ 80 cm from the ileocecal valve | mSES-CD <4 or SES-CD <4 | IFX, AZA, elemental diet, 5-ASA |
| Beigel, 2014 | Retrospective, single center | Germany | 152 | CD subgroup patients treated with anti-TNFα antibodies (IFX and/or ADA) | SES-CD = 0 | One anti-TNFα antibody or 2 anti-TNFα antibodies |
| Macedo Silva, 2022 | Retrospective, case control | Portugal | 47 | CD subgroup patients treated with anti-TNFα antibodies (IFX and/or ADA) | Lewis score ≤135 | Thiopurines, biologics, corticosteroids |
| Elosua, 2021 | Retrospective, single center | Spain | 432 | Established patients with CD with SBCE | Lewis score <135 | 5-ASA, steroids, immunomodulators (thiopurines and methotrexate) and biologics (IFX, ADA, ustekinumab, vedolizumab and certolizumab) |
| Ben-Horin, 2019 | Prospective, multicenter | Israel | 61 | Adult patients with CD involving the small bowel with confirmed small bowel patency | Lewis score <350 | Immunomodulators, biologics |
| Studies Reporting TH . | ||||||
| Study reference (author, year) | Study Design | Geography | N | Patient Population | Measure used and Criteria for Healing | Interventions |
| Halle, 2020 | Retrospective, single center | France | 115 | Small bowel CD | A radiological response was defined as (1) a complete response, if all inflammatory signs had disappeared, (2) a partial response if the length of the wall enhancement of any inflammatory segment decreased or if there was a clear decrease in the size of an abscess or fistula without worsening of the other inflammatory parameters, (3) progression if any inflammatory segment showed a clear increase in any of inflammatory parameters or if a new inflammatory lesion appeared, and (4) stable in other cases | Immunosuppressant, anti-TNF treatment, vedolizumab, ustekinumab, steroids |
| Ma, 2021 | Prospective, single center, cohort | China | 77 | Patients with CD who had been consecutively hospitalized without use of CD-related steroids, immunosuppressants, or biologics for ≥6 months prior | BWT ≤3 mm in any place, with the normalization of stratification, no hypervascularization, the resolution of mesenteric inflammatory fat, and no signs of active inflammation (abscesses or fistulas) | NR |
| Messadeg, 2020 | Prospective, multicenter | France | 46 | Adult patients with CD | Early transmural response, defined as a 25% decrease of either Clermont score or MaRIA. Clermont score: 1.646 × bowel thickness − 1.321 × ADC + 5.613 × edema + 8.306 × ulcers + 5.039 MaRIA: 1.5 × wall thickening [mm] + 0.02 × RCE + 5 × edema + 10 × ulceration Transmural response: Cumulative score using 5 parameters (disappearance of ulcerations; disappearance in enlarged lymph nodes, disappearance of sclerolipomatosis; 30% decrease of RCE; 10% increase in ADC) | IFX or ADA |
| Deepak, 2016 | Retrospective, single center | US | 150 | Patients with small bowel CD who had pre-therapy CTE/MRE with follow-up CTE or MRE after 6 months, or 2 CTE/MREs ≥6 months apart while on maintenance therapy | CTE or MRE | NR |
| Laterza, 2018 | Prospective, single center | Italy | 57 | Patients with CD who underwent a clinical, endoscopic, and radiological assessment of disease within 1 month | CTE Absence of lesions | NR |
| Castiglione, 2019 | Prospective, single center, longitudinal | Italy | 218 | Patients with CD achieving TH following treatment with biologics Patients reaching only MH or no healing | Normalization of BWT of all inflamed segments involved in CD; BWT ≤3 mm | IFX and ADA |
| Paredes, 2019 | Prospective, single center | Spain | 36 | Adult patients with CD who were treated with biologics | Normalization of BWT (<3 mm) | IFX or ADA |
| Sagami, 2019 | Retrospective, single center | Japan | 50 | Patients with CD who underwent ileocolonoscopy and MRE | MaRIA score of <50 | 5-ASA, glucocorticoid, AZA, 6-MP, IFX, ADA, elemental diet, antibiotics, none |
| Fernandes, 2017 | Retrospective, single center | Portugal | 214 | Patients with CD | Both a normal MRE and normal endoscopy: complete healing of all the bowel layers (including the mucosa) | Thiopurines, methotrexate, anti-TNF therapy |
| Lafeuille, 2021 | Retrospective, database review | France | 154 | Adult patients with CD | Combination of endoscopic MH and MRI healing (absence of mucosal ulceration including aphtoid erosions and no sign of active inflammation (ie, ulceration, edema, diffusion-weighted hyperintensity, increased contrast enhancement), no extra-enteric sign (ie, fat creeping, enlarged lymph nodes, comb sign), and no CD-related complications (ie, stricture, fistula, or abscess)) | 5-ASA, corticoids, immunosuppressants, anti-TNF agents, IFX, ADA, golimumab, vedolizumab, ustekinumab |
| Helwig, 2022 | Prospective, noninterventional, multicenter | Germany | 180 | Adult patients with ileocecal or colonic CD | Simplified TH: Normalization of BWT (terminal ileum ≤2mm, colon ≤3mm) and the absence of an amplified color Doppler signal (Limberg 1/2) Extended TH: Normalized BWT and normalized color Doppler signal or no loss of stratification or no fibrofatty proliferation Complete TH: All 4 factors normalized | Systemic corticosteroids, AZA/6-MP, anti-TNF, anti-integrin, anti IL12/23 |
| Studies Reporting MH Using Validated Measures . | ||||||
|---|---|---|---|---|---|---|
| Study Reference (author, year) | Study Design | Geography | N | Patient Population | Validated Measure Used and Cut-off Score | Interventions |
| Nishikawa, 2020 | Retrospective, single center | Japan | 60 | Patients with CD who underwent capsule endoscopy | Lewis score <270 | Immunomodulator, anti-TNFα agent, prednisolone |
| Hoekman, 2018 | Retrospective, multicenter | Belgium, the Netherlands, Germany | 49 | Adult patients with mild to moderate CD | SES-CD = 0 | Corticosteroids, anti-TNF agents |
| Laterza, 2018 | Prospective, single center | Italy | 57 | Patients with CD | SES-CD ≤2 or Rutgeert’s score i0-i1 (for patients with history of previous surgery) | NR |
| Castiglione, 2019 | Prospective, single center, longitudinal | Italy | 218 | Patients with CD achieving TH following treatment with biologics Patients reaching only MH or no healing | SES-CD ≤2 | Infliximab (IFX) and adalimumab (ADA) |
| Takenaka, 2018 | Prospective, single center | Japan | 116 | Patients with CD in clinical-serological remission | SES-CDa = 0 | Concomitant treatment of steroids, immunomodulator, or anti-TNF inhibitor |
| Sagami, 2019 | Retrospective, single center | Japan | 50 | Patients with CD who underwent ileocolonoscopy and MRE | SES-CD <5 | 5-aminosalicylic acid (5-ASA), glucocorticoid, azathioprine (AZA), 6-mercaptopurine (6-MP), IFX, ADA, elemental diet, antibiotics, none |
| Naganuma, 2016 | Retrospective, single center | Japan | 131 | Patients with CD with clinical remission and who underwent conventional ileocolonoscopy | SES-CD ≤2 | Surgical, nonsurgical |
| Yzet, 2019 | Retrospective, single center | France | 84 | Patients with CD | CDEIS = 0 | IFX, ADA or vedolizumab |
| Sakuraba, 2013 | Retrospective, single center, case review | US | 32 | Patients with CD who underwent ≥1 colonoscopy before or during treatment | SES-CD >70% reduction | Natalizumab |
| Morise, 2015 | Retrospective, single center | Japan | 76 | Patients with CD who underwent transanal double-balloon endoscopy (DBE), included patients with stenosis which hampered passage of the scope and those who underwent DBE with observation for ≥ 80 cm from the ileocecal valve | mSES-CD <4 or SES-CD <4 | IFX, AZA, elemental diet, 5-ASA |
| Beigel, 2014 | Retrospective, single center | Germany | 152 | CD subgroup patients treated with anti-TNFα antibodies (IFX and/or ADA) | SES-CD = 0 | One anti-TNFα antibody or 2 anti-TNFα antibodies |
| Macedo Silva, 2022 | Retrospective, case control | Portugal | 47 | CD subgroup patients treated with anti-TNFα antibodies (IFX and/or ADA) | Lewis score ≤135 | Thiopurines, biologics, corticosteroids |
| Elosua, 2021 | Retrospective, single center | Spain | 432 | Established patients with CD with SBCE | Lewis score <135 | 5-ASA, steroids, immunomodulators (thiopurines and methotrexate) and biologics (IFX, ADA, ustekinumab, vedolizumab and certolizumab) |
| Ben-Horin, 2019 | Prospective, multicenter | Israel | 61 | Adult patients with CD involving the small bowel with confirmed small bowel patency | Lewis score <350 | Immunomodulators, biologics |
| Studies Reporting TH . | ||||||
| Study reference (author, year) | Study Design | Geography | N | Patient Population | Measure used and Criteria for Healing | Interventions |
| Halle, 2020 | Retrospective, single center | France | 115 | Small bowel CD | A radiological response was defined as (1) a complete response, if all inflammatory signs had disappeared, (2) a partial response if the length of the wall enhancement of any inflammatory segment decreased or if there was a clear decrease in the size of an abscess or fistula without worsening of the other inflammatory parameters, (3) progression if any inflammatory segment showed a clear increase in any of inflammatory parameters or if a new inflammatory lesion appeared, and (4) stable in other cases | Immunosuppressant, anti-TNF treatment, vedolizumab, ustekinumab, steroids |
| Ma, 2021 | Prospective, single center, cohort | China | 77 | Patients with CD who had been consecutively hospitalized without use of CD-related steroids, immunosuppressants, or biologics for ≥6 months prior | BWT ≤3 mm in any place, with the normalization of stratification, no hypervascularization, the resolution of mesenteric inflammatory fat, and no signs of active inflammation (abscesses or fistulas) | NR |
| Messadeg, 2020 | Prospective, multicenter | France | 46 | Adult patients with CD | Early transmural response, defined as a 25% decrease of either Clermont score or MaRIA. Clermont score: 1.646 × bowel thickness − 1.321 × ADC + 5.613 × edema + 8.306 × ulcers + 5.039 MaRIA: 1.5 × wall thickening [mm] + 0.02 × RCE + 5 × edema + 10 × ulceration Transmural response: Cumulative score using 5 parameters (disappearance of ulcerations; disappearance in enlarged lymph nodes, disappearance of sclerolipomatosis; 30% decrease of RCE; 10% increase in ADC) | IFX or ADA |
| Deepak, 2016 | Retrospective, single center | US | 150 | Patients with small bowel CD who had pre-therapy CTE/MRE with follow-up CTE or MRE after 6 months, or 2 CTE/MREs ≥6 months apart while on maintenance therapy | CTE or MRE | NR |
| Laterza, 2018 | Prospective, single center | Italy | 57 | Patients with CD who underwent a clinical, endoscopic, and radiological assessment of disease within 1 month | CTE Absence of lesions | NR |
| Castiglione, 2019 | Prospective, single center, longitudinal | Italy | 218 | Patients with CD achieving TH following treatment with biologics Patients reaching only MH or no healing | Normalization of BWT of all inflamed segments involved in CD; BWT ≤3 mm | IFX and ADA |
| Paredes, 2019 | Prospective, single center | Spain | 36 | Adult patients with CD who were treated with biologics | Normalization of BWT (<3 mm) | IFX or ADA |
| Sagami, 2019 | Retrospective, single center | Japan | 50 | Patients with CD who underwent ileocolonoscopy and MRE | MaRIA score of <50 | 5-ASA, glucocorticoid, AZA, 6-MP, IFX, ADA, elemental diet, antibiotics, none |
| Fernandes, 2017 | Retrospective, single center | Portugal | 214 | Patients with CD | Both a normal MRE and normal endoscopy: complete healing of all the bowel layers (including the mucosa) | Thiopurines, methotrexate, anti-TNF therapy |
| Lafeuille, 2021 | Retrospective, database review | France | 154 | Adult patients with CD | Combination of endoscopic MH and MRI healing (absence of mucosal ulceration including aphtoid erosions and no sign of active inflammation (ie, ulceration, edema, diffusion-weighted hyperintensity, increased contrast enhancement), no extra-enteric sign (ie, fat creeping, enlarged lymph nodes, comb sign), and no CD-related complications (ie, stricture, fistula, or abscess)) | 5-ASA, corticoids, immunosuppressants, anti-TNF agents, IFX, ADA, golimumab, vedolizumab, ustekinumab |
| Helwig, 2022 | Prospective, noninterventional, multicenter | Germany | 180 | Adult patients with ileocecal or colonic CD | Simplified TH: Normalization of BWT (terminal ileum ≤2mm, colon ≤3mm) and the absence of an amplified color Doppler signal (Limberg 1/2) Extended TH: Normalized BWT and normalized color Doppler signal or no loss of stratification or no fibrofatty proliferation Complete TH: All 4 factors normalized | Systemic corticosteroids, AZA/6-MP, anti-TNF, anti-integrin, anti IL12/23 |
Abbreviations: 5-ASA, 5-aminosalicylic acid; 6-MP, 6-mercaptoprine; ADA, adalimumab; AZA, azathioprine; ADC, apparent diffusion coefficient; BWT, bowel wall thickness; CD, Crohn’s disease; CDEIS, Crohn’s Disease Endoscopic Index of Severity; CTE, computed tomography enterography; DBE, double balloon endoscopy; IFX, Infliximab; MaRIA, Magnetic Resonance Index of Activity; MH, mucosal healing; MRE, magnetic resonance enterography; MRI, magnetic resonance imaging; mSES-CD, modified SES-CD; NR, not reported; RCE, relative contrast enhancement; SBCE, small bowel endoscopy; SES-CD, Simple Endoscopic Score for Crohn’s Disease; SES-CDa, Simple Endoscopic Active Score for Crohn’s Disease; TH, transmural healing; TNF, tumor necrosis factor; TNFα, tumor necrosis factor alpha.
| Studies Reporting MH Using Validated Measures . | ||||||
|---|---|---|---|---|---|---|
| Study Reference (author, year) | Study Design | Geography | N | Patient Population | Validated Measure Used and Cut-off Score | Interventions |
| Nishikawa, 2020 | Retrospective, single center | Japan | 60 | Patients with CD who underwent capsule endoscopy | Lewis score <270 | Immunomodulator, anti-TNFα agent, prednisolone |
| Hoekman, 2018 | Retrospective, multicenter | Belgium, the Netherlands, Germany | 49 | Adult patients with mild to moderate CD | SES-CD = 0 | Corticosteroids, anti-TNF agents |
| Laterza, 2018 | Prospective, single center | Italy | 57 | Patients with CD | SES-CD ≤2 or Rutgeert’s score i0-i1 (for patients with history of previous surgery) | NR |
| Castiglione, 2019 | Prospective, single center, longitudinal | Italy | 218 | Patients with CD achieving TH following treatment with biologics Patients reaching only MH or no healing | SES-CD ≤2 | Infliximab (IFX) and adalimumab (ADA) |
| Takenaka, 2018 | Prospective, single center | Japan | 116 | Patients with CD in clinical-serological remission | SES-CDa = 0 | Concomitant treatment of steroids, immunomodulator, or anti-TNF inhibitor |
| Sagami, 2019 | Retrospective, single center | Japan | 50 | Patients with CD who underwent ileocolonoscopy and MRE | SES-CD <5 | 5-aminosalicylic acid (5-ASA), glucocorticoid, azathioprine (AZA), 6-mercaptopurine (6-MP), IFX, ADA, elemental diet, antibiotics, none |
| Naganuma, 2016 | Retrospective, single center | Japan | 131 | Patients with CD with clinical remission and who underwent conventional ileocolonoscopy | SES-CD ≤2 | Surgical, nonsurgical |
| Yzet, 2019 | Retrospective, single center | France | 84 | Patients with CD | CDEIS = 0 | IFX, ADA or vedolizumab |
| Sakuraba, 2013 | Retrospective, single center, case review | US | 32 | Patients with CD who underwent ≥1 colonoscopy before or during treatment | SES-CD >70% reduction | Natalizumab |
| Morise, 2015 | Retrospective, single center | Japan | 76 | Patients with CD who underwent transanal double-balloon endoscopy (DBE), included patients with stenosis which hampered passage of the scope and those who underwent DBE with observation for ≥ 80 cm from the ileocecal valve | mSES-CD <4 or SES-CD <4 | IFX, AZA, elemental diet, 5-ASA |
| Beigel, 2014 | Retrospective, single center | Germany | 152 | CD subgroup patients treated with anti-TNFα antibodies (IFX and/or ADA) | SES-CD = 0 | One anti-TNFα antibody or 2 anti-TNFα antibodies |
| Macedo Silva, 2022 | Retrospective, case control | Portugal | 47 | CD subgroup patients treated with anti-TNFα antibodies (IFX and/or ADA) | Lewis score ≤135 | Thiopurines, biologics, corticosteroids |
| Elosua, 2021 | Retrospective, single center | Spain | 432 | Established patients with CD with SBCE | Lewis score <135 | 5-ASA, steroids, immunomodulators (thiopurines and methotrexate) and biologics (IFX, ADA, ustekinumab, vedolizumab and certolizumab) |
| Ben-Horin, 2019 | Prospective, multicenter | Israel | 61 | Adult patients with CD involving the small bowel with confirmed small bowel patency | Lewis score <350 | Immunomodulators, biologics |
| Studies Reporting TH . | ||||||
| Study reference (author, year) | Study Design | Geography | N | Patient Population | Measure used and Criteria for Healing | Interventions |
| Halle, 2020 | Retrospective, single center | France | 115 | Small bowel CD | A radiological response was defined as (1) a complete response, if all inflammatory signs had disappeared, (2) a partial response if the length of the wall enhancement of any inflammatory segment decreased or if there was a clear decrease in the size of an abscess or fistula without worsening of the other inflammatory parameters, (3) progression if any inflammatory segment showed a clear increase in any of inflammatory parameters or if a new inflammatory lesion appeared, and (4) stable in other cases | Immunosuppressant, anti-TNF treatment, vedolizumab, ustekinumab, steroids |
| Ma, 2021 | Prospective, single center, cohort | China | 77 | Patients with CD who had been consecutively hospitalized without use of CD-related steroids, immunosuppressants, or biologics for ≥6 months prior | BWT ≤3 mm in any place, with the normalization of stratification, no hypervascularization, the resolution of mesenteric inflammatory fat, and no signs of active inflammation (abscesses or fistulas) | NR |
| Messadeg, 2020 | Prospective, multicenter | France | 46 | Adult patients with CD | Early transmural response, defined as a 25% decrease of either Clermont score or MaRIA. Clermont score: 1.646 × bowel thickness − 1.321 × ADC + 5.613 × edema + 8.306 × ulcers + 5.039 MaRIA: 1.5 × wall thickening [mm] + 0.02 × RCE + 5 × edema + 10 × ulceration Transmural response: Cumulative score using 5 parameters (disappearance of ulcerations; disappearance in enlarged lymph nodes, disappearance of sclerolipomatosis; 30% decrease of RCE; 10% increase in ADC) | IFX or ADA |
| Deepak, 2016 | Retrospective, single center | US | 150 | Patients with small bowel CD who had pre-therapy CTE/MRE with follow-up CTE or MRE after 6 months, or 2 CTE/MREs ≥6 months apart while on maintenance therapy | CTE or MRE | NR |
| Laterza, 2018 | Prospective, single center | Italy | 57 | Patients with CD who underwent a clinical, endoscopic, and radiological assessment of disease within 1 month | CTE Absence of lesions | NR |
| Castiglione, 2019 | Prospective, single center, longitudinal | Italy | 218 | Patients with CD achieving TH following treatment with biologics Patients reaching only MH or no healing | Normalization of BWT of all inflamed segments involved in CD; BWT ≤3 mm | IFX and ADA |
| Paredes, 2019 | Prospective, single center | Spain | 36 | Adult patients with CD who were treated with biologics | Normalization of BWT (<3 mm) | IFX or ADA |
| Sagami, 2019 | Retrospective, single center | Japan | 50 | Patients with CD who underwent ileocolonoscopy and MRE | MaRIA score of <50 | 5-ASA, glucocorticoid, AZA, 6-MP, IFX, ADA, elemental diet, antibiotics, none |
| Fernandes, 2017 | Retrospective, single center | Portugal | 214 | Patients with CD | Both a normal MRE and normal endoscopy: complete healing of all the bowel layers (including the mucosa) | Thiopurines, methotrexate, anti-TNF therapy |
| Lafeuille, 2021 | Retrospective, database review | France | 154 | Adult patients with CD | Combination of endoscopic MH and MRI healing (absence of mucosal ulceration including aphtoid erosions and no sign of active inflammation (ie, ulceration, edema, diffusion-weighted hyperintensity, increased contrast enhancement), no extra-enteric sign (ie, fat creeping, enlarged lymph nodes, comb sign), and no CD-related complications (ie, stricture, fistula, or abscess)) | 5-ASA, corticoids, immunosuppressants, anti-TNF agents, IFX, ADA, golimumab, vedolizumab, ustekinumab |
| Helwig, 2022 | Prospective, noninterventional, multicenter | Germany | 180 | Adult patients with ileocecal or colonic CD | Simplified TH: Normalization of BWT (terminal ileum ≤2mm, colon ≤3mm) and the absence of an amplified color Doppler signal (Limberg 1/2) Extended TH: Normalized BWT and normalized color Doppler signal or no loss of stratification or no fibrofatty proliferation Complete TH: All 4 factors normalized | Systemic corticosteroids, AZA/6-MP, anti-TNF, anti-integrin, anti IL12/23 |
| Studies Reporting MH Using Validated Measures . | ||||||
|---|---|---|---|---|---|---|
| Study Reference (author, year) | Study Design | Geography | N | Patient Population | Validated Measure Used and Cut-off Score | Interventions |
| Nishikawa, 2020 | Retrospective, single center | Japan | 60 | Patients with CD who underwent capsule endoscopy | Lewis score <270 | Immunomodulator, anti-TNFα agent, prednisolone |
| Hoekman, 2018 | Retrospective, multicenter | Belgium, the Netherlands, Germany | 49 | Adult patients with mild to moderate CD | SES-CD = 0 | Corticosteroids, anti-TNF agents |
| Laterza, 2018 | Prospective, single center | Italy | 57 | Patients with CD | SES-CD ≤2 or Rutgeert’s score i0-i1 (for patients with history of previous surgery) | NR |
| Castiglione, 2019 | Prospective, single center, longitudinal | Italy | 218 | Patients with CD achieving TH following treatment with biologics Patients reaching only MH or no healing | SES-CD ≤2 | Infliximab (IFX) and adalimumab (ADA) |
| Takenaka, 2018 | Prospective, single center | Japan | 116 | Patients with CD in clinical-serological remission | SES-CDa = 0 | Concomitant treatment of steroids, immunomodulator, or anti-TNF inhibitor |
| Sagami, 2019 | Retrospective, single center | Japan | 50 | Patients with CD who underwent ileocolonoscopy and MRE | SES-CD <5 | 5-aminosalicylic acid (5-ASA), glucocorticoid, azathioprine (AZA), 6-mercaptopurine (6-MP), IFX, ADA, elemental diet, antibiotics, none |
| Naganuma, 2016 | Retrospective, single center | Japan | 131 | Patients with CD with clinical remission and who underwent conventional ileocolonoscopy | SES-CD ≤2 | Surgical, nonsurgical |
| Yzet, 2019 | Retrospective, single center | France | 84 | Patients with CD | CDEIS = 0 | IFX, ADA or vedolizumab |
| Sakuraba, 2013 | Retrospective, single center, case review | US | 32 | Patients with CD who underwent ≥1 colonoscopy before or during treatment | SES-CD >70% reduction | Natalizumab |
| Morise, 2015 | Retrospective, single center | Japan | 76 | Patients with CD who underwent transanal double-balloon endoscopy (DBE), included patients with stenosis which hampered passage of the scope and those who underwent DBE with observation for ≥ 80 cm from the ileocecal valve | mSES-CD <4 or SES-CD <4 | IFX, AZA, elemental diet, 5-ASA |
| Beigel, 2014 | Retrospective, single center | Germany | 152 | CD subgroup patients treated with anti-TNFα antibodies (IFX and/or ADA) | SES-CD = 0 | One anti-TNFα antibody or 2 anti-TNFα antibodies |
| Macedo Silva, 2022 | Retrospective, case control | Portugal | 47 | CD subgroup patients treated with anti-TNFα antibodies (IFX and/or ADA) | Lewis score ≤135 | Thiopurines, biologics, corticosteroids |
| Elosua, 2021 | Retrospective, single center | Spain | 432 | Established patients with CD with SBCE | Lewis score <135 | 5-ASA, steroids, immunomodulators (thiopurines and methotrexate) and biologics (IFX, ADA, ustekinumab, vedolizumab and certolizumab) |
| Ben-Horin, 2019 | Prospective, multicenter | Israel | 61 | Adult patients with CD involving the small bowel with confirmed small bowel patency | Lewis score <350 | Immunomodulators, biologics |
| Studies Reporting TH . | ||||||
| Study reference (author, year) | Study Design | Geography | N | Patient Population | Measure used and Criteria for Healing | Interventions |
| Halle, 2020 | Retrospective, single center | France | 115 | Small bowel CD | A radiological response was defined as (1) a complete response, if all inflammatory signs had disappeared, (2) a partial response if the length of the wall enhancement of any inflammatory segment decreased or if there was a clear decrease in the size of an abscess or fistula without worsening of the other inflammatory parameters, (3) progression if any inflammatory segment showed a clear increase in any of inflammatory parameters or if a new inflammatory lesion appeared, and (4) stable in other cases | Immunosuppressant, anti-TNF treatment, vedolizumab, ustekinumab, steroids |
| Ma, 2021 | Prospective, single center, cohort | China | 77 | Patients with CD who had been consecutively hospitalized without use of CD-related steroids, immunosuppressants, or biologics for ≥6 months prior | BWT ≤3 mm in any place, with the normalization of stratification, no hypervascularization, the resolution of mesenteric inflammatory fat, and no signs of active inflammation (abscesses or fistulas) | NR |
| Messadeg, 2020 | Prospective, multicenter | France | 46 | Adult patients with CD | Early transmural response, defined as a 25% decrease of either Clermont score or MaRIA. Clermont score: 1.646 × bowel thickness − 1.321 × ADC + 5.613 × edema + 8.306 × ulcers + 5.039 MaRIA: 1.5 × wall thickening [mm] + 0.02 × RCE + 5 × edema + 10 × ulceration Transmural response: Cumulative score using 5 parameters (disappearance of ulcerations; disappearance in enlarged lymph nodes, disappearance of sclerolipomatosis; 30% decrease of RCE; 10% increase in ADC) | IFX or ADA |
| Deepak, 2016 | Retrospective, single center | US | 150 | Patients with small bowel CD who had pre-therapy CTE/MRE with follow-up CTE or MRE after 6 months, or 2 CTE/MREs ≥6 months apart while on maintenance therapy | CTE or MRE | NR |
| Laterza, 2018 | Prospective, single center | Italy | 57 | Patients with CD who underwent a clinical, endoscopic, and radiological assessment of disease within 1 month | CTE Absence of lesions | NR |
| Castiglione, 2019 | Prospective, single center, longitudinal | Italy | 218 | Patients with CD achieving TH following treatment with biologics Patients reaching only MH or no healing | Normalization of BWT of all inflamed segments involved in CD; BWT ≤3 mm | IFX and ADA |
| Paredes, 2019 | Prospective, single center | Spain | 36 | Adult patients with CD who were treated with biologics | Normalization of BWT (<3 mm) | IFX or ADA |
| Sagami, 2019 | Retrospective, single center | Japan | 50 | Patients with CD who underwent ileocolonoscopy and MRE | MaRIA score of <50 | 5-ASA, glucocorticoid, AZA, 6-MP, IFX, ADA, elemental diet, antibiotics, none |
| Fernandes, 2017 | Retrospective, single center | Portugal | 214 | Patients with CD | Both a normal MRE and normal endoscopy: complete healing of all the bowel layers (including the mucosa) | Thiopurines, methotrexate, anti-TNF therapy |
| Lafeuille, 2021 | Retrospective, database review | France | 154 | Adult patients with CD | Combination of endoscopic MH and MRI healing (absence of mucosal ulceration including aphtoid erosions and no sign of active inflammation (ie, ulceration, edema, diffusion-weighted hyperintensity, increased contrast enhancement), no extra-enteric sign (ie, fat creeping, enlarged lymph nodes, comb sign), and no CD-related complications (ie, stricture, fistula, or abscess)) | 5-ASA, corticoids, immunosuppressants, anti-TNF agents, IFX, ADA, golimumab, vedolizumab, ustekinumab |
| Helwig, 2022 | Prospective, noninterventional, multicenter | Germany | 180 | Adult patients with ileocecal or colonic CD | Simplified TH: Normalization of BWT (terminal ileum ≤2mm, colon ≤3mm) and the absence of an amplified color Doppler signal (Limberg 1/2) Extended TH: Normalized BWT and normalized color Doppler signal or no loss of stratification or no fibrofatty proliferation Complete TH: All 4 factors normalized | Systemic corticosteroids, AZA/6-MP, anti-TNF, anti-integrin, anti IL12/23 |
Abbreviations: 5-ASA, 5-aminosalicylic acid; 6-MP, 6-mercaptoprine; ADA, adalimumab; AZA, azathioprine; ADC, apparent diffusion coefficient; BWT, bowel wall thickness; CD, Crohn’s disease; CDEIS, Crohn’s Disease Endoscopic Index of Severity; CTE, computed tomography enterography; DBE, double balloon endoscopy; IFX, Infliximab; MaRIA, Magnetic Resonance Index of Activity; MH, mucosal healing; MRE, magnetic resonance enterography; MRI, magnetic resonance imaging; mSES-CD, modified SES-CD; NR, not reported; RCE, relative contrast enhancement; SBCE, small bowel endoscopy; SES-CD, Simple Endoscopic Score for Crohn’s Disease; SES-CDa, Simple Endoscopic Active Score for Crohn’s Disease; TH, transmural healing; TNF, tumor necrosis factor; TNFα, tumor necrosis factor alpha.
PRISMA flow diagram. Abbreviations: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RWE, real-world evidence
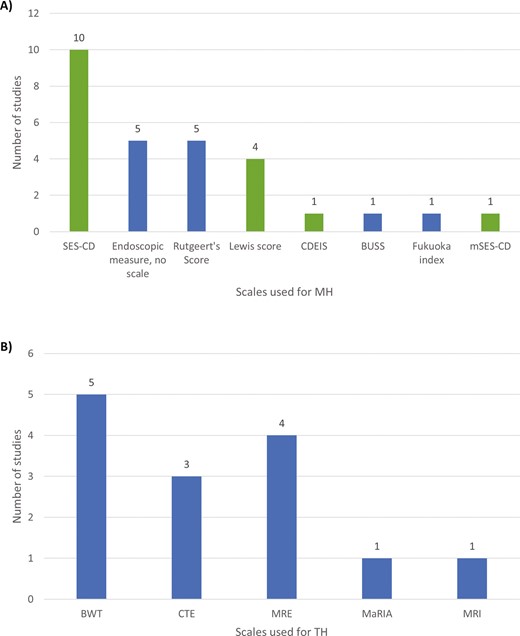
Scales used for MH and TH. A, Scales used for MH; B, Scales used for TH. Abbreviations: BUSS, Bowel Ultrasound Score; BWT, Bowel wall thickness; CDEIS, Crohn’s Disease Endoscopic Index of Severity; CTE, computed tomography enterography; MaRIA, Magnetic Resonance Index of Activity; MH, mucosal healing; MRE, magnetic resonance enterography; MRI, magnetic resonance imaging; mSES-CD, Modified Simple Endoscopic Score for Crohn’s Disease; SES-CD, Simple Endoscopic Score for Crohn’s Disease; TH, transmural healing.
Study Population and Follow-up Time
The study populations included a total of 5530 adult patients with a median disease duration ranging from 10.3 to 180 months. Median patient age ranged from 21.0 to 43.5 years. Validated endoscopic measures indicated a wide range of disease severity at baseline, as indicated by scores such as CDEIS <430 and SES-CD 0 to 18. Similarly, wide ranges in clinical measures at baseline were observed including BWT (≤3 mm and >3 mm)31; Crohn’s Disease Activity Index (CDAI; mean 106.7-450)27,32,33; Harvey–Bradshaw Index (HBI; mean 2.0 [SD: 2.3] to median 11 [IQR: 8-15])34,35; and Rutgeert’s score36 (i0-i1: 11/57 Table 1). Four studies included patients with a disease duration of 2 years or less, all of whom had full or partial MH at baseline.12,30,33,37
Follow-up time ranged from 16 weeks to 11 years. Cost and work productivity data were not reported by studies using validated scales. Quality of life data were not reported by studies comparing MH and non-MH using a validated scale or by studies comparing TH and non-TH.
Mucosal Healing
Thirty-nine studies reported on long-term outcomes among patients with MH with follow-up times ranging from 5 to 132 months (Table 2); however, definitions of MH and outcomes used to assess MH varied across studies (Supplementary Table 3). Studies that reported MH outcomes without using validated scales typically described the presence of MH based on visible inflammation as observed through endoscopy; these studies were not included in our analyses. The remaining 14 studies reported on long-term outcomes using established definitions of MH that included the SES-CD (9 studies), CDEIS (1 study), and Lewis (4 studies) scores.
| Study Reference (author, year) . | . | . | Results and significance . | |||||
|---|---|---|---|---|---|---|---|---|
| Follow-up Time (months) | Intervention of Interest* | Clinical remission | Relapse/ recurrence | Treatment Change | Hospitalization | Surgery | Event-free Survival | |
| Nishikawa, 2020 | ≤24 | None | NR | Timepoint: within 2 years LS ≥270 vs <270: • 54.5% vs 5.3% | Timepoint: within 2 years LS ≥270 vs <270: • 54.5% vs 2.6% | Timepoint: within 2 years LS ≥270 vs <270: • 27.3% vs 2.6% • Multivariate HR for patients with LS ≥270: 9.475 (95% CI, 2.596-34.59), P = .001 | NR | NR |
| Hoekman, 2018 | 24 | Early combined immunosuppression (top-down) vs conventional management (step-up) | Timepoint: 104 weeks MH vs no MH • Median: 70% vs 70% (P = .64) | Timepoint: 104 weeks MH vs no MH • Median: 13% vs 13% (P = .65) | Timepoint: 104 weeks • No difference between patients with MH vs no MH for use of rescue therapy (P = .48) or time to new fistula (P = .37) | Timepoint: 104 weeks • No difference between groups for time to CD-related hospitalization (P = .67) | Timepoint: 104 weeks • No difference between groups in time to CD-related surgery (P = .5) | NR |
| Laterza, 2018 | 36 | None | NR | NR | Timepoint: up to 36 months • Baseline mucosal activity associated with higher use of topical steroids (P < .01) • No differences found for the use of immunosuppressants, systemic steroids, and anti-TNFα | Timepoint: up to 36 months • No differences between groups based on mucosal activity | NR | NR |
| Castiglione, 2019 | 12 | Anti-TNF alpha agents | Timepoint: 1 year • MH vs NH: 75% vs 41% (P < .001) • Multivariate OR: o NH: 0.2 (95% CI, 0.1-0.6), P = .03 | Timepoint: 1 year • MH vs NH: 25% vs 59% (P < .001) • Multivariate OR: o MH: 0.06 (95% CI, 0.02‐0.1), P < .001 o NH: 7.79 (95% CI, 3.1‐19.5), P < .001 | Timepoint: 1 year (need for dose escalation) • MH vs NH: 33.3% vs 38.8% (P = .6) • Need for dose escalation, MH vs NH: HR = 0.96 (95% Cl, 0.73-1.24), P = .08 • “Switch or swap,” MH vs NH: 15% vs 26.6% (P = .1) | Timepoint: 1 year • MH vs NH: 28.3% vs 66.6% (P < .001) • Multivariate OR: o MH: 0.1 (95% Cl, 0.05‐0.3), P < .001 o NH: 3.6 (95% Cl, 1.6‐8.1), P = .002 • Need for hospitalization, MH vs NH: HR = 0.65 (95% Cl, 0.49-0.86), P = .01 | Timepoint: 1 year • MH vs NH: 10% vs 35.5% (P < .001) • Multivariate OR: o MH: 0.2 (95% Cl, 0.07‐0.6), P = .006 o NH: 3.8 (95% Cl, 1.9‐6.8), P < .001 • Later need for surgery, MH vs NH: HR = 0.83 (95% Cl, 0.76-0.92), P = .01 | NR |
| Takenaka, 2018 | 27 | None | NR | Timepoint: Median: 27 months • No/mild disease vs ulcerative disease o Clinical relapse: 9.2% vs 36.5% o Serological relapse: 31.6% vs 60.3% • Multivariate HR in ulcerative disease: o Clinical relapse: 5.34 (95% CI, 2.06-13.81), P = .001 o Serological relapse: 3.02 (95% CI, 1.65-5.51), P < .001 | NR | Timepoint: Median: 27 months • No/mild disease vs ulcerative disease: 18.4% vs 42.9% • Adjusted HR in ulcerative disease: 2.34 (95% CI, 1.13-4.83), P = .021 | Timepoint: Median: 27 months • No/mild disease vs ulcerative disease: 3.9% vs 20.6% • Adjusted HR for surgery in no/mild disease: 5.40 (95% CI, 1.41-20.67), P = .014 | NR |
| Sagami, 2019 | 15 | None | NR | NR | Timepoint: Median: 449 days • Time to treatment escalation for endoscopic remission vs endoscopic active disease: HR = 2.43 (95% CI, 1.09-5.42), P = .0302 | NR | NR | NR |
| Naganuma, 2016 | ≥ 6 | None | Timepoint: Mean: 23.5 months • Duration of clinical remission, MH vs non-MH: 44.8 vs 16.9 months (P < .01) • Multivariate HR for maintenance of remission in MH: HR = 0.17 (95% CI, 0.06-0.48), P = .001 | Timepoint: Mean: 23.5 months • Total population: 39% had clinical recurrence | NR | NR | NR | NR |
| Yzet, 2019 | 58 | Anti-TNF agents | NR | NR | • Median TTF for: o EH: 21.5 months o Partial MH: 13.5 months • Complete EH vs Partial MH o Treatment failure at 1 year: 9% vs 16% (P = .028) o Treatment failure at 3 years: 19% vs 37% (P = .049) • Multivariate HR for partial MH vs complete EH: 2.17 (95% CI, 1.01-4.65), P = .0204 | Increased risk of CD-related hospitalization with partial MH: P < .0246 | Increased risk of CD-related intestinal resection with partial MH: P = .0082 | NR |
| Sakuraba, 2013 | 5 | Natalizumab | NR | NR | • Proportion of patients continuing natalizumab significantly higher in patients with a significant vs partial vs no improvement in MH (P = .032)† | • RR for hospitalization per patient-year follow-up for MH with: o Significant vs no improvement: 0.17 (95% CI, 0.039-0.78) o Partial vs no improvement: 0.77 (95% CI, 0.24-2.5) | Median time after initiation of therapy: 5 months • Rate of surgery during or after natalizumab for patients with significant vs partial vs no improvement in MH: 66.7% vs 44.4% vs 27.3% | NR |
| Morise, 2015 | NR | None | NR | NR | NR | NR | • 34.2% of patients underwent surgery • Multivariate analysis: mSES-CD (≥4) associated with surgery-free survival: HR = 9.38 (95% CI, 1.20-73.5), P = .033 | NR |
| Beigel, 2014 | 58–87.5 | Infliximab and/or adalimumab | NR | NR | NR | Median time from baseline to follow-up colonoscopy: TNF1: 63 months TNF2: 64.5 months • Patients with CD hospitalized, MH vs no MH (n): o TNF1: 9 vs 26 (P = .82) o TNF2: 2 vs 11 (P = .42) | Median time from baseline to follow-up colonoscopy: TNF1: 63 months TNF2: 64.5 months • Patients with CD undergoing surgery, MH vs no MH (n): o TNF1: 3 vs 24 (P = .049) o TNF2: 0 vs 7 (P = .14) | NR |
| Macedo Silva, 2022 | ≥12 | None | NR | Timepoint: 1 year MH vs no MH • 25.5% vs 48.3% (P = .01) | NR | NR | NR | NR |
| Elosua, 2021 | 132 | None | NR | NR | Timepoint: At next clinic visit or within 3 months after capsule endoscopy MH vs mild-to-moderate mucosal inflammation vs moderate-to-severe mucosal inflammation • Treatment escalation o 6.3% vs 57.8% vs 89.5% (P < .001) • De-escalation of therapy o 13.9% vs 1.1% vs 0% (P < .001) | NR | NR | NR |
| Ben-Horin, 2019 | 24 | None | NR | NR | NR | NR | NR | NR |
| Study Reference (author, year) . | . | . | Results and significance . | |||||
|---|---|---|---|---|---|---|---|---|
| Follow-up Time (months) | Intervention of Interest* | Clinical remission | Relapse/ recurrence | Treatment Change | Hospitalization | Surgery | Event-free Survival | |
| Nishikawa, 2020 | ≤24 | None | NR | Timepoint: within 2 years LS ≥270 vs <270: • 54.5% vs 5.3% | Timepoint: within 2 years LS ≥270 vs <270: • 54.5% vs 2.6% | Timepoint: within 2 years LS ≥270 vs <270: • 27.3% vs 2.6% • Multivariate HR for patients with LS ≥270: 9.475 (95% CI, 2.596-34.59), P = .001 | NR | NR |
| Hoekman, 2018 | 24 | Early combined immunosuppression (top-down) vs conventional management (step-up) | Timepoint: 104 weeks MH vs no MH • Median: 70% vs 70% (P = .64) | Timepoint: 104 weeks MH vs no MH • Median: 13% vs 13% (P = .65) | Timepoint: 104 weeks • No difference between patients with MH vs no MH for use of rescue therapy (P = .48) or time to new fistula (P = .37) | Timepoint: 104 weeks • No difference between groups for time to CD-related hospitalization (P = .67) | Timepoint: 104 weeks • No difference between groups in time to CD-related surgery (P = .5) | NR |
| Laterza, 2018 | 36 | None | NR | NR | Timepoint: up to 36 months • Baseline mucosal activity associated with higher use of topical steroids (P < .01) • No differences found for the use of immunosuppressants, systemic steroids, and anti-TNFα | Timepoint: up to 36 months • No differences between groups based on mucosal activity | NR | NR |
| Castiglione, 2019 | 12 | Anti-TNF alpha agents | Timepoint: 1 year • MH vs NH: 75% vs 41% (P < .001) • Multivariate OR: o NH: 0.2 (95% CI, 0.1-0.6), P = .03 | Timepoint: 1 year • MH vs NH: 25% vs 59% (P < .001) • Multivariate OR: o MH: 0.06 (95% CI, 0.02‐0.1), P < .001 o NH: 7.79 (95% CI, 3.1‐19.5), P < .001 | Timepoint: 1 year (need for dose escalation) • MH vs NH: 33.3% vs 38.8% (P = .6) • Need for dose escalation, MH vs NH: HR = 0.96 (95% Cl, 0.73-1.24), P = .08 • “Switch or swap,” MH vs NH: 15% vs 26.6% (P = .1) | Timepoint: 1 year • MH vs NH: 28.3% vs 66.6% (P < .001) • Multivariate OR: o MH: 0.1 (95% Cl, 0.05‐0.3), P < .001 o NH: 3.6 (95% Cl, 1.6‐8.1), P = .002 • Need for hospitalization, MH vs NH: HR = 0.65 (95% Cl, 0.49-0.86), P = .01 | Timepoint: 1 year • MH vs NH: 10% vs 35.5% (P < .001) • Multivariate OR: o MH: 0.2 (95% Cl, 0.07‐0.6), P = .006 o NH: 3.8 (95% Cl, 1.9‐6.8), P < .001 • Later need for surgery, MH vs NH: HR = 0.83 (95% Cl, 0.76-0.92), P = .01 | NR |
| Takenaka, 2018 | 27 | None | NR | Timepoint: Median: 27 months • No/mild disease vs ulcerative disease o Clinical relapse: 9.2% vs 36.5% o Serological relapse: 31.6% vs 60.3% • Multivariate HR in ulcerative disease: o Clinical relapse: 5.34 (95% CI, 2.06-13.81), P = .001 o Serological relapse: 3.02 (95% CI, 1.65-5.51), P < .001 | NR | Timepoint: Median: 27 months • No/mild disease vs ulcerative disease: 18.4% vs 42.9% • Adjusted HR in ulcerative disease: 2.34 (95% CI, 1.13-4.83), P = .021 | Timepoint: Median: 27 months • No/mild disease vs ulcerative disease: 3.9% vs 20.6% • Adjusted HR for surgery in no/mild disease: 5.40 (95% CI, 1.41-20.67), P = .014 | NR |
| Sagami, 2019 | 15 | None | NR | NR | Timepoint: Median: 449 days • Time to treatment escalation for endoscopic remission vs endoscopic active disease: HR = 2.43 (95% CI, 1.09-5.42), P = .0302 | NR | NR | NR |
| Naganuma, 2016 | ≥ 6 | None | Timepoint: Mean: 23.5 months • Duration of clinical remission, MH vs non-MH: 44.8 vs 16.9 months (P < .01) • Multivariate HR for maintenance of remission in MH: HR = 0.17 (95% CI, 0.06-0.48), P = .001 | Timepoint: Mean: 23.5 months • Total population: 39% had clinical recurrence | NR | NR | NR | NR |
| Yzet, 2019 | 58 | Anti-TNF agents | NR | NR | • Median TTF for: o EH: 21.5 months o Partial MH: 13.5 months • Complete EH vs Partial MH o Treatment failure at 1 year: 9% vs 16% (P = .028) o Treatment failure at 3 years: 19% vs 37% (P = .049) • Multivariate HR for partial MH vs complete EH: 2.17 (95% CI, 1.01-4.65), P = .0204 | Increased risk of CD-related hospitalization with partial MH: P < .0246 | Increased risk of CD-related intestinal resection with partial MH: P = .0082 | NR |
| Sakuraba, 2013 | 5 | Natalizumab | NR | NR | • Proportion of patients continuing natalizumab significantly higher in patients with a significant vs partial vs no improvement in MH (P = .032)† | • RR for hospitalization per patient-year follow-up for MH with: o Significant vs no improvement: 0.17 (95% CI, 0.039-0.78) o Partial vs no improvement: 0.77 (95% CI, 0.24-2.5) | Median time after initiation of therapy: 5 months • Rate of surgery during or after natalizumab for patients with significant vs partial vs no improvement in MH: 66.7% vs 44.4% vs 27.3% | NR |
| Morise, 2015 | NR | None | NR | NR | NR | NR | • 34.2% of patients underwent surgery • Multivariate analysis: mSES-CD (≥4) associated with surgery-free survival: HR = 9.38 (95% CI, 1.20-73.5), P = .033 | NR |
| Beigel, 2014 | 58–87.5 | Infliximab and/or adalimumab | NR | NR | NR | Median time from baseline to follow-up colonoscopy: TNF1: 63 months TNF2: 64.5 months • Patients with CD hospitalized, MH vs no MH (n): o TNF1: 9 vs 26 (P = .82) o TNF2: 2 vs 11 (P = .42) | Median time from baseline to follow-up colonoscopy: TNF1: 63 months TNF2: 64.5 months • Patients with CD undergoing surgery, MH vs no MH (n): o TNF1: 3 vs 24 (P = .049) o TNF2: 0 vs 7 (P = .14) | NR |
| Macedo Silva, 2022 | ≥12 | None | NR | Timepoint: 1 year MH vs no MH • 25.5% vs 48.3% (P = .01) | NR | NR | NR | NR |
| Elosua, 2021 | 132 | None | NR | NR | Timepoint: At next clinic visit or within 3 months after capsule endoscopy MH vs mild-to-moderate mucosal inflammation vs moderate-to-severe mucosal inflammation • Treatment escalation o 6.3% vs 57.8% vs 89.5% (P < .001) • De-escalation of therapy o 13.9% vs 1.1% vs 0% (P < .001) | NR | NR | NR |
| Ben-Horin, 2019 | 24 | None | NR | NR | NR | NR | NR | NR |
Abbreviations: CD, Crohn’s disease; CI, confidence interval; DBE, double balloon endoscopy; EH, endoscopic healing; HR, hazard ratio; IQR, interquartile range; LS, Lewis score; MH, mucosal healing; mSES-CD, modified SES-CD; NA, not applicable; NH, no healing; NPV, negative predictive value; NR, not reported; OR, odds ratio; PPV, positive predictive value; RR, relative risk; SES-CD, Simple Endoscopic Score for Crohn’s Disease; TNF, tumor necrosis factor; TNFα, tumor necrosis factor alpha; TNF1, one TNFα antibody; TNF2, 2 TNFα antibodies; TTF, time to treatment failure.
*Studies without an intervention of interest included real-world and observational studies that did not limit the patient participation based on existing treatment regimens (ie, all-comers).
†MH with significant improvement: >70% reduction of SES-CD; MH with partial improvement: 20%-70% reduction of SES-CD; No improvement in MH: <20% reduction of SES-CD.
| Study Reference (author, year) . | . | . | Results and significance . | |||||
|---|---|---|---|---|---|---|---|---|
| Follow-up Time (months) | Intervention of Interest* | Clinical remission | Relapse/ recurrence | Treatment Change | Hospitalization | Surgery | Event-free Survival | |
| Nishikawa, 2020 | ≤24 | None | NR | Timepoint: within 2 years LS ≥270 vs <270: • 54.5% vs 5.3% | Timepoint: within 2 years LS ≥270 vs <270: • 54.5% vs 2.6% | Timepoint: within 2 years LS ≥270 vs <270: • 27.3% vs 2.6% • Multivariate HR for patients with LS ≥270: 9.475 (95% CI, 2.596-34.59), P = .001 | NR | NR |
| Hoekman, 2018 | 24 | Early combined immunosuppression (top-down) vs conventional management (step-up) | Timepoint: 104 weeks MH vs no MH • Median: 70% vs 70% (P = .64) | Timepoint: 104 weeks MH vs no MH • Median: 13% vs 13% (P = .65) | Timepoint: 104 weeks • No difference between patients with MH vs no MH for use of rescue therapy (P = .48) or time to new fistula (P = .37) | Timepoint: 104 weeks • No difference between groups for time to CD-related hospitalization (P = .67) | Timepoint: 104 weeks • No difference between groups in time to CD-related surgery (P = .5) | NR |
| Laterza, 2018 | 36 | None | NR | NR | Timepoint: up to 36 months • Baseline mucosal activity associated with higher use of topical steroids (P < .01) • No differences found for the use of immunosuppressants, systemic steroids, and anti-TNFα | Timepoint: up to 36 months • No differences between groups based on mucosal activity | NR | NR |
| Castiglione, 2019 | 12 | Anti-TNF alpha agents | Timepoint: 1 year • MH vs NH: 75% vs 41% (P < .001) • Multivariate OR: o NH: 0.2 (95% CI, 0.1-0.6), P = .03 | Timepoint: 1 year • MH vs NH: 25% vs 59% (P < .001) • Multivariate OR: o MH: 0.06 (95% CI, 0.02‐0.1), P < .001 o NH: 7.79 (95% CI, 3.1‐19.5), P < .001 | Timepoint: 1 year (need for dose escalation) • MH vs NH: 33.3% vs 38.8% (P = .6) • Need for dose escalation, MH vs NH: HR = 0.96 (95% Cl, 0.73-1.24), P = .08 • “Switch or swap,” MH vs NH: 15% vs 26.6% (P = .1) | Timepoint: 1 year • MH vs NH: 28.3% vs 66.6% (P < .001) • Multivariate OR: o MH: 0.1 (95% Cl, 0.05‐0.3), P < .001 o NH: 3.6 (95% Cl, 1.6‐8.1), P = .002 • Need for hospitalization, MH vs NH: HR = 0.65 (95% Cl, 0.49-0.86), P = .01 | Timepoint: 1 year • MH vs NH: 10% vs 35.5% (P < .001) • Multivariate OR: o MH: 0.2 (95% Cl, 0.07‐0.6), P = .006 o NH: 3.8 (95% Cl, 1.9‐6.8), P < .001 • Later need for surgery, MH vs NH: HR = 0.83 (95% Cl, 0.76-0.92), P = .01 | NR |
| Takenaka, 2018 | 27 | None | NR | Timepoint: Median: 27 months • No/mild disease vs ulcerative disease o Clinical relapse: 9.2% vs 36.5% o Serological relapse: 31.6% vs 60.3% • Multivariate HR in ulcerative disease: o Clinical relapse: 5.34 (95% CI, 2.06-13.81), P = .001 o Serological relapse: 3.02 (95% CI, 1.65-5.51), P < .001 | NR | Timepoint: Median: 27 months • No/mild disease vs ulcerative disease: 18.4% vs 42.9% • Adjusted HR in ulcerative disease: 2.34 (95% CI, 1.13-4.83), P = .021 | Timepoint: Median: 27 months • No/mild disease vs ulcerative disease: 3.9% vs 20.6% • Adjusted HR for surgery in no/mild disease: 5.40 (95% CI, 1.41-20.67), P = .014 | NR |
| Sagami, 2019 | 15 | None | NR | NR | Timepoint: Median: 449 days • Time to treatment escalation for endoscopic remission vs endoscopic active disease: HR = 2.43 (95% CI, 1.09-5.42), P = .0302 | NR | NR | NR |
| Naganuma, 2016 | ≥ 6 | None | Timepoint: Mean: 23.5 months • Duration of clinical remission, MH vs non-MH: 44.8 vs 16.9 months (P < .01) • Multivariate HR for maintenance of remission in MH: HR = 0.17 (95% CI, 0.06-0.48), P = .001 | Timepoint: Mean: 23.5 months • Total population: 39% had clinical recurrence | NR | NR | NR | NR |
| Yzet, 2019 | 58 | Anti-TNF agents | NR | NR | • Median TTF for: o EH: 21.5 months o Partial MH: 13.5 months • Complete EH vs Partial MH o Treatment failure at 1 year: 9% vs 16% (P = .028) o Treatment failure at 3 years: 19% vs 37% (P = .049) • Multivariate HR for partial MH vs complete EH: 2.17 (95% CI, 1.01-4.65), P = .0204 | Increased risk of CD-related hospitalization with partial MH: P < .0246 | Increased risk of CD-related intestinal resection with partial MH: P = .0082 | NR |
| Sakuraba, 2013 | 5 | Natalizumab | NR | NR | • Proportion of patients continuing natalizumab significantly higher in patients with a significant vs partial vs no improvement in MH (P = .032)† | • RR for hospitalization per patient-year follow-up for MH with: o Significant vs no improvement: 0.17 (95% CI, 0.039-0.78) o Partial vs no improvement: 0.77 (95% CI, 0.24-2.5) | Median time after initiation of therapy: 5 months • Rate of surgery during or after natalizumab for patients with significant vs partial vs no improvement in MH: 66.7% vs 44.4% vs 27.3% | NR |
| Morise, 2015 | NR | None | NR | NR | NR | NR | • 34.2% of patients underwent surgery • Multivariate analysis: mSES-CD (≥4) associated with surgery-free survival: HR = 9.38 (95% CI, 1.20-73.5), P = .033 | NR |
| Beigel, 2014 | 58–87.5 | Infliximab and/or adalimumab | NR | NR | NR | Median time from baseline to follow-up colonoscopy: TNF1: 63 months TNF2: 64.5 months • Patients with CD hospitalized, MH vs no MH (n): o TNF1: 9 vs 26 (P = .82) o TNF2: 2 vs 11 (P = .42) | Median time from baseline to follow-up colonoscopy: TNF1: 63 months TNF2: 64.5 months • Patients with CD undergoing surgery, MH vs no MH (n): o TNF1: 3 vs 24 (P = .049) o TNF2: 0 vs 7 (P = .14) | NR |
| Macedo Silva, 2022 | ≥12 | None | NR | Timepoint: 1 year MH vs no MH • 25.5% vs 48.3% (P = .01) | NR | NR | NR | NR |
| Elosua, 2021 | 132 | None | NR | NR | Timepoint: At next clinic visit or within 3 months after capsule endoscopy MH vs mild-to-moderate mucosal inflammation vs moderate-to-severe mucosal inflammation • Treatment escalation o 6.3% vs 57.8% vs 89.5% (P < .001) • De-escalation of therapy o 13.9% vs 1.1% vs 0% (P < .001) | NR | NR | NR |
| Ben-Horin, 2019 | 24 | None | NR | NR | NR | NR | NR | NR |
| Study Reference (author, year) . | . | . | Results and significance . | |||||
|---|---|---|---|---|---|---|---|---|
| Follow-up Time (months) | Intervention of Interest* | Clinical remission | Relapse/ recurrence | Treatment Change | Hospitalization | Surgery | Event-free Survival | |
| Nishikawa, 2020 | ≤24 | None | NR | Timepoint: within 2 years LS ≥270 vs <270: • 54.5% vs 5.3% | Timepoint: within 2 years LS ≥270 vs <270: • 54.5% vs 2.6% | Timepoint: within 2 years LS ≥270 vs <270: • 27.3% vs 2.6% • Multivariate HR for patients with LS ≥270: 9.475 (95% CI, 2.596-34.59), P = .001 | NR | NR |
| Hoekman, 2018 | 24 | Early combined immunosuppression (top-down) vs conventional management (step-up) | Timepoint: 104 weeks MH vs no MH • Median: 70% vs 70% (P = .64) | Timepoint: 104 weeks MH vs no MH • Median: 13% vs 13% (P = .65) | Timepoint: 104 weeks • No difference between patients with MH vs no MH for use of rescue therapy (P = .48) or time to new fistula (P = .37) | Timepoint: 104 weeks • No difference between groups for time to CD-related hospitalization (P = .67) | Timepoint: 104 weeks • No difference between groups in time to CD-related surgery (P = .5) | NR |
| Laterza, 2018 | 36 | None | NR | NR | Timepoint: up to 36 months • Baseline mucosal activity associated with higher use of topical steroids (P < .01) • No differences found for the use of immunosuppressants, systemic steroids, and anti-TNFα | Timepoint: up to 36 months • No differences between groups based on mucosal activity | NR | NR |
| Castiglione, 2019 | 12 | Anti-TNF alpha agents | Timepoint: 1 year • MH vs NH: 75% vs 41% (P < .001) • Multivariate OR: o NH: 0.2 (95% CI, 0.1-0.6), P = .03 | Timepoint: 1 year • MH vs NH: 25% vs 59% (P < .001) • Multivariate OR: o MH: 0.06 (95% CI, 0.02‐0.1), P < .001 o NH: 7.79 (95% CI, 3.1‐19.5), P < .001 | Timepoint: 1 year (need for dose escalation) • MH vs NH: 33.3% vs 38.8% (P = .6) • Need for dose escalation, MH vs NH: HR = 0.96 (95% Cl, 0.73-1.24), P = .08 • “Switch or swap,” MH vs NH: 15% vs 26.6% (P = .1) | Timepoint: 1 year • MH vs NH: 28.3% vs 66.6% (P < .001) • Multivariate OR: o MH: 0.1 (95% Cl, 0.05‐0.3), P < .001 o NH: 3.6 (95% Cl, 1.6‐8.1), P = .002 • Need for hospitalization, MH vs NH: HR = 0.65 (95% Cl, 0.49-0.86), P = .01 | Timepoint: 1 year • MH vs NH: 10% vs 35.5% (P < .001) • Multivariate OR: o MH: 0.2 (95% Cl, 0.07‐0.6), P = .006 o NH: 3.8 (95% Cl, 1.9‐6.8), P < .001 • Later need for surgery, MH vs NH: HR = 0.83 (95% Cl, 0.76-0.92), P = .01 | NR |
| Takenaka, 2018 | 27 | None | NR | Timepoint: Median: 27 months • No/mild disease vs ulcerative disease o Clinical relapse: 9.2% vs 36.5% o Serological relapse: 31.6% vs 60.3% • Multivariate HR in ulcerative disease: o Clinical relapse: 5.34 (95% CI, 2.06-13.81), P = .001 o Serological relapse: 3.02 (95% CI, 1.65-5.51), P < .001 | NR | Timepoint: Median: 27 months • No/mild disease vs ulcerative disease: 18.4% vs 42.9% • Adjusted HR in ulcerative disease: 2.34 (95% CI, 1.13-4.83), P = .021 | Timepoint: Median: 27 months • No/mild disease vs ulcerative disease: 3.9% vs 20.6% • Adjusted HR for surgery in no/mild disease: 5.40 (95% CI, 1.41-20.67), P = .014 | NR |
| Sagami, 2019 | 15 | None | NR | NR | Timepoint: Median: 449 days • Time to treatment escalation for endoscopic remission vs endoscopic active disease: HR = 2.43 (95% CI, 1.09-5.42), P = .0302 | NR | NR | NR |
| Naganuma, 2016 | ≥ 6 | None | Timepoint: Mean: 23.5 months • Duration of clinical remission, MH vs non-MH: 44.8 vs 16.9 months (P < .01) • Multivariate HR for maintenance of remission in MH: HR = 0.17 (95% CI, 0.06-0.48), P = .001 | Timepoint: Mean: 23.5 months • Total population: 39% had clinical recurrence | NR | NR | NR | NR |
| Yzet, 2019 | 58 | Anti-TNF agents | NR | NR | • Median TTF for: o EH: 21.5 months o Partial MH: 13.5 months • Complete EH vs Partial MH o Treatment failure at 1 year: 9% vs 16% (P = .028) o Treatment failure at 3 years: 19% vs 37% (P = .049) • Multivariate HR for partial MH vs complete EH: 2.17 (95% CI, 1.01-4.65), P = .0204 | Increased risk of CD-related hospitalization with partial MH: P < .0246 | Increased risk of CD-related intestinal resection with partial MH: P = .0082 | NR |
| Sakuraba, 2013 | 5 | Natalizumab | NR | NR | • Proportion of patients continuing natalizumab significantly higher in patients with a significant vs partial vs no improvement in MH (P = .032)† | • RR for hospitalization per patient-year follow-up for MH with: o Significant vs no improvement: 0.17 (95% CI, 0.039-0.78) o Partial vs no improvement: 0.77 (95% CI, 0.24-2.5) | Median time after initiation of therapy: 5 months • Rate of surgery during or after natalizumab for patients with significant vs partial vs no improvement in MH: 66.7% vs 44.4% vs 27.3% | NR |
| Morise, 2015 | NR | None | NR | NR | NR | NR | • 34.2% of patients underwent surgery • Multivariate analysis: mSES-CD (≥4) associated with surgery-free survival: HR = 9.38 (95% CI, 1.20-73.5), P = .033 | NR |
| Beigel, 2014 | 58–87.5 | Infliximab and/or adalimumab | NR | NR | NR | Median time from baseline to follow-up colonoscopy: TNF1: 63 months TNF2: 64.5 months • Patients with CD hospitalized, MH vs no MH (n): o TNF1: 9 vs 26 (P = .82) o TNF2: 2 vs 11 (P = .42) | Median time from baseline to follow-up colonoscopy: TNF1: 63 months TNF2: 64.5 months • Patients with CD undergoing surgery, MH vs no MH (n): o TNF1: 3 vs 24 (P = .049) o TNF2: 0 vs 7 (P = .14) | NR |
| Macedo Silva, 2022 | ≥12 | None | NR | Timepoint: 1 year MH vs no MH • 25.5% vs 48.3% (P = .01) | NR | NR | NR | NR |
| Elosua, 2021 | 132 | None | NR | NR | Timepoint: At next clinic visit or within 3 months after capsule endoscopy MH vs mild-to-moderate mucosal inflammation vs moderate-to-severe mucosal inflammation • Treatment escalation o 6.3% vs 57.8% vs 89.5% (P < .001) • De-escalation of therapy o 13.9% vs 1.1% vs 0% (P < .001) | NR | NR | NR |
| Ben-Horin, 2019 | 24 | None | NR | NR | NR | NR | NR | NR |
Abbreviations: CD, Crohn’s disease; CI, confidence interval; DBE, double balloon endoscopy; EH, endoscopic healing; HR, hazard ratio; IQR, interquartile range; LS, Lewis score; MH, mucosal healing; mSES-CD, modified SES-CD; NA, not applicable; NH, no healing; NPV, negative predictive value; NR, not reported; OR, odds ratio; PPV, positive predictive value; RR, relative risk; SES-CD, Simple Endoscopic Score for Crohn’s Disease; TNF, tumor necrosis factor; TNFα, tumor necrosis factor alpha; TNF1, one TNFα antibody; TNF2, 2 TNFα antibodies; TTF, time to treatment failure.
*Studies without an intervention of interest included real-world and observational studies that did not limit the patient participation based on existing treatment regimens (ie, all-comers).
†MH with significant improvement: >70% reduction of SES-CD; MH with partial improvement: 20%-70% reduction of SES-CD; No improvement in MH: <20% reduction of SES-CD.
Clinical outcomes
Twenty-five studies reported clinical outcomes among patients with and without MH; 12 of these studies used validated scales. Three studies31,33,38 reporting clinical remission used the SES-CD scale to measure MH, and only 1 of these studies38 showed that MH was able to predict sustained clinical remission in a multivariate analysis. Five studies reporting relapse/recurrence used validated scales to define MH (SES-CD, 3 studies31,33,39; and Lewis score, 2 studies40,41). Of these, 3 showed significantly lower relapse/recurrence rates among patients with MH vs without MH. Single-center studies in Italy31 and Japan39 used multivariate analysis to show that patients with MH were less likely to have a clinical relapse, and a retrospective study in Portugal40 showed that patients with MH were less likely to suffer a disease flare compared with those without MH.
Eight studies reported the need for treatment change as an outcome indicating active disease and used a validated scale to define MH (SES-CD, 5 studies31,33–36; CDEIS, 1 study30; and Lewis score, 2 studies41,42). Six of these studies reported significantly lower need for change in treatment (ie, treatment failure rates, tumor necrosis factor inhibitor [TNFi]-intensification, topical steroid specific) among patients achieving MH. Only Ben-Horin et al43 used a validated measure, the Lewis score, as a measure of MH to report survival without a disease flare. This study reported that patients with MH had significantly longer event-free survival (P < .001) compared with patients who did not achieve MH.
Healthcare resource utilization
Eighteen studies reported HCRU outcomes among patients with MH and without MH, among which 9 studies used validated scales (Table 2). Eight studies (3 prospective, 5 retrospective) reporting hospitalization used a validated scale to define MH (SES-CD, 6 studies31,33,35,36,39,44; CDEIS, 1 study30; and Lewis score, 1 study41). Of those 8 studies, 4 studies reported that patients with MH had a lower likelihood of requiring hospitalization.31,35,39,41 Seven studies reporting need for surgery used validated scales to define MH (SES-CD, 6 studies27,31,33,35,39,44; and CDEIS, 1 study30), including 4 studies that reported statistically lower rates of surgery or intestinal resection among patients with MH vs without MH.30,31,39,44
Transmural Healing
Thirteen studies reported long-term outcomes among patients with TH (Table 3), with follow-up time ranging from 12 to 48.5 months. Two studies20,45 did not include both TH and non-TH subgroups and were not included in SLR but are relevant to this review. One was a prospective, multicenter study in Germany45 that compared different definitions of TH (transmural response, simplified TH, extended TH, and complete TH), and the other was a prospective, single-center study in China20 that compared MH and TH. Definitions of TH and specific outcomes differed between included studies (Supplementary Table 3).
| Study Reference (author, year) . | . | Results and significance . | |||||
|---|---|---|---|---|---|---|---|
| Follow-up Time (months) | Clinical Remission | Relapse/ recurrence | Treatment Change | Hospitalization | Surgery | Event-free Survival | |
| Oh, 2022 | 18 | NR | Timepoint: median of 18.0 months (IQR, 15.0–21.0) • Relapse/recurrence in 7% of patients (no statistical tests used) | Timepoint: median of 18.0 months (IQR, 15.0-21.0) • Need for anti-TNF dose intensification: 5.3%; Switch to other biologics: 0%; (no statistical tests used) | Timepoint: median of 18.0 months (IQR, 15.0-21.0) • Need for hospitalization across 4 groups was different deep healing, endoscopic healing only, radiologic healing only, and nonhealing (P = .016), with those in the nonhealing group needing the most hospitalization | Timepoint: median of 18.0 months (IQR, 15.0-21.0) • Need for surgery in 0.9% of patients (no statistical tests used) | NR |
| Halle, 2020 | 17 | NR | NR | Median time to treatment adjustment in radiologic responders vs nonresponders: • 173 vs 110 days (P = .39) | Median time to hospitalization in radiologic responders vs nonresponders: • 173 vs 270 days (P = .77) | Median time to surgery or endoscopic procedure in radiologic responders vs nonresponders: • 684 vs 162 days (P = .04) | NR |
| Ma, 2021 | 19 | Timepoint: 19 months TH predicted SFCR: • Multivariate OR = 52.6 (95% CI, 9.7-167.2), P < .001 | NR | Timepoint: 19 months TH associated with lower risk of treatment escalation: • Multivariate OR = 0.1 (95% CI, 0.03-0.4), P = .002 | Timepoint: 19 months TH associated with lower risk of hospitalization: • Multivariate: OR = 0.05 (95% CI, 0.07-0.4), P = .005 | Timepoint: 19 months TH associated with longer time to surgery (P = .03) | NR |
| Messadeg, 2020 | Timepoint: 52 weeks SFCR rate with TM response score ≥2: 84.6% TM response score associated with steroid-free remission • OR: 81.2 (95% CI, 2.6-254.12, P = .012); | NR | NR | NR | NR | NR | |
| Deepak, 2016 | ≥12 | NR | NR | Timepoint: up to 10 years Multivariate HR = 0.37 (95% CI, 0.21-0.64) P < .001 | Timepoint: up to 10 years Complete response associated with reduced risk of hospitalization vs nonresponse • HR = 0.32 (95% CI, 0.17-0.61), P < .001 | Timepoint: up to 10 years Complete response associated with reduced risk of surgery vs nonresponse • Multivariate HR = 0.27 (95% CI, 0.15-0.48), P < .001 | NR |
| Laterza, 2018 | 36 | NR | NR | Timepoint: up to 36 months No significant differences in therapy adjustment based upon transmural activity stratification at baseline. | Timepoint: up to 36 months Higher transmural activity or more severe clinical activity at baseline required significantly more hospitalizations (all P < .01) | NR | NR |
| Castiglione, 2019 | 12 | Timepoint: 1 year SFCR rate: • TH: 95.6% vs • MH: 75%, P = .01 • NH: 41%, P < .001 TH associated with increased odds of SFCR vs NH: • Multivariate OR = 6.3 (95% CI, 1.5-25.7), P = .01 | Timepoint: 1 year • TH: 4.4% vs • MH: 25%, P = .03 • NH: 59%, P < .001 TH associated with reduced risk of relapse vs NH: • Multivariate OR = 0.02 (95% CI, 0.008‐0.07), P < .001 | Timepoint: 1 year Need for dose escalation • TH: 11.7% vs • MH: 33.3%, P = .005 • NH: 38.8%, P < .001 Need to switch or swap • TH: 10.2% vs • NH: 26.6%, P = .01 | Timepoint: 1 year Need for hospitalization • TH: 8.8% vs • MH: 28.3%, P = .004 • NH: 66.6%, P < .001 TH associated with lower risk of hospitalization vs NH: • Multivariate OR = 0.02 (0.009‐0.09), P < .001) | Timepoint: 1 year 0%, Need for surgery • TH: 0% vs • MH: 10.0%, P = .009 • NH: 35.5%, P < .001 TH associated with lower risk of hospitalization vs NH: • Multivariate OR: 0.1 (0.003‐0.4), P < .001) | NR |
| Paredes, 2019 | 48.5 | NR | NR | Timepoint: 1 year Required corticosteroid therapy: • TH: 0% vs • NH: 10.5%, P = .21 Required treatment intensification: • TH: 3.0% vs • NH: 26.3%, P = .9 | NR | Timepoint: 1 year Surgery rate: • TH: 0% vs • NH: 15.7%, P = .119 | NR |
| Sagami, 2019 | 15 | NR | NR | Incidence of treatment escalation higher for MaRIA score ≥50 than <50 • HR = 2.65 (95% CI, 1.23-5.70), P = .0121 | NR | NR | NR |
| Fernandes, 2017 | 42 | NR | NR | Timepoint: End of study, median: 3.5 years (IQR, 1-7.9) Need for treatment escalation: • TH: 15.2% vs • MH: 36.5%, P = .027 • NH: 54.2%, P < .001 | Timepoint: 1 year Need for hospitalization • TH: 3.0% vs • MH: 17.3%, P = .044 • NH: 24.0%, P = .003 | Timepoint: End of study, Median: 3.5 years (IQR, 1-7.9) Need for surgery • TH: 0% vs • MH: 11.5%, P = .047 • NH: 11.6%, P < .027 | NR |
| Lafeuille, 2021 | 29.1 | NR | Timepoint: Median follow-up 29.1 months (IQR, 12.9-52.3) Progression rates • TH: 3.6% vs • MH: 6.7% • MRI healing: 43.8% • NH: 57.5%, all P < .0001) TH and MRI healing associated with lower risk of progression: • TH HR = 0.04 (95% CI, 0.01-0.32), P < .001 • MRI healing HR = 0.07 (95% CI, 0.02-0.30), P < .0001 | Timepoint: Median follow-up: 29.1 months (IQR, 12.9-52.3) Required treatment escalation or experienced treatment failure • TH: 25% • MH: 56.3% • MRI healing: 50.0% • NH: 83.7% Risk of relapse-related discontinuation was lower with TH vs NH: • Multivariate HR = 0.14 (95% CI, 0.06-0.30), P < .001 | Timepoint: Median: 29.1 months (IQR, 12.9-52.3) Need for hospitalization • TH: 7.1% vs • MH: 23.3%, P = .19 • MRI healing: 25.0%, P = .28 • NH: 48.7%, 0.003 Risk of hospitalization lower with TH vs NH: • Univariate HR = 0.11 (95% CI, 0.03-0.47), P = .0001 | Timepoint: Median follow-up after the second examination: 29.1 months (IQR, 12.9-52.3) Need for surgery • TH: 0% vs • MH: 3.3%, P = .11 • MRI healing: 12.5%, P = .59 • NH: 33.7%,0.0013 | Bowel-damage progression-free survival was better for TH vs: • Endoscopic MH: P = .002 • NH: P = .001 |
| Helwig, 2022 | 12 | Timepoint: 12 weeks SFCR rate: • TR: 82%, univariate OR = 3.95 (95% CI, 1.40-11.1), P = .007 • Simplified TH: 42%, OR = 3.33 (95% CI, 1.09-10.2), P = .044 • Extended TH: 48%, OR = 3.39 (95% CI, 1.17-9.77), P = .021 • Complete TH: 36%, OR = 15.2 (95% CI, 1.90-121.3); P = .001 | NR | Timepoint: 12 weeks and 52 weeks Rates of treatment change (12 weeks, 52 weeks) • TR: 56%; 61% • TH: 26%, 36% | NR | NR | NR |
| Study Reference (author, year) . | . | Results and significance . | |||||
|---|---|---|---|---|---|---|---|
| Follow-up Time (months) | Clinical Remission | Relapse/ recurrence | Treatment Change | Hospitalization | Surgery | Event-free Survival | |
| Oh, 2022 | 18 | NR | Timepoint: median of 18.0 months (IQR, 15.0–21.0) • Relapse/recurrence in 7% of patients (no statistical tests used) | Timepoint: median of 18.0 months (IQR, 15.0-21.0) • Need for anti-TNF dose intensification: 5.3%; Switch to other biologics: 0%; (no statistical tests used) | Timepoint: median of 18.0 months (IQR, 15.0-21.0) • Need for hospitalization across 4 groups was different deep healing, endoscopic healing only, radiologic healing only, and nonhealing (P = .016), with those in the nonhealing group needing the most hospitalization | Timepoint: median of 18.0 months (IQR, 15.0-21.0) • Need for surgery in 0.9% of patients (no statistical tests used) | NR |
| Halle, 2020 | 17 | NR | NR | Median time to treatment adjustment in radiologic responders vs nonresponders: • 173 vs 110 days (P = .39) | Median time to hospitalization in radiologic responders vs nonresponders: • 173 vs 270 days (P = .77) | Median time to surgery or endoscopic procedure in radiologic responders vs nonresponders: • 684 vs 162 days (P = .04) | NR |
| Ma, 2021 | 19 | Timepoint: 19 months TH predicted SFCR: • Multivariate OR = 52.6 (95% CI, 9.7-167.2), P < .001 | NR | Timepoint: 19 months TH associated with lower risk of treatment escalation: • Multivariate OR = 0.1 (95% CI, 0.03-0.4), P = .002 | Timepoint: 19 months TH associated with lower risk of hospitalization: • Multivariate: OR = 0.05 (95% CI, 0.07-0.4), P = .005 | Timepoint: 19 months TH associated with longer time to surgery (P = .03) | NR |
| Messadeg, 2020 | Timepoint: 52 weeks SFCR rate with TM response score ≥2: 84.6% TM response score associated with steroid-free remission • OR: 81.2 (95% CI, 2.6-254.12, P = .012); | NR | NR | NR | NR | NR | |
| Deepak, 2016 | ≥12 | NR | NR | Timepoint: up to 10 years Multivariate HR = 0.37 (95% CI, 0.21-0.64) P < .001 | Timepoint: up to 10 years Complete response associated with reduced risk of hospitalization vs nonresponse • HR = 0.32 (95% CI, 0.17-0.61), P < .001 | Timepoint: up to 10 years Complete response associated with reduced risk of surgery vs nonresponse • Multivariate HR = 0.27 (95% CI, 0.15-0.48), P < .001 | NR |
| Laterza, 2018 | 36 | NR | NR | Timepoint: up to 36 months No significant differences in therapy adjustment based upon transmural activity stratification at baseline. | Timepoint: up to 36 months Higher transmural activity or more severe clinical activity at baseline required significantly more hospitalizations (all P < .01) | NR | NR |
| Castiglione, 2019 | 12 | Timepoint: 1 year SFCR rate: • TH: 95.6% vs • MH: 75%, P = .01 • NH: 41%, P < .001 TH associated with increased odds of SFCR vs NH: • Multivariate OR = 6.3 (95% CI, 1.5-25.7), P = .01 | Timepoint: 1 year • TH: 4.4% vs • MH: 25%, P = .03 • NH: 59%, P < .001 TH associated with reduced risk of relapse vs NH: • Multivariate OR = 0.02 (95% CI, 0.008‐0.07), P < .001 | Timepoint: 1 year Need for dose escalation • TH: 11.7% vs • MH: 33.3%, P = .005 • NH: 38.8%, P < .001 Need to switch or swap • TH: 10.2% vs • NH: 26.6%, P = .01 | Timepoint: 1 year Need for hospitalization • TH: 8.8% vs • MH: 28.3%, P = .004 • NH: 66.6%, P < .001 TH associated with lower risk of hospitalization vs NH: • Multivariate OR = 0.02 (0.009‐0.09), P < .001) | Timepoint: 1 year 0%, Need for surgery • TH: 0% vs • MH: 10.0%, P = .009 • NH: 35.5%, P < .001 TH associated with lower risk of hospitalization vs NH: • Multivariate OR: 0.1 (0.003‐0.4), P < .001) | NR |
| Paredes, 2019 | 48.5 | NR | NR | Timepoint: 1 year Required corticosteroid therapy: • TH: 0% vs • NH: 10.5%, P = .21 Required treatment intensification: • TH: 3.0% vs • NH: 26.3%, P = .9 | NR | Timepoint: 1 year Surgery rate: • TH: 0% vs • NH: 15.7%, P = .119 | NR |
| Sagami, 2019 | 15 | NR | NR | Incidence of treatment escalation higher for MaRIA score ≥50 than <50 • HR = 2.65 (95% CI, 1.23-5.70), P = .0121 | NR | NR | NR |
| Fernandes, 2017 | 42 | NR | NR | Timepoint: End of study, median: 3.5 years (IQR, 1-7.9) Need for treatment escalation: • TH: 15.2% vs • MH: 36.5%, P = .027 • NH: 54.2%, P < .001 | Timepoint: 1 year Need for hospitalization • TH: 3.0% vs • MH: 17.3%, P = .044 • NH: 24.0%, P = .003 | Timepoint: End of study, Median: 3.5 years (IQR, 1-7.9) Need for surgery • TH: 0% vs • MH: 11.5%, P = .047 • NH: 11.6%, P < .027 | NR |
| Lafeuille, 2021 | 29.1 | NR | Timepoint: Median follow-up 29.1 months (IQR, 12.9-52.3) Progression rates • TH: 3.6% vs • MH: 6.7% • MRI healing: 43.8% • NH: 57.5%, all P < .0001) TH and MRI healing associated with lower risk of progression: • TH HR = 0.04 (95% CI, 0.01-0.32), P < .001 • MRI healing HR = 0.07 (95% CI, 0.02-0.30), P < .0001 | Timepoint: Median follow-up: 29.1 months (IQR, 12.9-52.3) Required treatment escalation or experienced treatment failure • TH: 25% • MH: 56.3% • MRI healing: 50.0% • NH: 83.7% Risk of relapse-related discontinuation was lower with TH vs NH: • Multivariate HR = 0.14 (95% CI, 0.06-0.30), P < .001 | Timepoint: Median: 29.1 months (IQR, 12.9-52.3) Need for hospitalization • TH: 7.1% vs • MH: 23.3%, P = .19 • MRI healing: 25.0%, P = .28 • NH: 48.7%, 0.003 Risk of hospitalization lower with TH vs NH: • Univariate HR = 0.11 (95% CI, 0.03-0.47), P = .0001 | Timepoint: Median follow-up after the second examination: 29.1 months (IQR, 12.9-52.3) Need for surgery • TH: 0% vs • MH: 3.3%, P = .11 • MRI healing: 12.5%, P = .59 • NH: 33.7%,0.0013 | Bowel-damage progression-free survival was better for TH vs: • Endoscopic MH: P = .002 • NH: P = .001 |
| Helwig, 2022 | 12 | Timepoint: 12 weeks SFCR rate: • TR: 82%, univariate OR = 3.95 (95% CI, 1.40-11.1), P = .007 • Simplified TH: 42%, OR = 3.33 (95% CI, 1.09-10.2), P = .044 • Extended TH: 48%, OR = 3.39 (95% CI, 1.17-9.77), P = .021 • Complete TH: 36%, OR = 15.2 (95% CI, 1.90-121.3); P = .001 | NR | Timepoint: 12 weeks and 52 weeks Rates of treatment change (12 weeks, 52 weeks) • TR: 56%; 61% • TH: 26%, 36% | NR | NR | NR |
Abbreviations: CDAI, Crohn’s Disease Activity Index; CI, confidence interval; HR, hazard ratio; IQR, interquartile range; MH, mucosal healing; MRE, magnetic resonance enterography; MRI, magnetic resonance imaging; NH, no healing; NR, not reported; OR, odds ratio; SFCR, steroid-free clinical remission; TH, transmural healing; TNFα, tumor necrosis factor alpha; TR, transmural response.
| Study Reference (author, year) . | . | Results and significance . | |||||
|---|---|---|---|---|---|---|---|
| Follow-up Time (months) | Clinical Remission | Relapse/ recurrence | Treatment Change | Hospitalization | Surgery | Event-free Survival | |
| Oh, 2022 | 18 | NR | Timepoint: median of 18.0 months (IQR, 15.0–21.0) • Relapse/recurrence in 7% of patients (no statistical tests used) | Timepoint: median of 18.0 months (IQR, 15.0-21.0) • Need for anti-TNF dose intensification: 5.3%; Switch to other biologics: 0%; (no statistical tests used) | Timepoint: median of 18.0 months (IQR, 15.0-21.0) • Need for hospitalization across 4 groups was different deep healing, endoscopic healing only, radiologic healing only, and nonhealing (P = .016), with those in the nonhealing group needing the most hospitalization | Timepoint: median of 18.0 months (IQR, 15.0-21.0) • Need for surgery in 0.9% of patients (no statistical tests used) | NR |
| Halle, 2020 | 17 | NR | NR | Median time to treatment adjustment in radiologic responders vs nonresponders: • 173 vs 110 days (P = .39) | Median time to hospitalization in radiologic responders vs nonresponders: • 173 vs 270 days (P = .77) | Median time to surgery or endoscopic procedure in radiologic responders vs nonresponders: • 684 vs 162 days (P = .04) | NR |
| Ma, 2021 | 19 | Timepoint: 19 months TH predicted SFCR: • Multivariate OR = 52.6 (95% CI, 9.7-167.2), P < .001 | NR | Timepoint: 19 months TH associated with lower risk of treatment escalation: • Multivariate OR = 0.1 (95% CI, 0.03-0.4), P = .002 | Timepoint: 19 months TH associated with lower risk of hospitalization: • Multivariate: OR = 0.05 (95% CI, 0.07-0.4), P = .005 | Timepoint: 19 months TH associated with longer time to surgery (P = .03) | NR |
| Messadeg, 2020 | Timepoint: 52 weeks SFCR rate with TM response score ≥2: 84.6% TM response score associated with steroid-free remission • OR: 81.2 (95% CI, 2.6-254.12, P = .012); | NR | NR | NR | NR | NR | |
| Deepak, 2016 | ≥12 | NR | NR | Timepoint: up to 10 years Multivariate HR = 0.37 (95% CI, 0.21-0.64) P < .001 | Timepoint: up to 10 years Complete response associated with reduced risk of hospitalization vs nonresponse • HR = 0.32 (95% CI, 0.17-0.61), P < .001 | Timepoint: up to 10 years Complete response associated with reduced risk of surgery vs nonresponse • Multivariate HR = 0.27 (95% CI, 0.15-0.48), P < .001 | NR |
| Laterza, 2018 | 36 | NR | NR | Timepoint: up to 36 months No significant differences in therapy adjustment based upon transmural activity stratification at baseline. | Timepoint: up to 36 months Higher transmural activity or more severe clinical activity at baseline required significantly more hospitalizations (all P < .01) | NR | NR |
| Castiglione, 2019 | 12 | Timepoint: 1 year SFCR rate: • TH: 95.6% vs • MH: 75%, P = .01 • NH: 41%, P < .001 TH associated with increased odds of SFCR vs NH: • Multivariate OR = 6.3 (95% CI, 1.5-25.7), P = .01 | Timepoint: 1 year • TH: 4.4% vs • MH: 25%, P = .03 • NH: 59%, P < .001 TH associated with reduced risk of relapse vs NH: • Multivariate OR = 0.02 (95% CI, 0.008‐0.07), P < .001 | Timepoint: 1 year Need for dose escalation • TH: 11.7% vs • MH: 33.3%, P = .005 • NH: 38.8%, P < .001 Need to switch or swap • TH: 10.2% vs • NH: 26.6%, P = .01 | Timepoint: 1 year Need for hospitalization • TH: 8.8% vs • MH: 28.3%, P = .004 • NH: 66.6%, P < .001 TH associated with lower risk of hospitalization vs NH: • Multivariate OR = 0.02 (0.009‐0.09), P < .001) | Timepoint: 1 year 0%, Need for surgery • TH: 0% vs • MH: 10.0%, P = .009 • NH: 35.5%, P < .001 TH associated with lower risk of hospitalization vs NH: • Multivariate OR: 0.1 (0.003‐0.4), P < .001) | NR |
| Paredes, 2019 | 48.5 | NR | NR | Timepoint: 1 year Required corticosteroid therapy: • TH: 0% vs • NH: 10.5%, P = .21 Required treatment intensification: • TH: 3.0% vs • NH: 26.3%, P = .9 | NR | Timepoint: 1 year Surgery rate: • TH: 0% vs • NH: 15.7%, P = .119 | NR |
| Sagami, 2019 | 15 | NR | NR | Incidence of treatment escalation higher for MaRIA score ≥50 than <50 • HR = 2.65 (95% CI, 1.23-5.70), P = .0121 | NR | NR | NR |
| Fernandes, 2017 | 42 | NR | NR | Timepoint: End of study, median: 3.5 years (IQR, 1-7.9) Need for treatment escalation: • TH: 15.2% vs • MH: 36.5%, P = .027 • NH: 54.2%, P < .001 | Timepoint: 1 year Need for hospitalization • TH: 3.0% vs • MH: 17.3%, P = .044 • NH: 24.0%, P = .003 | Timepoint: End of study, Median: 3.5 years (IQR, 1-7.9) Need for surgery • TH: 0% vs • MH: 11.5%, P = .047 • NH: 11.6%, P < .027 | NR |
| Lafeuille, 2021 | 29.1 | NR | Timepoint: Median follow-up 29.1 months (IQR, 12.9-52.3) Progression rates • TH: 3.6% vs • MH: 6.7% • MRI healing: 43.8% • NH: 57.5%, all P < .0001) TH and MRI healing associated with lower risk of progression: • TH HR = 0.04 (95% CI, 0.01-0.32), P < .001 • MRI healing HR = 0.07 (95% CI, 0.02-0.30), P < .0001 | Timepoint: Median follow-up: 29.1 months (IQR, 12.9-52.3) Required treatment escalation or experienced treatment failure • TH: 25% • MH: 56.3% • MRI healing: 50.0% • NH: 83.7% Risk of relapse-related discontinuation was lower with TH vs NH: • Multivariate HR = 0.14 (95% CI, 0.06-0.30), P < .001 | Timepoint: Median: 29.1 months (IQR, 12.9-52.3) Need for hospitalization • TH: 7.1% vs • MH: 23.3%, P = .19 • MRI healing: 25.0%, P = .28 • NH: 48.7%, 0.003 Risk of hospitalization lower with TH vs NH: • Univariate HR = 0.11 (95% CI, 0.03-0.47), P = .0001 | Timepoint: Median follow-up after the second examination: 29.1 months (IQR, 12.9-52.3) Need for surgery • TH: 0% vs • MH: 3.3%, P = .11 • MRI healing: 12.5%, P = .59 • NH: 33.7%,0.0013 | Bowel-damage progression-free survival was better for TH vs: • Endoscopic MH: P = .002 • NH: P = .001 |
| Helwig, 2022 | 12 | Timepoint: 12 weeks SFCR rate: • TR: 82%, univariate OR = 3.95 (95% CI, 1.40-11.1), P = .007 • Simplified TH: 42%, OR = 3.33 (95% CI, 1.09-10.2), P = .044 • Extended TH: 48%, OR = 3.39 (95% CI, 1.17-9.77), P = .021 • Complete TH: 36%, OR = 15.2 (95% CI, 1.90-121.3); P = .001 | NR | Timepoint: 12 weeks and 52 weeks Rates of treatment change (12 weeks, 52 weeks) • TR: 56%; 61% • TH: 26%, 36% | NR | NR | NR |
| Study Reference (author, year) . | . | Results and significance . | |||||
|---|---|---|---|---|---|---|---|
| Follow-up Time (months) | Clinical Remission | Relapse/ recurrence | Treatment Change | Hospitalization | Surgery | Event-free Survival | |
| Oh, 2022 | 18 | NR | Timepoint: median of 18.0 months (IQR, 15.0–21.0) • Relapse/recurrence in 7% of patients (no statistical tests used) | Timepoint: median of 18.0 months (IQR, 15.0-21.0) • Need for anti-TNF dose intensification: 5.3%; Switch to other biologics: 0%; (no statistical tests used) | Timepoint: median of 18.0 months (IQR, 15.0-21.0) • Need for hospitalization across 4 groups was different deep healing, endoscopic healing only, radiologic healing only, and nonhealing (P = .016), with those in the nonhealing group needing the most hospitalization | Timepoint: median of 18.0 months (IQR, 15.0-21.0) • Need for surgery in 0.9% of patients (no statistical tests used) | NR |
| Halle, 2020 | 17 | NR | NR | Median time to treatment adjustment in radiologic responders vs nonresponders: • 173 vs 110 days (P = .39) | Median time to hospitalization in radiologic responders vs nonresponders: • 173 vs 270 days (P = .77) | Median time to surgery or endoscopic procedure in radiologic responders vs nonresponders: • 684 vs 162 days (P = .04) | NR |
| Ma, 2021 | 19 | Timepoint: 19 months TH predicted SFCR: • Multivariate OR = 52.6 (95% CI, 9.7-167.2), P < .001 | NR | Timepoint: 19 months TH associated with lower risk of treatment escalation: • Multivariate OR = 0.1 (95% CI, 0.03-0.4), P = .002 | Timepoint: 19 months TH associated with lower risk of hospitalization: • Multivariate: OR = 0.05 (95% CI, 0.07-0.4), P = .005 | Timepoint: 19 months TH associated with longer time to surgery (P = .03) | NR |
| Messadeg, 2020 | Timepoint: 52 weeks SFCR rate with TM response score ≥2: 84.6% TM response score associated with steroid-free remission • OR: 81.2 (95% CI, 2.6-254.12, P = .012); | NR | NR | NR | NR | NR | |
| Deepak, 2016 | ≥12 | NR | NR | Timepoint: up to 10 years Multivariate HR = 0.37 (95% CI, 0.21-0.64) P < .001 | Timepoint: up to 10 years Complete response associated with reduced risk of hospitalization vs nonresponse • HR = 0.32 (95% CI, 0.17-0.61), P < .001 | Timepoint: up to 10 years Complete response associated with reduced risk of surgery vs nonresponse • Multivariate HR = 0.27 (95% CI, 0.15-0.48), P < .001 | NR |
| Laterza, 2018 | 36 | NR | NR | Timepoint: up to 36 months No significant differences in therapy adjustment based upon transmural activity stratification at baseline. | Timepoint: up to 36 months Higher transmural activity or more severe clinical activity at baseline required significantly more hospitalizations (all P < .01) | NR | NR |
| Castiglione, 2019 | 12 | Timepoint: 1 year SFCR rate: • TH: 95.6% vs • MH: 75%, P = .01 • NH: 41%, P < .001 TH associated with increased odds of SFCR vs NH: • Multivariate OR = 6.3 (95% CI, 1.5-25.7), P = .01 | Timepoint: 1 year • TH: 4.4% vs • MH: 25%, P = .03 • NH: 59%, P < .001 TH associated with reduced risk of relapse vs NH: • Multivariate OR = 0.02 (95% CI, 0.008‐0.07), P < .001 | Timepoint: 1 year Need for dose escalation • TH: 11.7% vs • MH: 33.3%, P = .005 • NH: 38.8%, P < .001 Need to switch or swap • TH: 10.2% vs • NH: 26.6%, P = .01 | Timepoint: 1 year Need for hospitalization • TH: 8.8% vs • MH: 28.3%, P = .004 • NH: 66.6%, P < .001 TH associated with lower risk of hospitalization vs NH: • Multivariate OR = 0.02 (0.009‐0.09), P < .001) | Timepoint: 1 year 0%, Need for surgery • TH: 0% vs • MH: 10.0%, P = .009 • NH: 35.5%, P < .001 TH associated with lower risk of hospitalization vs NH: • Multivariate OR: 0.1 (0.003‐0.4), P < .001) | NR |
| Paredes, 2019 | 48.5 | NR | NR | Timepoint: 1 year Required corticosteroid therapy: • TH: 0% vs • NH: 10.5%, P = .21 Required treatment intensification: • TH: 3.0% vs • NH: 26.3%, P = .9 | NR | Timepoint: 1 year Surgery rate: • TH: 0% vs • NH: 15.7%, P = .119 | NR |
| Sagami, 2019 | 15 | NR | NR | Incidence of treatment escalation higher for MaRIA score ≥50 than <50 • HR = 2.65 (95% CI, 1.23-5.70), P = .0121 | NR | NR | NR |
| Fernandes, 2017 | 42 | NR | NR | Timepoint: End of study, median: 3.5 years (IQR, 1-7.9) Need for treatment escalation: • TH: 15.2% vs • MH: 36.5%, P = .027 • NH: 54.2%, P < .001 | Timepoint: 1 year Need for hospitalization • TH: 3.0% vs • MH: 17.3%, P = .044 • NH: 24.0%, P = .003 | Timepoint: End of study, Median: 3.5 years (IQR, 1-7.9) Need for surgery • TH: 0% vs • MH: 11.5%, P = .047 • NH: 11.6%, P < .027 | NR |
| Lafeuille, 2021 | 29.1 | NR | Timepoint: Median follow-up 29.1 months (IQR, 12.9-52.3) Progression rates • TH: 3.6% vs • MH: 6.7% • MRI healing: 43.8% • NH: 57.5%, all P < .0001) TH and MRI healing associated with lower risk of progression: • TH HR = 0.04 (95% CI, 0.01-0.32), P < .001 • MRI healing HR = 0.07 (95% CI, 0.02-0.30), P < .0001 | Timepoint: Median follow-up: 29.1 months (IQR, 12.9-52.3) Required treatment escalation or experienced treatment failure • TH: 25% • MH: 56.3% • MRI healing: 50.0% • NH: 83.7% Risk of relapse-related discontinuation was lower with TH vs NH: • Multivariate HR = 0.14 (95% CI, 0.06-0.30), P < .001 | Timepoint: Median: 29.1 months (IQR, 12.9-52.3) Need for hospitalization • TH: 7.1% vs • MH: 23.3%, P = .19 • MRI healing: 25.0%, P = .28 • NH: 48.7%, 0.003 Risk of hospitalization lower with TH vs NH: • Univariate HR = 0.11 (95% CI, 0.03-0.47), P = .0001 | Timepoint: Median follow-up after the second examination: 29.1 months (IQR, 12.9-52.3) Need for surgery • TH: 0% vs • MH: 3.3%, P = .11 • MRI healing: 12.5%, P = .59 • NH: 33.7%,0.0013 | Bowel-damage progression-free survival was better for TH vs: • Endoscopic MH: P = .002 • NH: P = .001 |
| Helwig, 2022 | 12 | Timepoint: 12 weeks SFCR rate: • TR: 82%, univariate OR = 3.95 (95% CI, 1.40-11.1), P = .007 • Simplified TH: 42%, OR = 3.33 (95% CI, 1.09-10.2), P = .044 • Extended TH: 48%, OR = 3.39 (95% CI, 1.17-9.77), P = .021 • Complete TH: 36%, OR = 15.2 (95% CI, 1.90-121.3); P = .001 | NR | Timepoint: 12 weeks and 52 weeks Rates of treatment change (12 weeks, 52 weeks) • TR: 56%; 61% • TH: 26%, 36% | NR | NR | NR |
Abbreviations: CDAI, Crohn’s Disease Activity Index; CI, confidence interval; HR, hazard ratio; IQR, interquartile range; MH, mucosal healing; MRE, magnetic resonance enterography; MRI, magnetic resonance imaging; NH, no healing; NR, not reported; OR, odds ratio; SFCR, steroid-free clinical remission; TH, transmural healing; TNFα, tumor necrosis factor alpha; TR, transmural response.
Clinical outcomes
Eleven studies reported clinical outcomes among patients with and without TH (Table 3). Only 2 studies reported rates of clinical remission for TH, and both showed a significantly higher likelihood of remission in patients with TH.31,46 Similarly, only 3 studies reported the proportion of patients experiencing a relapse,31,47,48 with all 3 studies showing that patients with TH had lower rates of relapse or a composite of healing indicators.47 Ten studies reported changes in treatment, including dose escalations, escalation to another treatment, the need for corticosteroids, switching from one biologic to another, and relapse-related drug discontinuation. Of these, 6 studies31,34,48–51 showed significantly fewer treatment escalations (including the probability of 1-year dose escalation-free survival, time to treatment escalation, risk of relapse-related drug discontinuation, association to medication escalation, and steroid use) among patients achieving TH. Event-free survival rates were not reported by studies with TH subgroups.
Healthcare resource utilization
Nine studies reported HCRU outcomes among patients with and without TH (Table 3). Of 8 studies reporting hospitalizations, 6 studies reported that patients with TH were less likely to require hospitalization.31,36,48–51 Surgery outcomes were reported in 8 studies, with 6 studies showing significantly lower rates of surgery or intestinal resection among patients with TH vs without TH.31,48–52
Transmural Healing vs Mucosal Healing
Seven studies reported outcomes for both TH and MH (Table 4); 4 of these studies20,31,34,36 used SES-CD to define MH. Three of the 4 studies showed that TH was better able to predict long-term clinical outcomes compared with MH. A prospective, single-center study in China20 used a multivariate analysis to determine that TH was associated with steroid-free clinical remission, longer time to treatment escalation, and longer time to hospitalization, whereas MH was only associated with later drug escalation. In an Italian, single-center, prospective study,36 MH predicted some changes in treatments; however, there was no changes among those with TH. Conversely, TH was predictive for hospitalization, but MH was not. A separate Italian, single-center, prospective study31 directly compared TH to MH and demonstrated that TH was associated with a higher rate of steroid-free clinical remission, lower rates of hospitalization, and reduced need for surgery at 1 year compared with MH and no healing. In this study, TH was also associated with longer intervals until clinical relapse, hospitalization, and surgery compared with MH. A retrospective study of patients with CD in Japan who underwent ileocolonoscopy and MRE34 reported that incomplete TH is associated with the need for treatment escalation even among patients with MH.
| Study Reference (author, year) . | Clinical Remission . | Hospitalization . | Treatment Change . | Surgery . | ||||
|---|---|---|---|---|---|---|---|---|
| MH | TH | MH | TH | MH | TH | MH | TH | |
| Castiglione et al, 2019 | 75% of patients in steroid-free CR at 1-year follow-up • P < .001 Lower risk of clinical relapse MH vs NH • HR 0.49 (95% CI, 0.36-0.68), P = .01 | 95.58% of patients in steroid-free CR at 1-year follow-up • P < .001 Later clinical relapse TH vs MH • HR 0.87 (95% CI, 0.68-0.96), P = .008 | 28.3% of patients needed hospitalization • P < .001 Later hospitalization MH vs NH • HR 0.65 (95% CI, 0.49-0.86), P = .01 | 8.8% of patients needed hospitalization • P < .001 Later hospitalization TH vs MH • HR 0.88 (95% CI, 0.69-0.95), P = .007 | 33.3% of patients required change in treatment • MH vs NH P = .64 No difference in MH vs NH • HR 0.96 (95% CI, 0.73-1.24), P = .08 | 11.7% of patients required change in treatment • TH vs MH P = .008 Later dose escalation TH vs MH • HR 0.86 (95% CI, 0.66-0.94), P = .006 | 10% of patients needed surgery • P = .007 Later surgery MH vs NH • HR 0.83 (95% CI, 0.76-0.92), P = .01 | 0% of patients needed surgery • P = .007 Later surgery TH vs MH • HR 0.94 (95% CI, 0.84-0.98), P = .009 |
| Laterza et al, 2018 | NR | NR | No differences between groups | Greater transmural activity required more hospitalizations • P < .01 | Mucosal activity at baseline associated with higher use of topical steroids • P < .01 | No significant differences | NR | NR |
| Ma et al, 2021 | Significantly higher cumulative probability of steroid-free CR MH vs no-MH • P = .003 | Significantly higher cumulative probability of steroid-free CR TH vs no-TH • P < .001 • OR 52.6 (95% CI, 9.7-167.2), P < .001 | Later hospitalization MH vs no-MH • P = .09 | Later hospitalization TH vs no-TH • P = .005 • OR 0.05 (95% CI, 0.07-0.4), P = .005 | Later drug escalation MH vs no-MH • P = .06 | Later drug escalation TH vs no-TH • P = .003 • OR 0.1 (95% CI, 0.03-0.4), P = .002 | Later surgery MH vs no-MH • P = .06 | Later surgery TH vs no-TH • P = .03 |
| Sagami et al, 2019 | NR | NR | NR | NR | Later treatment escalation MH vs no-MH • HR 2.43 (95% CI, 1.09-5.42), P = .0302 Shorter time to treatment escalation for MH with active disease in MRE (no-TH), • HR, 4.14 (95% CI, 1.09-15.6), P = .04 | Higher incidence of treatment escalation associated with active disease in MRE than with disease remission (TH) • HR 2.65 (95% CI, 1.23-5.70), P = .0121 | NR | NR |
| Study Reference (author, year) . | Clinical Remission . | Hospitalization . | Treatment Change . | Surgery . | ||||
|---|---|---|---|---|---|---|---|---|
| MH | TH | MH | TH | MH | TH | MH | TH | |
| Castiglione et al, 2019 | 75% of patients in steroid-free CR at 1-year follow-up • P < .001 Lower risk of clinical relapse MH vs NH • HR 0.49 (95% CI, 0.36-0.68), P = .01 | 95.58% of patients in steroid-free CR at 1-year follow-up • P < .001 Later clinical relapse TH vs MH • HR 0.87 (95% CI, 0.68-0.96), P = .008 | 28.3% of patients needed hospitalization • P < .001 Later hospitalization MH vs NH • HR 0.65 (95% CI, 0.49-0.86), P = .01 | 8.8% of patients needed hospitalization • P < .001 Later hospitalization TH vs MH • HR 0.88 (95% CI, 0.69-0.95), P = .007 | 33.3% of patients required change in treatment • MH vs NH P = .64 No difference in MH vs NH • HR 0.96 (95% CI, 0.73-1.24), P = .08 | 11.7% of patients required change in treatment • TH vs MH P = .008 Later dose escalation TH vs MH • HR 0.86 (95% CI, 0.66-0.94), P = .006 | 10% of patients needed surgery • P = .007 Later surgery MH vs NH • HR 0.83 (95% CI, 0.76-0.92), P = .01 | 0% of patients needed surgery • P = .007 Later surgery TH vs MH • HR 0.94 (95% CI, 0.84-0.98), P = .009 |
| Laterza et al, 2018 | NR | NR | No differences between groups | Greater transmural activity required more hospitalizations • P < .01 | Mucosal activity at baseline associated with higher use of topical steroids • P < .01 | No significant differences | NR | NR |
| Ma et al, 2021 | Significantly higher cumulative probability of steroid-free CR MH vs no-MH • P = .003 | Significantly higher cumulative probability of steroid-free CR TH vs no-TH • P < .001 • OR 52.6 (95% CI, 9.7-167.2), P < .001 | Later hospitalization MH vs no-MH • P = .09 | Later hospitalization TH vs no-TH • P = .005 • OR 0.05 (95% CI, 0.07-0.4), P = .005 | Later drug escalation MH vs no-MH • P = .06 | Later drug escalation TH vs no-TH • P = .003 • OR 0.1 (95% CI, 0.03-0.4), P = .002 | Later surgery MH vs no-MH • P = .06 | Later surgery TH vs no-TH • P = .03 |
| Sagami et al, 2019 | NR | NR | NR | NR | Later treatment escalation MH vs no-MH • HR 2.43 (95% CI, 1.09-5.42), P = .0302 Shorter time to treatment escalation for MH with active disease in MRE (no-TH), • HR, 4.14 (95% CI, 1.09-15.6), P = .04 | Higher incidence of treatment escalation associated with active disease in MRE than with disease remission (TH) • HR 2.65 (95% CI, 1.23-5.70), P = .0121 | NR | NR |
Abbreviations: CI, confidence interval; CR, clinical remission; HR, hazard ratio; MH, mucosal healing; MRE, magnetic resonance enterography; NA, not applicable; NH, no healing; NR, not reported; OR, odds ratio; TH, transmural healing.
| Study Reference (author, year) . | Clinical Remission . | Hospitalization . | Treatment Change . | Surgery . | ||||
|---|---|---|---|---|---|---|---|---|
| MH | TH | MH | TH | MH | TH | MH | TH | |
| Castiglione et al, 2019 | 75% of patients in steroid-free CR at 1-year follow-up • P < .001 Lower risk of clinical relapse MH vs NH • HR 0.49 (95% CI, 0.36-0.68), P = .01 | 95.58% of patients in steroid-free CR at 1-year follow-up • P < .001 Later clinical relapse TH vs MH • HR 0.87 (95% CI, 0.68-0.96), P = .008 | 28.3% of patients needed hospitalization • P < .001 Later hospitalization MH vs NH • HR 0.65 (95% CI, 0.49-0.86), P = .01 | 8.8% of patients needed hospitalization • P < .001 Later hospitalization TH vs MH • HR 0.88 (95% CI, 0.69-0.95), P = .007 | 33.3% of patients required change in treatment • MH vs NH P = .64 No difference in MH vs NH • HR 0.96 (95% CI, 0.73-1.24), P = .08 | 11.7% of patients required change in treatment • TH vs MH P = .008 Later dose escalation TH vs MH • HR 0.86 (95% CI, 0.66-0.94), P = .006 | 10% of patients needed surgery • P = .007 Later surgery MH vs NH • HR 0.83 (95% CI, 0.76-0.92), P = .01 | 0% of patients needed surgery • P = .007 Later surgery TH vs MH • HR 0.94 (95% CI, 0.84-0.98), P = .009 |
| Laterza et al, 2018 | NR | NR | No differences between groups | Greater transmural activity required more hospitalizations • P < .01 | Mucosal activity at baseline associated with higher use of topical steroids • P < .01 | No significant differences | NR | NR |
| Ma et al, 2021 | Significantly higher cumulative probability of steroid-free CR MH vs no-MH • P = .003 | Significantly higher cumulative probability of steroid-free CR TH vs no-TH • P < .001 • OR 52.6 (95% CI, 9.7-167.2), P < .001 | Later hospitalization MH vs no-MH • P = .09 | Later hospitalization TH vs no-TH • P = .005 • OR 0.05 (95% CI, 0.07-0.4), P = .005 | Later drug escalation MH vs no-MH • P = .06 | Later drug escalation TH vs no-TH • P = .003 • OR 0.1 (95% CI, 0.03-0.4), P = .002 | Later surgery MH vs no-MH • P = .06 | Later surgery TH vs no-TH • P = .03 |
| Sagami et al, 2019 | NR | NR | NR | NR | Later treatment escalation MH vs no-MH • HR 2.43 (95% CI, 1.09-5.42), P = .0302 Shorter time to treatment escalation for MH with active disease in MRE (no-TH), • HR, 4.14 (95% CI, 1.09-15.6), P = .04 | Higher incidence of treatment escalation associated with active disease in MRE than with disease remission (TH) • HR 2.65 (95% CI, 1.23-5.70), P = .0121 | NR | NR |
| Study Reference (author, year) . | Clinical Remission . | Hospitalization . | Treatment Change . | Surgery . | ||||
|---|---|---|---|---|---|---|---|---|
| MH | TH | MH | TH | MH | TH | MH | TH | |
| Castiglione et al, 2019 | 75% of patients in steroid-free CR at 1-year follow-up • P < .001 Lower risk of clinical relapse MH vs NH • HR 0.49 (95% CI, 0.36-0.68), P = .01 | 95.58% of patients in steroid-free CR at 1-year follow-up • P < .001 Later clinical relapse TH vs MH • HR 0.87 (95% CI, 0.68-0.96), P = .008 | 28.3% of patients needed hospitalization • P < .001 Later hospitalization MH vs NH • HR 0.65 (95% CI, 0.49-0.86), P = .01 | 8.8% of patients needed hospitalization • P < .001 Later hospitalization TH vs MH • HR 0.88 (95% CI, 0.69-0.95), P = .007 | 33.3% of patients required change in treatment • MH vs NH P = .64 No difference in MH vs NH • HR 0.96 (95% CI, 0.73-1.24), P = .08 | 11.7% of patients required change in treatment • TH vs MH P = .008 Later dose escalation TH vs MH • HR 0.86 (95% CI, 0.66-0.94), P = .006 | 10% of patients needed surgery • P = .007 Later surgery MH vs NH • HR 0.83 (95% CI, 0.76-0.92), P = .01 | 0% of patients needed surgery • P = .007 Later surgery TH vs MH • HR 0.94 (95% CI, 0.84-0.98), P = .009 |
| Laterza et al, 2018 | NR | NR | No differences between groups | Greater transmural activity required more hospitalizations • P < .01 | Mucosal activity at baseline associated with higher use of topical steroids • P < .01 | No significant differences | NR | NR |
| Ma et al, 2021 | Significantly higher cumulative probability of steroid-free CR MH vs no-MH • P = .003 | Significantly higher cumulative probability of steroid-free CR TH vs no-TH • P < .001 • OR 52.6 (95% CI, 9.7-167.2), P < .001 | Later hospitalization MH vs no-MH • P = .09 | Later hospitalization TH vs no-TH • P = .005 • OR 0.05 (95% CI, 0.07-0.4), P = .005 | Later drug escalation MH vs no-MH • P = .06 | Later drug escalation TH vs no-TH • P = .003 • OR 0.1 (95% CI, 0.03-0.4), P = .002 | Later surgery MH vs no-MH • P = .06 | Later surgery TH vs no-TH • P = .03 |
| Sagami et al, 2019 | NR | NR | NR | NR | Later treatment escalation MH vs no-MH • HR 2.43 (95% CI, 1.09-5.42), P = .0302 Shorter time to treatment escalation for MH with active disease in MRE (no-TH), • HR, 4.14 (95% CI, 1.09-15.6), P = .04 | Higher incidence of treatment escalation associated with active disease in MRE than with disease remission (TH) • HR 2.65 (95% CI, 1.23-5.70), P = .0121 | NR | NR |
Abbreviations: CI, confidence interval; CR, clinical remission; HR, hazard ratio; MH, mucosal healing; MRE, magnetic resonance enterography; NA, not applicable; NH, no healing; NR, not reported; OR, odds ratio; TH, transmural healing.
Discussion
The purpose of this review was to identify and summarize the evidence relating MH and TH and report associated clinical, economic, and quality of life outcomes. The majority of studies that used validated scales reported that patients with vs without MH had improved clinical outcomes, including increased rates of clinical remission and decreased rates of relapse or treatment escalation. The clinical benefit of MH provides a clear longer-term target for CD. However, because MH did not always correspond to positive HCRU outcomes such as reduced rates of surgeries and hospitalizations,20 evidence of MH may only be indicative of partial healing.
Given that CD represents a transmural disease with active intramural inflammation,53 TH may represent a more suitable target than MH in CD. However, no consensus has been available on how to assess the relevance of TH for CD. However, increasing evidence is now available for TH, and the majority of studies in this review reported that patients with vs without TH had improved outcomes including increased clinical remission rates, decreased rates of relapse or treatment escalation, and reduced HCRU (hospitalizations and surgeries).20,21
The present study assesses how MH and TH impact long-term outcomes and highlights studies that compared MH and TH. Previous reviews have also assessed the relationship between MH54 or TH55 and long-term clinical outcomes with similar conclusions to the present study. In the recent review by Geyl et al,55 only the correlation between MH and TH was assessed and shown in only a small number of studies.
Definitions of MH and TH
Our findings highlight the variation in the definitions of MH and TH used in current clinical assessments.2 Studies that did not use validated measures defined MH descriptively, such as “remission or mild inflammatory mucosal activity in the most affected area of the GI tract, without ulcerations,”12 or used cut-off scores in unvalidated measures. Studies in this review that employed validated measures also used various definitions of MH, using SES-CD cut-off scores that ranged from 0 to <5, and on the Lewis scale from <135 to <350 (Table 1), among others. Another recent SLR on MH in CD also reported varying definitions of end points but noted that the majority of studies used the absence of ulcers or erosions as the definition of MH and/or an SES-CD score of ≤4.22
For TH, some studies in this review suggested it may be defined as the normalization of BWT with no inflammation or hypervascularization and no fibrofatty proliferation,20,45,48 while others defined TH as simply the reduction of intestinal parietal thickness to <3 mm32,56 (Table 1).
Although these definitions are not standardized, recent studies have suggested that MH and TH, however they are defined, lead to better outcomes and may be good long-term treatment targets for CD, an idea supported by the findings of this review. Of the small number of studies that compared MH and TH (n = 7), TH was more closely associated with positive clinical outcomes, including reduced hospitalization, steroid-free clinical remission, and reduced need for surgery.
Health Assessment Instruments and Thresholds
Health assessment instruments and their corresponding thresholds to determine MH have been the main method used to assess healing in CD to date, but even within these thresholds, definitions of healing vary. For example, biomarkers such as fecal calprotectin (FC) have been used to determine MH/endoscopic remission, but FC is not specific to CD.1 Also, thresholds for FC levels vary across studies: <272 μg/g, <70 μg/g, 82 to 168 μg/g, and 50 to 250 μg/g,2 and the 2021 Delphi panel consulted in STRIDE-II used a cut-off value of 150 μg/g.10 C-reactive protein, the other commonly used biomarker, can indicate increased inflammation; however, up to 25% of patients may have inflammation evident in endoscopy and not demonstrate elevated CRP levels.1,2
Endoscopy, used for direct visualization of the mucosa to evaluate mucosal inflammation, is the most frequently employed assessment instrument for CD. The FDA recently produced guidance regarding the use of endoscopic assessments and CDAI for assessing CD, in which endoscopic remission (ie, MH) was defined as an SES-CD score of 0 to 2 or a score of 0 to 4 with no individual subscore greater than 1.11 The first therapies to include endoscopy in their FDA-approved labeling, risankizumab57,58 and upadacitinib,59,60 used SES-CD-determined endoscopic response as a coprimary end point, along with CDAI-determined clinical remission. The SES-CD threshold recommended by the FDA largely aligns with expert guidance from the STRIDE-II Delphi panel study that defined endoscopic remission as a combination of SES-CD ≤2 or CDEIS <3 and lack of ulcerations.10 However, Klenske et al pointed out that the limitation of the SES-CD is that the validation, responsiveness, and reliability to assess inflammation in CD are still unclear.1 They also noted that remission has been defined variously in different studies as either a score of 0 to 2 or 0 to 3. Absence of ulceration as assessed through endoscopy has also been used to define endoscopic healing/MH, but some studies have used endoscopic response, a reduction in SES-CD of >50% from baseline as a predictor of improved outcomes.1 Drawing from endoscopic results, the CDEIS is also used to evaluate endoscopic outcomes in CD, and thresholds for endoscopic remission have varied between studies, including scores of <4, 0, and 1 to 4.2
Increasingly, the IBD community is interested in the use of intestinal ultrasound to allow a cross-sectional view of the bowel wall and assess TH, though it is not yet a completely accepted standard. Ultrasonography is more convenient than endoscopy in terms of cost and accessibility due to its noninvasive nature. Ultrasound-assessed healing is approached by evaluating thickened bowel walls, fibrofatty proliferation, ulcers, and abscesses.1,15,20 Doppler ultrasound can be used to assess the vascular pattern in the bowel wall and indicate whether there is increased blood supply to the area that would signify inflammation, with variable results depending on factors such as food ingestion.21 Despite the advantages of ultrasonography for the monitoring of CD, there is currently no standardized threshold for the thickness measurement that would indicate healing, nor are there standardized placements for where or how (cross-sectionally or longitudinally) such measurements should be taken.21 Likewise, there is no standardized measure for the fatty tissue that would correlate with disease severity.21 Several scoring indices have been proposed to address the lack of standardization in ultrasound interpretation, including the simple intestinal ultrasound (IUS) score and bowel ultrasound score, but these require additional validation and/or sensitivity assessment.16 Despite the effectiveness of ultrasound and other imaging tools, the STRIDE-II Delphi panel agreed that these should be used as an adjunct assessment due to the limited ability of currently available treatments to achieve TH.10
The Capsule Endoscopy Crohn’s Disease Activity Index (CECDAI) is another validated scale used in assessing CD, which was not identified among included studies in the SLR; depending on uptake of capsule endoscopy in the future, it may be of interest for further exploration. Small bowel enteroscopy is another potentially useful tool for evaluating CD, with several studies reporting its use to measure MH with a modified Rutgeerts’ scoring system61,62 or the Fukuoka Index.63
The strengths and weaknesses of each of these instruments in detecting inflammation due to CD influence how healing has been defined and assessed; and the use of each instrument may vary by availability, access, reimbursement, and geography.19 Additionally, clinicians may not use some of the outcome instruments in practice because of impracticability, regardless of their effectiveness in assessing healing. At a minimum, standard thresholds for each assessment instrument would be helpful for making decisions about treatment selection for patients with CD and will be necessary for streamlining research towards deep healing. Forthcoming artificial intelligence technologies may help to address this issue and limit inter- and intraclinician variation in assessment interpretation.
Future Directions and Recommendations
As new diagnostic tools have surfaced over the past decade that allow for more precise measurement of disease state and healing, there has been some official recognition of the need for a standardized definition of healing in CD and standardized long-term goals of treatment. IOIBD’s STRIDE initiative recommended TH as an informal assessment target, pointing out that new biologic and small molecule therapies and new, less invasive, diagnostic tools have created the opportunity to adjust treatment goals for CD while noting the need for defining said goals.10 Currently, patient reticence to experience invasive endoscopic testing, paired with interclinician variability in interpretation, can reduce the likelihood of endoscopic monitoring and complicate the healthcare process. Increased standardization, plus better education for clinicians on guidelines and scoring mechanisms, would reduce some inefficiency even without improved health assessment techniques.
While studies are beginning to evaluate how MH and TH impact long-term clinical outcomes, limited evidence exists for the impact of MH and TH on patient quality of life and medical costs. Additional evidence is also needed on the effect of MH and TH on health-related quality of life or healthcare costs. These could include but are not limited to the assessment instrument and whether endoscopy findings correlate with cross-sectional imaging, as the use of ultrasound in place of endoscopy could relieve some of the humanistic and/or economic burden of invasive monitoring on patients with CD.
Additionally, further studies of TH as an indicator of long-term outcomes of CD are warranted, particularly with standardized assessment thresholds to aid in comparative analysis.
Short- and long-term treatment targets, as recommended by STRIDE-II, may be the most effective treatment strategy for CD, with the ultimate goal of disease control and remission. The combination of goals prioritizes quick symptomatic response, thereby improving patient quality of life and minimizing mucosal damage, but also recognizes that without complete healing of the bowel wall and mucosa, the risk of relapse and surgery may still be high. Because MH and TH have the potential to affect disease trajectory by reducing mucosal inflammation, there is a need for studies that assess MH and TH as prognostic parameters for a milder disease course in CD. Finally, prospective trials linking interventions to the induction of MH and modulation of the natural disease course, reducing the need for hospitalizations and surgeries, would mark an important step in defining the utility of MH for clinical practice.
Several limitations remain in our study. Most of the included studies (n = 28) were retrospective, with limited information on the time to measure MH. Additionally, studies included various follow-up times, ranging from 16 weeks to 11 years, and heterogenous populations. Included studies covered the CD population with mild, moderate, or mixed disease severity, as well as populations restricted to those who achieved MH or TH at study start and those undergoing specific therapeutic interventions. Such heterogeneity in populations likely impacts the outcomes reported in this study. As reported in previously published studies,54,55 variable definitions of MH and TH as well as heterogeneity in outcome measures remain a limitation of both our and other comprehensive reviews.
Conclusion
This SLR examined the evidence linking MH and TH with long-term clinical outcomes in CD. The majority of studies reported that patients with MH and TH (as measured by validated scales) had improved clinical outcomes, including increased rates of clinical remission, decreased rates of relapse or treatment escalation, and fewer hospitalizations and surgeries. MH and TH are important objective indicators of long-term disease control in CD, as both represent the potential for an altered disease course with reduced inflammation and fewer complications. Standardized definitions of MH and TH as well as outcome instrument assessment thresholds will be paramount in adopting MH and TH as targets of therapy.
Supplementary Data
Supplementary data is available at Inflammatory Bowel Diseases online.
Acknowledgments
Writing support for the development of this article was funded by AbbVie and provided by Jordan Godwin, MA, Allison Brackley, PhD, Elizabeth Hubscher, PhD, and Sally Neath, MA, of Cytel Inc.
Author Contributions
All authors were involved in the planning/conduct of study, collecting and/or interpreting data, drafting manuscript, and provided approval to submit this work for publication.
Funding
Financial support for the study was provided by AbbVie. AbbVie participated in the interpretation of data, review, and approval of the manuscript. All authors contributed to the development of the article and maintained control over the final content. No honoraria or payments were made for authorship.
Conflicts of Interest
B.S. has served as a consultant or received speaker’s fees from AbbVie, Abivax, Adiso Therapeutics, Alimentiv, Amgen, Arena Pharmaceuticals, Artizan Biosciences, Artugen Therapeutics, AstraZeneca, Bacainn Therapeutics, Biora Therapeutics, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol Myers Squibb, Calibr, Celltrion Healthcare, ClostraBio, Connect Biopharm, Cytoki Pharma, Eli Lilly and Company, Entera, Evommune, Ferring, Fresenius Kabi, Galapagos, Gilead, Genentech, Glaxo SmithKline, Gossamer Bio, HMP Acquisition, Imhotex, Immunic, InDex Pharmaceuticals, Innovation Pharmaceuticals, Inotrem, Ironwood Pharmaceuticals, Janssen, Johnson & Johnson, Kaleido, Kalyope, Merck, MiroBio, Morphic Therapeutic, MRM Health, OSE Immunotherapeutics, Pfizer, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonist Therapeutics, Q32 Bio, RedHill Biopharma, Sun Pharma Global, Surrozen, Synlogic Operating Company, Takeda, Target RWE, Theravance Biopharma R&D, TLL Pharmaceutical, USWM Enterprises, Ventyx Biosciences, Viela Bio, and has stock options from Ventyx Biosciences.
S.D. reports consultancy fees from AbbVie, Alimentiv, Allergan, Amgen, AstraZeneca, Athos Therapeutics, Biogen, Boehringer Ingelheim, Celgene, Celltrion, Eli Lilly, Enthera, Ferring Pharmaceuticals, Gilead, Hospira, Inotrem, Janssen, Johnson & Johnson, MSD, Mundipharma, Mylan, Pfizer, Roche, Sandoz, Sublimity Therapeutics, Takeda, TiGenix, UCB, and Vifor; and reports lecture fees from Abbvie, Amgen, Dr Falk Pharma, Ferring Pharmaceuticals, Gilead, Janssen, Mylan, Pfizer, and Takeda.
J.C.C. reports having served as a consultant of AbbVie, Medtronic; Advisory Board of AbbVie, Pfizer, Takeda; Speaker Bureau for AbbVie, Bristol Myers Squib, Janssen, Pfizer, Takeda.
S.G. and D.T. are employees of Cytel, Inc, which was a paid consultant on this project.
K.G. was an employee of Cytel Inc. at the time of the research.
J.G., N.J., and K.K. are employees of AbbVie.
A.D. reports fees for participation in clinical trials, review activities such as data monitoring boards, statistical analysis, and end point committees from Abivax, AbbVie, Arena Pharmaceuticals, Bristol Myers Squibb/Celgene, Dr Falk Foundation, Galapagos, Gilead, Janssen, and Pfizer; consultancy fees from AbbVie, Amgen, Arena Pharmaceuticals, Biogen, Boehringer Ingelheim, Bristol Myers Squibb/Celgene, Celltrion, Dr Falk Foundation, Ferring Pharmaceuticals, Fresenius Kabi, Galapagos, Janssen, Lilly, MSD, Pfizer, Pharmacosmos, Roche/Genentech, Sandoz/Hexal, Takeda, Tillotts, and Vifor Pharma; payment from lectures including service on speakers bureaus from AbbVie, Biogen, CED Service GmbH, Celltrion, Falk Foundation, Ferring, Galapagos, Gilead, High5MD, Janssen, Materia Prima, MedToday, MSD, Pfizer, Streamed-Up, Takeda, Tillotts, and Vifor Pharma; payment for manuscript preparation from Falk Foundation, Takeda, Thieme, and UniMed Verlag.
Data Availability
The full results from this study are available upon request.



