-
PDF
- Split View
-
Views
-
Cite
Cite
Benjamin Dreskin, Ingrid Jurickova, Elizabeth Angerman, Erin Bonkowski, Lee Denson, EICOSATETRAYNOIC ACID REGULATES PRO-FIBROTIC PATHWAYS IN AN INDUCED PLURIPOTENT STEM CELL DERIVED MACROPHAGE:HUMAN INTESTINAL ORGANOID MODEL OF ILEAL CROHN’S DISEASE, Inflammatory Bowel Diseases, Volume 29, Issue Supplement_1, February 2023, Pages S76–S77, https://doi.org/10.1093/ibd/izac247.147
Close - Share Icon Share
Abstract
Biologics targeting TNF are the mainstay of therapy for children with Crohn’s Disease (CD). However, a subset of patients do not respond, progressing to intestinal fibrosis requiring surgical resection. Prior studies have defined an ileal gene expression module linked to future strictures, and identified small molecules which may reverse this gene signature and suppress fibroblast activation. In the current study we developed a pre-clinical model system and tested a lead candidate, eicosatetraynoic acid (ETYA), a Peroxisome Proliferator-Activated Receptor (PPAR) agonist and arachidonic acid metabolism inhibitor.
Peripheral blood samples were collected from pediatric CD patients and induced pluripotent stem cell (iPSC) lines were generated. iPSC were differentiated into human intestinal organoids (HIOs) and macrophage-like cells. Macrophage:HIO co-cultures were exposed to lipopolysaccharide (LPS) with and without eicosatetraynoic acid (ETYA) pre-treatment. Flow cytometry and cytospin characterized macrophage activation markers and morphology. Co-culture populations were harvested, and RNA and conditioned media were isolated for downstream analysis. TaqMan Low Density Array, Luminex multiplex assay, immunohistologic staining, and sirius red polarized light microscopy were performed to quantify measures of inflammation and fibrosis, and to test whether introduction of ETYA abated any of these inflammatory or fibrotic changes.
iPSC-derived macrophages exhibited morphology similar to primary macrophages, and expressed inflammatory macrophage cell surface markers including CD68 (Fig. 1A). LPS-stimulated iPSC-derived macrophages expressed a global pattern of gene expression by RNA sequencing enriched in CD ileal inflammatory macrophages (ToppCell Atlas, p=4.397E-117), and produced cytokines and chemokines implicated in refractory disease (Fig. 1B). Co-culture of LPS-primed macrophages with HIO led to up-regulation of the fibroblast activation genes ACTA2 and COL1A1 (Fig. 2). Under these conditions, HIO collagen content measured by sirius red staining and polarized light microscopy was increased (Fig. 2). ETYA pre-treatment prevented these pro-fibrotic effects of LPS-primed macrophages upon HIO gene expression and collagen content. However, LPS induction of macrophage IL1B, TNF, and OSM production was not suppressed by ETYA, suggesting an alternative mechanism of action upon HIO fibroblast activation and collagen content in the co-culture system.
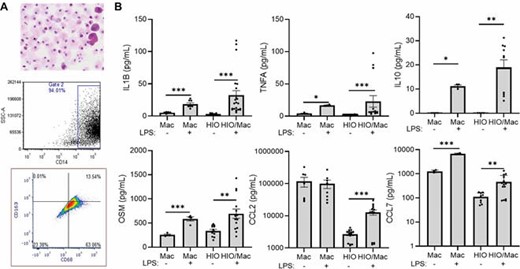
iPSC-derived macrophages exhibit a pro-inflammatory pattern of cytokine and chemokine production implicated in anti-TNF non-response. A) Induced pluripotent stem cells (iPSC) were differentiated into macrophage-like cells over 25 days. Cell morphology was assessed using cytospin, and CD14 expression, and CD68 and CD163 expression on CD14+ cells, was determined using flow cytometry. Representative images of scatter plots are shown, n=14. B) iPSC-derived macrophages (Mac) or human intestinal organoids (HIO) were assayed under basal conditions, or following macrophage:HIO (HIO/Mac) co-culture ± LPS, 100 ng/mL for 72 hours. Cytokine and chemokine secretion was measured using a luminex assay. Data are shown as the mean (SEM), n=4-14 per group, *p<0.001.

Eicosatetraynoic acid (ETYA) prevents up-regulation of HIO ECM gene expression and collagen content by LPS primed macrophages in co-culture. Human intestinal organoids (HIOs) were studied under basal conditions, with LPS primed macrophage (Macrophage/Mac) 72 hour co-culture, or following ETYA exposure, 50 mM for 14 days, preceding LPS primed macrophage 72 hour co-culture (ETYA/Macrophage & Mac/EA). A) Representative images are shown for alpha smooth muscle actin (ACTA), vimentin (VIM), and DAPI staining, and for Sirius red staining with polarized light microscopy (arrow). B) Human intestinal organoid ACTA2, VIM, and collagen (COL1A1) gene expression were determined by PCR. Collagen protein was detected in HIO using Sirius red staining and polarized light microscopy (arrows). Organoid collagen content was quantified using ImageJ. Data are shown as the mean (SEM), n=9-16 per group for PCR and collagen content data,*p<0.001.
ETYA inhibits effects of LPS-primed macrophages upon HIO pro-fibrotic gene expression and collagen production. This was not associated with an effect of ETYA upon macrophage inflammatory cytokine production. Future studies will test alternate pathways including PPAR activation and arachidonic acid metabolism which may mediate this response.



