-
PDF
- Split View
-
Views
-
Cite
Cite
David Rubin, Scott D Lee, Lucky Flores, Gustavo Albizu-Angulo, Ellen Scherl, Surinder Saini, Iming Cho, Shih-Yao Lin, Simona Reed, Jesse Hall, A PHASE II OPEN LABEL STUDY OF NEIHULIZUMAB, AN ANTI-CD162 (PSGL-1) ANTIBODY, IN PATIENTS WITH MODERATE TO SEVERE ACTIVE, ANTI-TNFα AND/OR ANTI-INTEGRIN REFRACTORY ULCERATIVE COLITIS, Inflammatory Bowel Diseases, Volume 29, Issue Supplement_1, February 2023, Pages S77–S78, https://doi.org/10.1093/ibd/izac247.149
Close - Share Icon Share
Abstract
Neihulizumab (ALTB-168) is a novel immune checkpoint agonistic antibody that binds to human CD162 (PSGL-1), and leads to downregulation of activated T-cells. Early-phase trials of neihulizumab have suggested a benefit in patients with immune conditions, including psoriasis, psoriatic arthritis and steroid-refractory acute graft-versus-host disease. Here, we report the results of a Phase II study investigating the use of neihulizumab for ulcerative colitis (UC) (ClinicalTrials.gov Identifier: NCT03298022).
The aims of this study were to evaluate efficacy, safety, tolerability and immunogenicity of neihulizumab in patients with moderate to severe active UC who are refractory or intolerant to anti-TNFα and/or anti-integrin therapy.
Two regimens were tested: 5 weekly IV doses plus 3 bi-weekly IV doses of 9 mg/kg neihulizumab (5 + 3 regimen), and 8 weekly IV doses plus 2 bi-weekly IV doses of 9 mg/kg neihulizumab (8 + 2 regimen). The primary efficacy outcome is the proportion of patients with clinical response at Week 12. The key secondary efficacy outcomes are the proportion of patients with clinical remission, mucosa healing, histological healing and the change in Inflammatory Bowel Disease Questionnaire (IBDQ).
24 subjects enrolled in this study; study demographics and baseline disease characteristics are shown in Table 1.
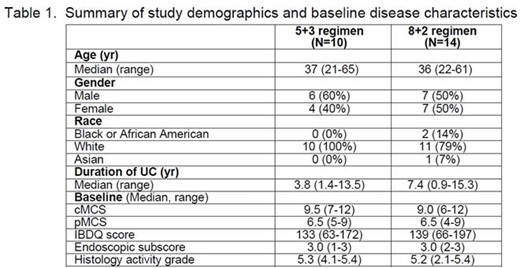
In the 5 + 3 regimen, 10 subjects were enrolled into this study and 2 (20%) subjects completed the study. The mean duration of study treatment exposure was 59.5 days. Among 10 enrolled subjects, 9 subjects were included in the efficacy analysis: 2 (22%) subjects achieved clinical response at Week 12 and 1 (11%) subject achieved clinical response at Week 2. The overall efficacy is summarized in Table 2. In this regimen, 9/10 (90%) subjects reported an AE: 7 (70%) subjects with AEs related to study treatment, 9 (90%) subjects with AEs that were not related to study treatment, 1 (10%) subject with an SAE not related to study treatment, and no deaths were reported in this regimen.
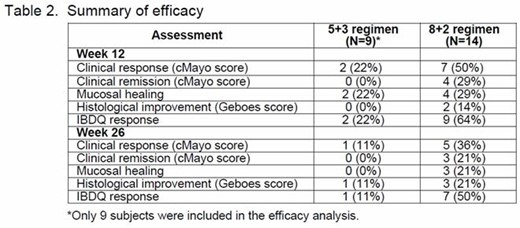
In the 8 + 2 regimen, 14 subjects were enrolled into this study and 9 (64%) subjects completed the study. The mean duration of study treatment exposure was 78.1 days. Among 14 enrolled subjects, 7 (50%) subjects achieved clinical response at Week 12 and 5 (36%) subjects achieved clinical response at Week 26 (Table 2). In this regimen, 13/14 (93%) of subjects reported an AE: 2 (14%) subjects with AEs related to study treatment and 12 (86%) subjects with AEs that were not related to study treatment; there was no SAE reported and no deaths.
This phase 2 study of neihulizumab suggest efficacy and safety signals in treatment-refractory UC that warrant further investigation.
- integrins
- inflammatory bowel disease
- ulcerative colitis
- arthritis, psoriatic
- phase 2 clinical trials
- demography
- down-regulation
- psoriasis
- safety
- steroids
- t-lymphocytes
- antibodies
- mucous membrane
- graft-versus-host disease, acute
- cell cycle checkpoint
- binding (molecular function)
- disease remission
- immunogenicity



