-
PDF
- Split View
-
Views
-
Cite
Cite
Dahham Alsoud, Bram Verstockt, Séverine Vermeire, Ustekinumab and Vedolizumab Exposure Is Unaffected by Pharmacogenetic Determinants of Anti-TNFs Pharmacokinetics, Inflammatory Bowel Diseases, Volume 30, Issue 5, May 2024, Pages 874–875, https://doi.org/10.1093/ibd/izae035
Close - Share Icon Share
To the Editors,
Optimizing currently available monoclonal antibodies for the treatment of inflammatory bowel disease (IBD) requires a better understanding of factors affecting their pharmacokinetics and pharmacodynamics.1 Throughout the long experience with antitumor necrosis factor (anti-TNF) biologics, several pharmacogenetic variants have been associated with increased risk of immunogenicity, accelerated clearance, and subsequent loss of response (LOR).2–4 By contrast, little is known about the association of such pharmacogenetic determinants with the pharmacokinetics of ustekinumab and vedolizumab.5
We therefore read with interest the post hoc analysis of the IM-UNITI and UNIFI cohorts, in which the authors investigated whether carriage of the human leukocyte antigen (HLA)-DQA1*05 allele is associated with increased risk of immunogenicity, lower ustekinumab trough concentrations, and LOR.6 In contrast to previous findings with anti-TNF biologics, the investigators did not observe similar associations in patients receiving ustekinumab.
In parallel to the HLA-DQA1*05 allele, a polymorphism in the neonatal Fc-receptor (FcRn) gene has also been found to affect anti-TNFs’ pharmacokinetics. Specifically, the variable number of tandem repeats (VNTR)2/VNTR3 genotype correlates with lower drug exposure compared with the wild VNTR3/VNTR3 genotype.3 To investigate whether the VNTR2/3 genotype is also associated with lower drug exposure of ustekinumab and vedolizumab, we retrospectively retrieved exposure data and DNA samples from IBD patients followed at the IBD clinic of the University Hospitals Leuven (Belgium) who initiated these biologics between January 2015 and September 2020. We identified patients who had low (quartile [Q]1) or high (Q4) serum levels, as defined by previous studies in the same population.7,8 Polymerase chain reaction assays were designed to genotype the VNTR polymorphism in the FcRn gene. Forty-two patients were included in the ustekinumab cohort, of whom 24 (57.1%) and 18 (42.9%) showed Q1 and Q4 serum levels, respectively. The vedolizumab cohort included 83 patients, of whom 53 (63.9%) and 30 (36.1%) had Q1 and Q4 levels, respectively. Ten (23.8%) and 18 (21.7%) patients in the ustekinumab and vedolizumab cohorts, respectively, carried the VNTR2/VNTR3 genotype. No significant association was observed between the VNTR2/VNTR3 genotype and serum levels of ustekinumab or vedolizumab during induction or maintenance (Figure 1).
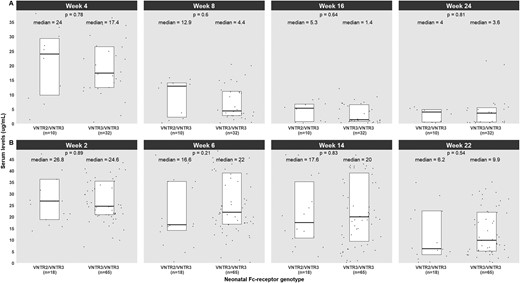
Differences in trough levels of ustekinumab (A) and vedolizumab (B) based on polymorphism in the neonatal Fc-receptor gene. VNTR, variable number of tandem repeats.
Further investigation into additional factors influencing the pharmacokinetics of ustekinumab and vedolizumab is essential for refining existing pharmacokinetic-pharmacodynamic models.9,10 By integrating relevant pharmacogenetic variants with patient and disease characteristics in such models, a more personalized dosing approach can be developed, which will optimize drug exposure and improve therapeutic outcomes.5
Funding
No funding was received for this study.
Conflicts of Interest
B.V. is supported by the Clinical Research Fund (KOOR) at the University Hospitals Leuven and the Research Council at the KU Leuven. S.V. and B.V. received a research grant from the Leona M. and Harry B. Helmsley Charitable Trust, and D.A. is funded by this grant. D.A. received a research grant from the IBD Patient’s Association Flanders (CCV VZW).
B.V. has received research support from AbbVie, Biora Therapeutics, Landos, Pfizer, Sossei Heptares and Takeda; speaker’s fees from Abbvie, Biogen, Bristol Myers Squibb, Celltrion, Chiesi, Falk, Ferring, Galapagos, Janssen, Lily, MSD, Pfizer, R-Biopharm, Sandoz, Takeda, Tillots Pharma, Truvion and Viatris; consultancy fees from Abbvie, Alimentiv, Applied Strategic, Atheneum, BenevolentAI, Biora Therapeutics, Bristol Myers Squibb, Galapagos, Guidepont, Landos, Lily, Mylan, Inotrem, Ipsos, Janssen, Pfizer, Progenity, Sandoz, Santa Ana Bio, Sosei Heptares, Takeda, Tillots Pharma, and Viatris.
S.V. has received grants from AbbVie, J&J, Pfizer, Galapagos and Takeda; consulting and/or speaking fees from AbbVie, Abivax, AbolerIS Pharma, AgomAb, Alimentiv, Arena Pharmaceuticals, AstraZeneca, Avaxia, BMS, Boehringer Ingelheim, Celgene, CVasThera, Dr Falk Pharma, Ferring, Galapagos, Genentech-Roche, Gilead, GSK, Hospira, Imidomics, Janssen, J&J, Lilly, Materia Prima, MiroBio, Morphic, MrMHealth, Mundipharma, MSD, Pfizer, Prodigest, Progenity, Prometheus, Robarts Clinical Trials, Second Genome, Shire, Surrozen, Takeda, Theravance, Tillots Pharma AG, and Zealand Pharma.
D.A. declares no conflicts of interest.



