-
PDF
- Split View
-
Views
-
Cite
Cite
Edward V Loftus, Daniel C Baumgart, Krisztina Gecse, Jami A Kinnucan, Susan B Connelly, Leonardo Salese, Chinyu Su, Kenneth K Kwok, John C Woolcott, Alessandro Armuzzi, Clostridium difficile Infection in Patients with Ulcerative Colitis Treated with Tofacitinib in the Ulcerative Colitis Program, Inflammatory Bowel Diseases, Volume 29, Issue 5, May 2023, Pages 744–751, https://doi.org/10.1093/ibd/izac139
Close - Share Icon Share
Abstract
Tofacitinib is an oral, small molecule Janus kinase inhibitor for the treatment of ulcerative colitis (UC). Patients with inflammatory bowel disease are susceptible to Clostridium difficile infection (CDI). Here, we evaluate CDI in the tofacitinib UC clinical program.
Events from 4 randomized, placebo-controlled studies (phase [P] 2 or P3 induction [NCT00787202; NCT01465763; NCT01458951], P3 maintenance [NCT01458574]) and an open-label, long-term extension (OLE) study (NCT01470612), were analyzed as 3 cohorts: Induction (P2/P3 induction), Maintenance (P3 maintenance), and Overall (patients receiving tofacitinib 5 or 10 mg twice daily [BID] in P2, P3, and OLE studies; including final data from the OLE study, as of August 24, 2020). Proportions and incidence rates (unique patients with events per 100 patient-years of exposure) of CDI were evaluated.
The overall cohort comprised 1157 patients who received ≥1 dose of tofacitinib 5 or 10 mg BID, with a total of 2814.4 patient-years of tofacitinib exposure and up to 7.8 years of treatment. A total of 82.6% of patients received predominantly tofacitinib 10 mg BID. In the induction, maintenance, and overall cohorts, 3 (2 tofacitinib treated, 1 placebo treated), 3 (all placebo treated), and 9 patients had CDI, respectively; the overall cohort incidence rate was 0.31 (95% confidence interval, 0.14-0.59). CDI were all mild–moderate in severity and resolved with treatment in 8 patients. Six of 9 patients continued tofacitinib treatment without interruption. Two patients had events reported as serious due to hospitalization. Two patients were receiving corticosteroids when the CDI occurred.
CDIs among patients with UC receiving tofacitinib were infrequent, cases were mild–moderate in severity, and most resolved with treatment.
Lay Summary
The incidence of Clostridium difficile infection in the tofacitinib ulcerative colitis clinical program was evaluated. C. difficile infection among patients with ulcerative colitis receiving tofacitinib were infrequent; cases were mild–moderate in severity, and most resolved with treatment.
What is already known?
Incidence of Clostridium difficile infection (CDI) is increasing in patients with inflammatory bowel disease (IBD), and is associated with higher morbidity and mortality vs patients without IBD.
What is new here?
CDIs were infrequent in the tofacitinib ulcerative colitis (UC) clinical program, patients continued tofacitinib therapy after CDI resolution, and those treated with tofacitinib were at similar or slightly lower risk of CDI than patients treated with placebo.
How can this study help patient care?
In this analysis, the majority of patients with UC and a CDI did not require interruption to their tofacitinib treatment.
Introduction
Studies have shown that incidence of Clostridium difficile infection (CDI) is increasing, particularly in the population with inflammatory bowel disease.1 Inflammatory bowel disease itself has been shown to be an independent risk factor for CDI.2,3 It has been shown that CDI in patients with inflammatory bowel disease is associated with higher morbidity and mortality, compared with CDI in patients without inflammatory bowel disease, by as much as 4.8-fold.4,5 CDI is also associated with increased risk of colectomy.6 Although biologics and small molecules have improved the management of ulcerative colitis (UC),7 these treatments may make patients more susceptible to opportunistic infections.2 Specifically, it has been reported that corticosteroids can increase the risk of CDI,8 and other studies raise the question that advanced therapies for UC, such as tumor necrosis factor inhibitors (TNFi) and vedolizumab, could also increase the risk of CDI.9,10 Similarly, CDI can increase the difficulty of UC management.11,12
Tofacitinib is an oral, small molecule Janus kinase inhibitor for the treatment of UC. The efficacy and safety of tofacitinib have been evaluated in an 8-week, phase 2 induction study13; 3 phase 3, randomized, placebo-controlled studies (Oral Clinical Trials for tofAcitinib in ulceratiVE colitis [OCTAVE] Induction 1 and 2 studies and OCTAVE Sustain study),14 and an open-label, long-term extension study (OCTAVE Open study) in patients with moderately to severely active UC.15
Here, we present an analysis of CDI in the tofacitinib UC clinical program, including final data from the OCTAVE Open study (as of August 24, 2020).
Methods
Patients and Study Design
Full details of the phase 2 induction study (NCT00787202), OCTAVE Induction 1 and 2 studies (NCT01465763 and NCT01458951), OCTAVE Sustain study (NCT01458574), and OCTAVE Open study (NCT01470612) have been reported previously.13-15 Patients who had clinical response at week 8 of the OCTAVE Induction 1 or 2 study were eligible to participate in the OCTAVE Sustain study. Nonresponders from the OCTAVE Induction 1 and 2 studies, as well as completers and treatment failures from the OCTAVE Sustain study, were eligible to enroll in the OCTAVE Open study. All patients underwent C. difficile screening (test for C. difficile toxin) at entry into the tofacitinib UC clinical program; a positive test excluded patients from phase 3 program entry.
Patients in the induction, maintenance, and overall (including final data from the OCTAVE Open study, as of August 24, 2020) cohorts were included in these analyses. The induction cohort included patients who received placebo or tofacitinib 10 mg twice daily (BID) in the 8-week, phase 2 study and the two 8-week, phase 3 (OCTAVE Induction 1 and 2 study) studies. The maintenance cohort included patients who received placebo, tofacitinib 5 mg BID, or tofacitinib 10 mg BID in the 52-week, phase 3 maintenance study (OCTAVE Sustain study). The overall cohort included all patients who received ≥1 dose of tofacitinib 5 or 10 mg BID in the phase 2 and 3 induction and maintenance studies, and the open-label, long-term extension study.
Inclusion and exclusion criteria have been described previously.13-15 Briefly, for patients to be eligible to enter the studies, they had to be >18 years of age with a diagnosis of UC for ≥3 months (phase 2) or ≥4 months (phase 3 OCTAVE Induction 1 and 2 studies, and OCTAVE Sustain study) and have had moderately to severely active UC at induction program entry (defined as a total Mayo score of 6-12, with a rectal bleeding subscore of 1-3 [phase 3] and an endoscopic subscore of 2 or 3).13,14
Some patients entering into the studies had prior TNFi exposure or prior TNFi failure. TNFi failure was determined by investigators and did not specify a minimum dose or treatment duration with TNFi. This includes failure with ≥1 TNFi and includes patients who had primary or secondary nonresponse to a TNFi. At baseline of the OCTAVE Induction 1 and 2 studies, oral corticosteroids (prednisone-equivalent up to 25 mg/d; budesonide up to 9 mg/d; stable dose for at least 2 weeks prior to baseline and during the study period) were permitted. During the OCTAVE Sustain and OCTAVE Open studies, tapering of oral corticosteroids was mandatory. Patients with worsening UC were permitted to increase their corticosteroid dose to the preceding week’s daily dosage; this could only be done once in the study. For patients receiving corticosteroids, the daily dose of prednisone or equivalent had to be decreased at a rate of 5 mg/wk until the dose reached 20 mg/d, then by 2.5 to 5 mg/wk until the dose reached 10 mg/d, and then by 2.5 mg/wk until the dose reached 0 mg/d. For patients receiving budesonide, the daily dose of budesonide had to be decreased at a rate of 3 mg every 3 weeks until the dose reached 0 mg.
Identification of CDI
CDI was identified in patients with Medical Dictionary for Regulatory Activities preferred terms of CDI, C. difficile colitis, or C. difficile test–positive (positive test for difficile toxin), who had received concomitant oral metronidazole or vancomycin (counted once per cohort).
The investigator was responsible for describing the maximum severity of the adverse event. When the CDI had no interference with patients’ usual function, severity was classified as mild; when the CDI had interference to some extent with patients’ usual function, severity was classified as moderate; and when the CDI had significant interference with patients’ usual function, severity was classified as severe. Additional information, including CDI outcome, study drug action, hospitalization, history of CDI, and concomitant medication (including antibiotic use prior to the CDI event), was also collected. CDI was defined as a serious adverse event if the event resulted in death, was life-threatening, required inpatient hospitalization or prolonged existing hospitalization, resulted in persistent or significant disability or incapacity (substantial disruption of the ability to conduct normal life functions), or resulted in congenital anomaly or birth defect.
Statistical Analysis
In the overall cohort, a tofacitinib all population (including patients receiving tofacitinib 5 or 10 mg BID) was analyzed. Patients in the overall cohort were also categorized based on the average daily dose of tofacitinib (placebo exposure was not included). Patients with a predominant dose (PD) of tofacitinib 5 mg BID had an average daily dose <15 mg, and patients with a PD of tofacitinib 10 mg BID had an average daily dose >15 mg. Proportions and incidence rates (IRs) (unique patients with events per 100 patient-years of exposure) were calculated for CDI. Events that occurred during and up to 28 days posttreatment were included in the proportions and IR calculations.
Ethical Considerations
All studies were registered with ClinicalTrials.gov (NCT00787202; NCT01465763; NCT01458951; NCT01458574; NCT01470612), conducted in compliance with the Declaration of Helsinki and the International Council for Harmonisation Good Clinical Practice Guidelines, and approved by the Institutional Review Board and/or Independent Ethics Committee at each investigational center participating in the studies or at a central Institutional Review Board. All patients provided written informed consent.
Results
Patients
The induction cohort included 1220 patients, 282 of whom received at least 1 dose of placebo and 938 of whom received at least 1 dose of tofacitinib 10 mg BID. This cohort included 194 patients from the phase 2 induction study, 48 of whom received placebo and 146 of whom received tofacitinib. The maintenance cohort included 592 patients, 198 of whom received placebo, 198 of whom received tofacitinib 5 mg BID, and 196 of whom received tofacitinib 10 mg BID. The overall cohort comprised 1157 patients, with 2814.4 patient-years of tofacitinib exposure and up to a maximum of 7.8 years of treatment. Of these patients, 956 (82.6%) received a PD of tofacitinib 10 mg BID and 201 (17.4%) received a PD of tofacitinib 5 mg BID.
In general, patient demographics and baseline characteristics in the tofacitinib group were similar to the placebo group and across the 3 cohorts (Table 1). Briefly, mean age across cohorts ranged from 40.6 ± 13.7 years to 44.6 ± 14.5 years. Between 44.8% and 56.6% of patients had prior TNFi exposure, and between 41.3% and 54.2% of patients had TNFi failure at baseline. Across the 3 cohorts, between 40.3% and 51.0% receiving corticosteroids at baseline, with a mean daily dose (prednisone equivalent) ranging from 14.5 to 16.9 mg/d, and about 71% of patients were receiving concomitant 5-aminosalicylates (5-ASA) therapy at baseline of phase 3 induction studies.
Demographics and Baseline Clinical Characteristics of the Induction, Maintenance, and Overall Cohorts
| . | Induction Cohort . | Maintenance Cohort . | Overall Cohorta . | |||||
|---|---|---|---|---|---|---|---|---|
| Placebo (N = 282) . | Tofacitinib 10 mg BID (N = 938) . | Placebo (N = 198) . | Tofacitinib 5 mg BID (N = 198) . | Tofacitinib 10 mg BID (N = 196) . | PD Tofacitinib 5 mg BID (N = 201) . | PD Tofacitinib 10 mg BID (N = 956) . | Tofacitinib All (N = 1157) . | |
| Total patient-years of exposure | 44.8 | 156.2 | 100.4 | 146.2 | 154.3 | 776.4 | 2038 | 2814.4 |
| Age, mean (SD), y | 41.4 ± 14.4 | 41.3 ± 13.8 | 43.4 ± 14.0 | 41.9 ± 13.7 | 43 ± 14.4 | 44.6 ± 14.5 | 40.6 ± 13.7 | 41.3 ± 13.9 |
| Male, n (%) | 155 (55.0) | 557 (59.4) | 116 (58.6) | 103 (52.0) | 110 (56.1) | 115 (57.2) | 564 (59.0) | 679 (58.7) |
| Race, n (%) | ||||||||
| White | 229 (81.2) | 756 (80.6) | 155 (78.3) | 164 (82.8) | 153 (78.1) | 159 (79.1) | 768 (80.3) | 927 (80.1) |
| Black | 4 (1.4) | 6 (0.6) | 3 (1.5) | 2 (1.0) | 0 (0.0) | 1 (0.5) | 9 (0.9) | 10 (0.9) |
| Asian | 28 (9.9) | 114 (12.2) | 26 (13.1) | 23 (11.6) | 25 (12.8) | 28 (13.9) | 116 (12.1) | 144 (12.4) |
| Other | 11 (3.9) | 36 (3.8) | 9 (4.5) | 5 (2.5) | 9 (4.6) | 9 (4.5) | 33 (3.5) | 42 (3.6) |
| Unspecified | 10 (3.5) | 26 (2.8) | 5 (2.5) | 4 (2.0) | 9 (4.6) | 4 (2.0) | 30 (3.1) | 34 (2.9) |
| Disease duration, mean (SD), y | 8.2 ± 6.8 | 8.2 ± 7.0 | 8.8 ± 7.5 | 8.3 ± 7.2 | 8.7 ± 7.0 | 8.3 ± 6.5 | 8.2 ± 7.1 | 8.2 ± 7.0 |
| Extent of disease, n (%)b | ||||||||
| Proctosigmoiditis | 35 (15.0) | 132 (14.6) | 21 (10.6) | 28 (14.3) | 33 (16.9) | 34 (17.0) | 129 (14.0) | 163 (14.5) |
| Left-sided colitis | 76 (32.6) | 307 (34.0) | 68 (34.4) | 66 (33.7) | 60 (30.8) | 69 (34.5) | 311 (33.8) | 380 (33.9) |
| Extensive/pancolitis | 122 (52.4) | 463 (51.3) | 108 (54.5) | 102 (52.3) | 102 (52.3) | 97 (48.5) | 480 (52.1) | 577 (51.5) |
| Proctitis | 0 (0.0) | 1 (0.1) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 1 (0.1) |
| Total Mayo score at baseline, mean (SD) | 8.9 ± 1.5 | 9.0 ± 1.5 | 3.3 ± 1.8 | 3.3 ± 1.8 | 3.4 ± 1.8 | 7.8 ± 2.5 | 8.8 ± 1.8 | 8.6 ± 2.0 |
| C-reactive protein, mg/L, median (range) | 5.3 (2.2-12.7) | 4.6 (1.8-11.6) | 1.0 (0.3-3.5) | 0.7 (0.3-2.3) | 0.9 (0.4-3.0) | 3.2 (0.1-122.5) | 4.9 (0.1-208.4) | 4.5 (0.1-208.4) |
| Prior history of CDI, n (%)c | 2 (0.7) | 20 (2.1) | 4 (2.0) | 3 (1.5) | 8 (4.1) | NR | NR | NR |
| Prior TNFi exposure, n (%)b,d | 130 (55.6) | 488 (53.9) | 92 (46.5) | 90 (45.5) | 100 (51.0) | 90 (44.8) | 522 (56.6) | 612 (54.4) |
| Prior TNFi failure, n (%)b,e | 124 (53.0) | 465 (51.4) | 89 (44.9) | 83 (41.9) | 92 (46.9) | 83 (41.3) | 500 (54.2) | 583 (51.9) |
| Prior immunosuppressant use, n (%)b,f,g | 160 (68.4) | 683 (75.5) | 134 (67.7) | 149 (75.3) | 144 (73.5) | 136 (67.7) | 702 (76.1) | 838 (74.6) |
| Prior immunosuppressant failure, n (%)b,f,g | 158 (67.5) | 661 (73.0) | 129 (65.2) | 143 (72.2) | 141 (71.9) | 130 (64.7) | 683 (74.0) | 813 (72.3) |
| Immunosuppressant use within 8 wk prior to baseline, n (%)b,g | 56 (23.9) | 259 (28.6) | 44 (22.2) | 45 (22.7) | 56 (28.6) | 47 (23.4) | 266 (28.8) | 313 (27.8) |
| 5-ASA use at baseline, n (%)f | 167 (71.4) | 650 (71.8) | NR | NR | NR | NR | NR | NR |
| Oral corticosteroid use at baseline, n (%)h | 127 (45.0) | 430 (45.8) | 100 (50.5) | 101 (51.0) | 86 (43.9) | 81 (40.3) | 442 (46.2) | 523 (45.2) |
| Oral corticosteroid daily dose at baseline—prednisone equivalent, mg/d, mean (SD) | 16.9 ± 6.2 | 16.0 ± 6.4 | 15.9 ± 6.2 | 14.9 ± 6.2 | 14.5 ± 5.9 | 14.9 ± 6.1 | 16.1 ± 6.4 | 16.0 ± 6.3 |
| . | Induction Cohort . | Maintenance Cohort . | Overall Cohorta . | |||||
|---|---|---|---|---|---|---|---|---|
| Placebo (N = 282) . | Tofacitinib 10 mg BID (N = 938) . | Placebo (N = 198) . | Tofacitinib 5 mg BID (N = 198) . | Tofacitinib 10 mg BID (N = 196) . | PD Tofacitinib 5 mg BID (N = 201) . | PD Tofacitinib 10 mg BID (N = 956) . | Tofacitinib All (N = 1157) . | |
| Total patient-years of exposure | 44.8 | 156.2 | 100.4 | 146.2 | 154.3 | 776.4 | 2038 | 2814.4 |
| Age, mean (SD), y | 41.4 ± 14.4 | 41.3 ± 13.8 | 43.4 ± 14.0 | 41.9 ± 13.7 | 43 ± 14.4 | 44.6 ± 14.5 | 40.6 ± 13.7 | 41.3 ± 13.9 |
| Male, n (%) | 155 (55.0) | 557 (59.4) | 116 (58.6) | 103 (52.0) | 110 (56.1) | 115 (57.2) | 564 (59.0) | 679 (58.7) |
| Race, n (%) | ||||||||
| White | 229 (81.2) | 756 (80.6) | 155 (78.3) | 164 (82.8) | 153 (78.1) | 159 (79.1) | 768 (80.3) | 927 (80.1) |
| Black | 4 (1.4) | 6 (0.6) | 3 (1.5) | 2 (1.0) | 0 (0.0) | 1 (0.5) | 9 (0.9) | 10 (0.9) |
| Asian | 28 (9.9) | 114 (12.2) | 26 (13.1) | 23 (11.6) | 25 (12.8) | 28 (13.9) | 116 (12.1) | 144 (12.4) |
| Other | 11 (3.9) | 36 (3.8) | 9 (4.5) | 5 (2.5) | 9 (4.6) | 9 (4.5) | 33 (3.5) | 42 (3.6) |
| Unspecified | 10 (3.5) | 26 (2.8) | 5 (2.5) | 4 (2.0) | 9 (4.6) | 4 (2.0) | 30 (3.1) | 34 (2.9) |
| Disease duration, mean (SD), y | 8.2 ± 6.8 | 8.2 ± 7.0 | 8.8 ± 7.5 | 8.3 ± 7.2 | 8.7 ± 7.0 | 8.3 ± 6.5 | 8.2 ± 7.1 | 8.2 ± 7.0 |
| Extent of disease, n (%)b | ||||||||
| Proctosigmoiditis | 35 (15.0) | 132 (14.6) | 21 (10.6) | 28 (14.3) | 33 (16.9) | 34 (17.0) | 129 (14.0) | 163 (14.5) |
| Left-sided colitis | 76 (32.6) | 307 (34.0) | 68 (34.4) | 66 (33.7) | 60 (30.8) | 69 (34.5) | 311 (33.8) | 380 (33.9) |
| Extensive/pancolitis | 122 (52.4) | 463 (51.3) | 108 (54.5) | 102 (52.3) | 102 (52.3) | 97 (48.5) | 480 (52.1) | 577 (51.5) |
| Proctitis | 0 (0.0) | 1 (0.1) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 1 (0.1) |
| Total Mayo score at baseline, mean (SD) | 8.9 ± 1.5 | 9.0 ± 1.5 | 3.3 ± 1.8 | 3.3 ± 1.8 | 3.4 ± 1.8 | 7.8 ± 2.5 | 8.8 ± 1.8 | 8.6 ± 2.0 |
| C-reactive protein, mg/L, median (range) | 5.3 (2.2-12.7) | 4.6 (1.8-11.6) | 1.0 (0.3-3.5) | 0.7 (0.3-2.3) | 0.9 (0.4-3.0) | 3.2 (0.1-122.5) | 4.9 (0.1-208.4) | 4.5 (0.1-208.4) |
| Prior history of CDI, n (%)c | 2 (0.7) | 20 (2.1) | 4 (2.0) | 3 (1.5) | 8 (4.1) | NR | NR | NR |
| Prior TNFi exposure, n (%)b,d | 130 (55.6) | 488 (53.9) | 92 (46.5) | 90 (45.5) | 100 (51.0) | 90 (44.8) | 522 (56.6) | 612 (54.4) |
| Prior TNFi failure, n (%)b,e | 124 (53.0) | 465 (51.4) | 89 (44.9) | 83 (41.9) | 92 (46.9) | 83 (41.3) | 500 (54.2) | 583 (51.9) |
| Prior immunosuppressant use, n (%)b,f,g | 160 (68.4) | 683 (75.5) | 134 (67.7) | 149 (75.3) | 144 (73.5) | 136 (67.7) | 702 (76.1) | 838 (74.6) |
| Prior immunosuppressant failure, n (%)b,f,g | 158 (67.5) | 661 (73.0) | 129 (65.2) | 143 (72.2) | 141 (71.9) | 130 (64.7) | 683 (74.0) | 813 (72.3) |
| Immunosuppressant use within 8 wk prior to baseline, n (%)b,g | 56 (23.9) | 259 (28.6) | 44 (22.2) | 45 (22.7) | 56 (28.6) | 47 (23.4) | 266 (28.8) | 313 (27.8) |
| 5-ASA use at baseline, n (%)f | 167 (71.4) | 650 (71.8) | NR | NR | NR | NR | NR | NR |
| Oral corticosteroid use at baseline, n (%)h | 127 (45.0) | 430 (45.8) | 100 (50.5) | 101 (51.0) | 86 (43.9) | 81 (40.3) | 442 (46.2) | 523 (45.2) |
| Oral corticosteroid daily dose at baseline—prednisone equivalent, mg/d, mean (SD) | 16.9 ± 6.2 | 16.0 ± 6.4 | 15.9 ± 6.2 | 14.9 ± 6.2 | 14.5 ± 5.9 | 14.9 ± 6.1 | 16.1 ± 6.4 | 16.0 ± 6.3 |
The N value may be slightly different for each variable; percentages were based on nonmissing values.
Abbreviations: 5-ASA, 5-aminosalicylates; BID, twice daily; CDI, Clostridium difficile infection; N, total number of patients in the treatment group; n, number of patients in the specified category; NR, not reported; PD, predominant dose; SD, standard deviation; TNFi, tumor necrosis factor inhibitor.
aFor overall cohort analysis, patients were categorized based on the average daily dose of tofacitinib (placebo exposure was not included): PD tofacitinib 5 mg BID (average total daily dose <15 mg) and PD tofacitinib 10 mg BID (average total daily dose ≥15 mg).
bData were collected at the start of the phase 3 induction studies.
cSite reported cases for prior medical history.
dPrior TNFi treatment was defined as any prior treatment with TNFi.
ePrior TNFi failure was determined by investigators and did not specify a minimum dose or treatment duration with TNFi.
fData are from phase 3 induction studies only.
gImmunosuppressants include nonbiologic agents such as azathioprine, 6-mercaptopurine, and cyclosporine.
hInduction baseline; based on prednisone-equivalent total daily dose, and excludes medications such as budesonide and beclomethasone.
Demographics and Baseline Clinical Characteristics of the Induction, Maintenance, and Overall Cohorts
| . | Induction Cohort . | Maintenance Cohort . | Overall Cohorta . | |||||
|---|---|---|---|---|---|---|---|---|
| Placebo (N = 282) . | Tofacitinib 10 mg BID (N = 938) . | Placebo (N = 198) . | Tofacitinib 5 mg BID (N = 198) . | Tofacitinib 10 mg BID (N = 196) . | PD Tofacitinib 5 mg BID (N = 201) . | PD Tofacitinib 10 mg BID (N = 956) . | Tofacitinib All (N = 1157) . | |
| Total patient-years of exposure | 44.8 | 156.2 | 100.4 | 146.2 | 154.3 | 776.4 | 2038 | 2814.4 |
| Age, mean (SD), y | 41.4 ± 14.4 | 41.3 ± 13.8 | 43.4 ± 14.0 | 41.9 ± 13.7 | 43 ± 14.4 | 44.6 ± 14.5 | 40.6 ± 13.7 | 41.3 ± 13.9 |
| Male, n (%) | 155 (55.0) | 557 (59.4) | 116 (58.6) | 103 (52.0) | 110 (56.1) | 115 (57.2) | 564 (59.0) | 679 (58.7) |
| Race, n (%) | ||||||||
| White | 229 (81.2) | 756 (80.6) | 155 (78.3) | 164 (82.8) | 153 (78.1) | 159 (79.1) | 768 (80.3) | 927 (80.1) |
| Black | 4 (1.4) | 6 (0.6) | 3 (1.5) | 2 (1.0) | 0 (0.0) | 1 (0.5) | 9 (0.9) | 10 (0.9) |
| Asian | 28 (9.9) | 114 (12.2) | 26 (13.1) | 23 (11.6) | 25 (12.8) | 28 (13.9) | 116 (12.1) | 144 (12.4) |
| Other | 11 (3.9) | 36 (3.8) | 9 (4.5) | 5 (2.5) | 9 (4.6) | 9 (4.5) | 33 (3.5) | 42 (3.6) |
| Unspecified | 10 (3.5) | 26 (2.8) | 5 (2.5) | 4 (2.0) | 9 (4.6) | 4 (2.0) | 30 (3.1) | 34 (2.9) |
| Disease duration, mean (SD), y | 8.2 ± 6.8 | 8.2 ± 7.0 | 8.8 ± 7.5 | 8.3 ± 7.2 | 8.7 ± 7.0 | 8.3 ± 6.5 | 8.2 ± 7.1 | 8.2 ± 7.0 |
| Extent of disease, n (%)b | ||||||||
| Proctosigmoiditis | 35 (15.0) | 132 (14.6) | 21 (10.6) | 28 (14.3) | 33 (16.9) | 34 (17.0) | 129 (14.0) | 163 (14.5) |
| Left-sided colitis | 76 (32.6) | 307 (34.0) | 68 (34.4) | 66 (33.7) | 60 (30.8) | 69 (34.5) | 311 (33.8) | 380 (33.9) |
| Extensive/pancolitis | 122 (52.4) | 463 (51.3) | 108 (54.5) | 102 (52.3) | 102 (52.3) | 97 (48.5) | 480 (52.1) | 577 (51.5) |
| Proctitis | 0 (0.0) | 1 (0.1) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 1 (0.1) |
| Total Mayo score at baseline, mean (SD) | 8.9 ± 1.5 | 9.0 ± 1.5 | 3.3 ± 1.8 | 3.3 ± 1.8 | 3.4 ± 1.8 | 7.8 ± 2.5 | 8.8 ± 1.8 | 8.6 ± 2.0 |
| C-reactive protein, mg/L, median (range) | 5.3 (2.2-12.7) | 4.6 (1.8-11.6) | 1.0 (0.3-3.5) | 0.7 (0.3-2.3) | 0.9 (0.4-3.0) | 3.2 (0.1-122.5) | 4.9 (0.1-208.4) | 4.5 (0.1-208.4) |
| Prior history of CDI, n (%)c | 2 (0.7) | 20 (2.1) | 4 (2.0) | 3 (1.5) | 8 (4.1) | NR | NR | NR |
| Prior TNFi exposure, n (%)b,d | 130 (55.6) | 488 (53.9) | 92 (46.5) | 90 (45.5) | 100 (51.0) | 90 (44.8) | 522 (56.6) | 612 (54.4) |
| Prior TNFi failure, n (%)b,e | 124 (53.0) | 465 (51.4) | 89 (44.9) | 83 (41.9) | 92 (46.9) | 83 (41.3) | 500 (54.2) | 583 (51.9) |
| Prior immunosuppressant use, n (%)b,f,g | 160 (68.4) | 683 (75.5) | 134 (67.7) | 149 (75.3) | 144 (73.5) | 136 (67.7) | 702 (76.1) | 838 (74.6) |
| Prior immunosuppressant failure, n (%)b,f,g | 158 (67.5) | 661 (73.0) | 129 (65.2) | 143 (72.2) | 141 (71.9) | 130 (64.7) | 683 (74.0) | 813 (72.3) |
| Immunosuppressant use within 8 wk prior to baseline, n (%)b,g | 56 (23.9) | 259 (28.6) | 44 (22.2) | 45 (22.7) | 56 (28.6) | 47 (23.4) | 266 (28.8) | 313 (27.8) |
| 5-ASA use at baseline, n (%)f | 167 (71.4) | 650 (71.8) | NR | NR | NR | NR | NR | NR |
| Oral corticosteroid use at baseline, n (%)h | 127 (45.0) | 430 (45.8) | 100 (50.5) | 101 (51.0) | 86 (43.9) | 81 (40.3) | 442 (46.2) | 523 (45.2) |
| Oral corticosteroid daily dose at baseline—prednisone equivalent, mg/d, mean (SD) | 16.9 ± 6.2 | 16.0 ± 6.4 | 15.9 ± 6.2 | 14.9 ± 6.2 | 14.5 ± 5.9 | 14.9 ± 6.1 | 16.1 ± 6.4 | 16.0 ± 6.3 |
| . | Induction Cohort . | Maintenance Cohort . | Overall Cohorta . | |||||
|---|---|---|---|---|---|---|---|---|
| Placebo (N = 282) . | Tofacitinib 10 mg BID (N = 938) . | Placebo (N = 198) . | Tofacitinib 5 mg BID (N = 198) . | Tofacitinib 10 mg BID (N = 196) . | PD Tofacitinib 5 mg BID (N = 201) . | PD Tofacitinib 10 mg BID (N = 956) . | Tofacitinib All (N = 1157) . | |
| Total patient-years of exposure | 44.8 | 156.2 | 100.4 | 146.2 | 154.3 | 776.4 | 2038 | 2814.4 |
| Age, mean (SD), y | 41.4 ± 14.4 | 41.3 ± 13.8 | 43.4 ± 14.0 | 41.9 ± 13.7 | 43 ± 14.4 | 44.6 ± 14.5 | 40.6 ± 13.7 | 41.3 ± 13.9 |
| Male, n (%) | 155 (55.0) | 557 (59.4) | 116 (58.6) | 103 (52.0) | 110 (56.1) | 115 (57.2) | 564 (59.0) | 679 (58.7) |
| Race, n (%) | ||||||||
| White | 229 (81.2) | 756 (80.6) | 155 (78.3) | 164 (82.8) | 153 (78.1) | 159 (79.1) | 768 (80.3) | 927 (80.1) |
| Black | 4 (1.4) | 6 (0.6) | 3 (1.5) | 2 (1.0) | 0 (0.0) | 1 (0.5) | 9 (0.9) | 10 (0.9) |
| Asian | 28 (9.9) | 114 (12.2) | 26 (13.1) | 23 (11.6) | 25 (12.8) | 28 (13.9) | 116 (12.1) | 144 (12.4) |
| Other | 11 (3.9) | 36 (3.8) | 9 (4.5) | 5 (2.5) | 9 (4.6) | 9 (4.5) | 33 (3.5) | 42 (3.6) |
| Unspecified | 10 (3.5) | 26 (2.8) | 5 (2.5) | 4 (2.0) | 9 (4.6) | 4 (2.0) | 30 (3.1) | 34 (2.9) |
| Disease duration, mean (SD), y | 8.2 ± 6.8 | 8.2 ± 7.0 | 8.8 ± 7.5 | 8.3 ± 7.2 | 8.7 ± 7.0 | 8.3 ± 6.5 | 8.2 ± 7.1 | 8.2 ± 7.0 |
| Extent of disease, n (%)b | ||||||||
| Proctosigmoiditis | 35 (15.0) | 132 (14.6) | 21 (10.6) | 28 (14.3) | 33 (16.9) | 34 (17.0) | 129 (14.0) | 163 (14.5) |
| Left-sided colitis | 76 (32.6) | 307 (34.0) | 68 (34.4) | 66 (33.7) | 60 (30.8) | 69 (34.5) | 311 (33.8) | 380 (33.9) |
| Extensive/pancolitis | 122 (52.4) | 463 (51.3) | 108 (54.5) | 102 (52.3) | 102 (52.3) | 97 (48.5) | 480 (52.1) | 577 (51.5) |
| Proctitis | 0 (0.0) | 1 (0.1) | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.1) | 1 (0.1) |
| Total Mayo score at baseline, mean (SD) | 8.9 ± 1.5 | 9.0 ± 1.5 | 3.3 ± 1.8 | 3.3 ± 1.8 | 3.4 ± 1.8 | 7.8 ± 2.5 | 8.8 ± 1.8 | 8.6 ± 2.0 |
| C-reactive protein, mg/L, median (range) | 5.3 (2.2-12.7) | 4.6 (1.8-11.6) | 1.0 (0.3-3.5) | 0.7 (0.3-2.3) | 0.9 (0.4-3.0) | 3.2 (0.1-122.5) | 4.9 (0.1-208.4) | 4.5 (0.1-208.4) |
| Prior history of CDI, n (%)c | 2 (0.7) | 20 (2.1) | 4 (2.0) | 3 (1.5) | 8 (4.1) | NR | NR | NR |
| Prior TNFi exposure, n (%)b,d | 130 (55.6) | 488 (53.9) | 92 (46.5) | 90 (45.5) | 100 (51.0) | 90 (44.8) | 522 (56.6) | 612 (54.4) |
| Prior TNFi failure, n (%)b,e | 124 (53.0) | 465 (51.4) | 89 (44.9) | 83 (41.9) | 92 (46.9) | 83 (41.3) | 500 (54.2) | 583 (51.9) |
| Prior immunosuppressant use, n (%)b,f,g | 160 (68.4) | 683 (75.5) | 134 (67.7) | 149 (75.3) | 144 (73.5) | 136 (67.7) | 702 (76.1) | 838 (74.6) |
| Prior immunosuppressant failure, n (%)b,f,g | 158 (67.5) | 661 (73.0) | 129 (65.2) | 143 (72.2) | 141 (71.9) | 130 (64.7) | 683 (74.0) | 813 (72.3) |
| Immunosuppressant use within 8 wk prior to baseline, n (%)b,g | 56 (23.9) | 259 (28.6) | 44 (22.2) | 45 (22.7) | 56 (28.6) | 47 (23.4) | 266 (28.8) | 313 (27.8) |
| 5-ASA use at baseline, n (%)f | 167 (71.4) | 650 (71.8) | NR | NR | NR | NR | NR | NR |
| Oral corticosteroid use at baseline, n (%)h | 127 (45.0) | 430 (45.8) | 100 (50.5) | 101 (51.0) | 86 (43.9) | 81 (40.3) | 442 (46.2) | 523 (45.2) |
| Oral corticosteroid daily dose at baseline—prednisone equivalent, mg/d, mean (SD) | 16.9 ± 6.2 | 16.0 ± 6.4 | 15.9 ± 6.2 | 14.9 ± 6.2 | 14.5 ± 5.9 | 14.9 ± 6.1 | 16.1 ± 6.4 | 16.0 ± 6.3 |
The N value may be slightly different for each variable; percentages were based on nonmissing values.
Abbreviations: 5-ASA, 5-aminosalicylates; BID, twice daily; CDI, Clostridium difficile infection; N, total number of patients in the treatment group; n, number of patients in the specified category; NR, not reported; PD, predominant dose; SD, standard deviation; TNFi, tumor necrosis factor inhibitor.
aFor overall cohort analysis, patients were categorized based on the average daily dose of tofacitinib (placebo exposure was not included): PD tofacitinib 5 mg BID (average total daily dose <15 mg) and PD tofacitinib 10 mg BID (average total daily dose ≥15 mg).
bData were collected at the start of the phase 3 induction studies.
cSite reported cases for prior medical history.
dPrior TNFi treatment was defined as any prior treatment with TNFi.
ePrior TNFi failure was determined by investigators and did not specify a minimum dose or treatment duration with TNFi.
fData are from phase 3 induction studies only.
gImmunosuppressants include nonbiologic agents such as azathioprine, 6-mercaptopurine, and cyclosporine.
hInduction baseline; based on prednisone-equivalent total daily dose, and excludes medications such as budesonide and beclomethasone.
Induction Cohort
In the induction cohort, 3 patients reported CDI, with a numerically higher proportion of patients with CDI in the placebo group (0.4%) than in the tofacitinib-all group (0.2%) (Figure 1). Of the 3 patients in the induction cohort with CDI, 2 were treated with tofacitinib 10 mg BID (IR, 1.21 [95% confidence interval (CI), 0.15-4.35]) and 1 was treated with placebo (IR, 1.98 [95% CI, 0.05-11.04]). CDI events occurred on days (day of induction study) 25 and 35 after initiation with tofacitinib treatment, and on day 16 after initiation with placebo treatment. One CDI event was classified as a serious adverse event and no patients had to stop taking their study medication, either temporarily or permanently. One patient was receiving concomitant corticosteroids and had concomitant antibiotic use. No patients had prior history of CDI or were hospitalized at the time of the CDI. CDI was resolved in 2 patients and was ongoing in 1 patient at the time of study end and at last follow-up, per protocol. The patient with ongoing CDI was treated with placebo.
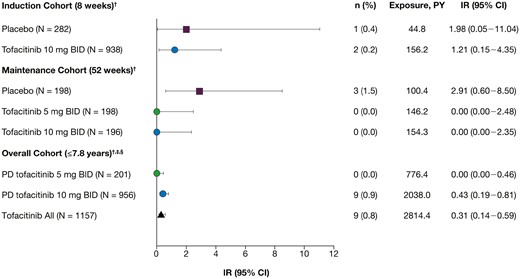
Incidence rates (IRs) of Clostridium difficile infection (CDI) in the induction, maintenance, and overall cohorts. Includes final data from the Oral Clinical Trials for tofAcitinib in ulceratiVE colitis (OCTAVE) Open study, as of August 24, 2020. Incidence rates reflect unique patients with events per 100 patient-years (PY) of exposure. †Excluding events occurring after 28 days from the last dose of the corresponding cohort. ‡For overall cohort analysis, patients were categorized based on the average daily dose of tofacitinib (placebo exposure was not included): predominant dose (PD) tofacitinib 5 mg twice daily (BID) (average total daily dose <15 mg) and PD tofacitinib 10 mg BID (average total daily dose ≥15 mg). §For CDI identification in the overall cohort, concomitant oral fidaxomicin was also included alongside concomitant oral metronidazole and vancomycin. No CDIs were identified from patients with concomitant fidaxomicin. CI, confidence interval; N, total number of patients in the treatment group; n, number of patients in the specified category.
Maintenance Cohort
In the maintenance cohort, there were no cases of CDI in patients treated with tofacitinib (Figure 1). In total, 3 patients in the maintenance cohort had a CDI, all of whom were treated with placebo (IR, 2.91 [95% CI, 0.60-8.50]). All of them had received tofacitinib 10 mg BID during induction. CDI events occurred on days (day of maintenance study) 68, 81, and 98 after initiation of placebo treatment in the OCTAVE Sustain study. No CDI events were classified as serious adverse events and no patients were receiving concomitant corticosteroids, had prior antibiotic use, or were hospitalized at the time of the CDI. CDI was resolved in 2 patients and ongoing in 1 patient at the time of study end and at last follow-up, per protocol.
Overall Cohort
In total, 9 patients in the overall cohort had CDI (IR, 0.31 [95% CI, 0.14-0.59]) (Figure 1). All patients had a PD of tofacitinib 10 mg BID. The day of onset (defined as the difference between onset date of event and date of first dose of study drug +1) ranged from 25 to 891. Two CDI events were classified as serious adverse events. Only 1 patient had prior history of CDI (Figure 2). All cases were mild or moderate in severity, and only 3 patients had to discontinue tofacitinib treatment due to CDI: 1 temporarily and 2 permanently (Figure 2).
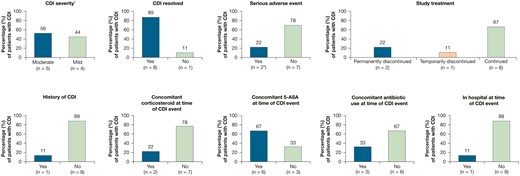
Characteristics of Clostridium difficile infection (CDI) in the 9 patients from the overall cohort. The overall cohort (tofacitinib all) includes final data from the Oral Clinical Trials for tofAcitinib in ulceratiVE colitis (OCTAVE) Open study, as of August 24, 2020. *Severity assessment: mild indicates no interference with patients’ usual function; moderate indicates interference to some extent with patients’ usual function. †Owing to hospitalization. 5-ASA, 5-aminosalicylates.
Patients reporting a CDI were between 23 and 41 years of age; the majority were male (78%) or had pancolitis at induction baseline (67%) (Table 2). The CDI day of onset in most patients was after >200 days of tofacitinib treatment, with the latest CDI event occurring on day 891. The day of onset did not appear to determine the severity of the CDI. Of the 2 patients treated with concomitant corticosteroids (40 mg and 60 mg prednisolone), 1 had CDI classed as mild in severity and 1 had CDI classed as moderate. The patient with a history of CDI had a CDI event prior to their enrollment in the induction study but also had recurrent CDI events in the open-label, long-term extension study. This patient was also receiving concomitant 5-ASA and concomitant antibiotics (for their previous CDI event) at the time of the most recent CDI event. Two other patients were using antibiotics at the time of their CDI event and 1 patient stopped using antibiotics 1 day before their CDI event.
Characteristics of Patients With CDI in the Overall Cohort, by Individual Patient
| Age (y) . | Sex . | Extent of Disease at Induction Baseline . | Day of Onseta . | Severity of CDI . | Serious Adverse Event . | Outcome . | Concomitant Corticosteroids . | Concomitant 5-ASA . | Concomitant Antibiotic Use . | History of CDI . | In Hospital at Time of CDI . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 26 | Male | NR | 870 | Moderate | No | Still presentb | Yesc | Yes | Yes | Yesd | No |
| 38 | Male | Proctitis, proctosigmoiditis | 616 | Mild | No | Resolved | No | No | No | No | No |
| 38 | Male | Proctitis, proctosigmoiditis, left-sided colitis | 219 | Mild | No | Resolved | No | No | No | No | No |
| 34 | Male | Pancolitis | 92 | Moderate | No | Resolved | No | No | Noe | No | No |
| 26 | Male | Pancolitis | 243 | Mild | No | Resolved | No | Yes | No | No | No |
| 24 | Male | Pancolitis | 25 | Mild | Yes | Resolved | Yesf | Yes | Yes | No | No |
| 23 | Male | Pancolitis | 891 | Moderate | No | Resolved | No | Yes | No | No | No |
| 39 | Female | Pancolitis | 482 | Moderate | Yes | Resolved | No | Yes | Yesg | No | Yes |
| 41 | Female | Pancolitis | 35 | Moderate | No | Resolved | No | Yes | No | No | No |
| Age (y) . | Sex . | Extent of Disease at Induction Baseline . | Day of Onseta . | Severity of CDI . | Serious Adverse Event . | Outcome . | Concomitant Corticosteroids . | Concomitant 5-ASA . | Concomitant Antibiotic Use . | History of CDI . | In Hospital at Time of CDI . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 26 | Male | NR | 870 | Moderate | No | Still presentb | Yesc | Yes | Yes | Yesd | No |
| 38 | Male | Proctitis, proctosigmoiditis | 616 | Mild | No | Resolved | No | No | No | No | No |
| 38 | Male | Proctitis, proctosigmoiditis, left-sided colitis | 219 | Mild | No | Resolved | No | No | No | No | No |
| 34 | Male | Pancolitis | 92 | Moderate | No | Resolved | No | No | Noe | No | No |
| 26 | Male | Pancolitis | 243 | Mild | No | Resolved | No | Yes | No | No | No |
| 24 | Male | Pancolitis | 25 | Mild | Yes | Resolved | Yesf | Yes | Yes | No | No |
| 23 | Male | Pancolitis | 891 | Moderate | No | Resolved | No | Yes | No | No | No |
| 39 | Female | Pancolitis | 482 | Moderate | Yes | Resolved | No | Yes | Yesg | No | Yes |
| 41 | Female | Pancolitis | 35 | Moderate | No | Resolved | No | Yes | No | No | No |
Abbreviations: 5-ASA, 5-aminosalicylate; CDI, Clostridium difficile infection; NR, not reported.
aOnset day is computed as the difference between onset date of event and date of first dose of study drug +1.
bAt the time of study completion.
cDose of 40 mg prednisolone.
dThis patient had a CDI event before enrollment in the induction study and also had recurrent CDI in the open-label, long-term extension study.
eAntibiotic use stopped 1 day prior to CDI event.
fDose of 60 mg prednisolone.
gThis patient was given metronidazole for ulcerative colitis flare and CDI 3 days before the CDI diagnosis was recorded.
Characteristics of Patients With CDI in the Overall Cohort, by Individual Patient
| Age (y) . | Sex . | Extent of Disease at Induction Baseline . | Day of Onseta . | Severity of CDI . | Serious Adverse Event . | Outcome . | Concomitant Corticosteroids . | Concomitant 5-ASA . | Concomitant Antibiotic Use . | History of CDI . | In Hospital at Time of CDI . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 26 | Male | NR | 870 | Moderate | No | Still presentb | Yesc | Yes | Yes | Yesd | No |
| 38 | Male | Proctitis, proctosigmoiditis | 616 | Mild | No | Resolved | No | No | No | No | No |
| 38 | Male | Proctitis, proctosigmoiditis, left-sided colitis | 219 | Mild | No | Resolved | No | No | No | No | No |
| 34 | Male | Pancolitis | 92 | Moderate | No | Resolved | No | No | Noe | No | No |
| 26 | Male | Pancolitis | 243 | Mild | No | Resolved | No | Yes | No | No | No |
| 24 | Male | Pancolitis | 25 | Mild | Yes | Resolved | Yesf | Yes | Yes | No | No |
| 23 | Male | Pancolitis | 891 | Moderate | No | Resolved | No | Yes | No | No | No |
| 39 | Female | Pancolitis | 482 | Moderate | Yes | Resolved | No | Yes | Yesg | No | Yes |
| 41 | Female | Pancolitis | 35 | Moderate | No | Resolved | No | Yes | No | No | No |
| Age (y) . | Sex . | Extent of Disease at Induction Baseline . | Day of Onseta . | Severity of CDI . | Serious Adverse Event . | Outcome . | Concomitant Corticosteroids . | Concomitant 5-ASA . | Concomitant Antibiotic Use . | History of CDI . | In Hospital at Time of CDI . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 26 | Male | NR | 870 | Moderate | No | Still presentb | Yesc | Yes | Yes | Yesd | No |
| 38 | Male | Proctitis, proctosigmoiditis | 616 | Mild | No | Resolved | No | No | No | No | No |
| 38 | Male | Proctitis, proctosigmoiditis, left-sided colitis | 219 | Mild | No | Resolved | No | No | No | No | No |
| 34 | Male | Pancolitis | 92 | Moderate | No | Resolved | No | No | Noe | No | No |
| 26 | Male | Pancolitis | 243 | Mild | No | Resolved | No | Yes | No | No | No |
| 24 | Male | Pancolitis | 25 | Mild | Yes | Resolved | Yesf | Yes | Yes | No | No |
| 23 | Male | Pancolitis | 891 | Moderate | No | Resolved | No | Yes | No | No | No |
| 39 | Female | Pancolitis | 482 | Moderate | Yes | Resolved | No | Yes | Yesg | No | Yes |
| 41 | Female | Pancolitis | 35 | Moderate | No | Resolved | No | Yes | No | No | No |
Abbreviations: 5-ASA, 5-aminosalicylate; CDI, Clostridium difficile infection; NR, not reported.
aOnset day is computed as the difference between onset date of event and date of first dose of study drug +1.
bAt the time of study completion.
cDose of 40 mg prednisolone.
dThis patient had a CDI event before enrollment in the induction study and also had recurrent CDI in the open-label, long-term extension study.
eAntibiotic use stopped 1 day prior to CDI event.
fDose of 60 mg prednisolone.
gThis patient was given metronidazole for ulcerative colitis flare and CDI 3 days before the CDI diagnosis was recorded.
Discussion
In this analysis, we investigated the proportions and incidence rates of CDI in patients receiving tofacitinib or placebo in the UC clinical program. These analyses demonstrate that CDI events are rare in the UC clinical program and suggest that patients treated with tofacitinib during induction and maintenance therapy were at similar or slightly lower risk of CDI than patients treated with placebo. Given that inflammatory bowel disease is a known risk factor for infections,16 and that patients with inactive disease are less likely to develop CDI,17 it is possible that the lower rates of CDI in the OCTAVE clinical program may be due to better-controlled disease in patients treated with tofacitinib, thus reducing susceptibility to infection.14
Importantly, most patients with a CDI in the UC clinical program were able to continue with tofacitinib therapy after the CDI was resolved. Among patients receiving tofacitinib, only 3 of the 9 patients with CDI required treatment disruption or discontinuation. Most patients who had CDI had no prior history of CDI and were not in the hospital when the CDI occurred. Also, there was only 1 case of recurrent CDI, which has become an increasing challenge in the management of CDI.18
Immunosuppression (in particular, corticosteroid use) may be associated with higher risk of CDI.8 Although limited by a small number of events, concomitant corticosteroids appeared to have little influence on the incidence of CDI in the overall cohort. Concomitant antibiotic use was slightly more common than concomitant corticosteroid use among patients who developed CDI during the OCTAVE clinical program; however, overall, the majority of patients who developed CDI were not on either concomitant therapy. This may be noteworthy, given published data suggesting that patients with inflammatory bowel disease who develop CDI frequently lack the traditional risk factors associated with CDI, such as antibiotic use or hospitalization, and therefore it is recommended that a high index of suspicion for CDI be maintained.19,20 In the phase 3 induction studies, the proportions of patients treated with placebo and tofacitinib 10 mg BID with 5-ASA use at baseline were almost equal, and 66.6% (n = 6 of 9) of patients with CDI in the overall cohort were receiving concomitant 5-ASA at the time of CDI in these studies. It is difficult to conclude the influence of concomitant 5‐ASA use on the CDI incidence rate observed in this cohort.
Overall, the proportions of CDI reported here from the overall cohort are similar to or lower than those among all patients with inflammatory bowel disease, with 1 retrospective cohort study of patients with inflammatory bowel disease (UC or Crohn’s disease) reporting that CDI occurred in 4.6% of patients with inflammatory bowel disease over 1 year (regardless of disease activity or background treatment).21,22
Optimal management of CDI in patients with inflammatory bowel disease is continuously evolving. An expert review from the Clinical Practice Updates Committee of the American Gastroenterological Association Institute offers several recommendations.23 All signs and symptoms of a flare should be tested for CDI. Hospitalization should be considered for close monitoring and management of patients with inflammatory bowel disease and CDI who have profuse diarrhea, acute abdominal pain, a significant increase in peripheral blood leukocyte count, or other signs of sepsis. Escalation of steroids and other immunosuppressant agents may be suspended during acute CDI until therapy for CDI has been initiated. However, the decision to withhold or continue immunosuppression in patients with inflammatory bowel disease and CDI should be tailored to the individual, as there is limited robust literature on which to develop firm recommendations. When considering treatment, initial therapy with oral vancomycin should be considered instead of metronidazole, and treating for at least 21 days should also be considered.23,24 This is longer than treatment guidance for the general population as longer duration of oral vancomycin in patients with inflammatory bowel disease is associated with lower rates of CDI recurrence.24 Finally, clinicians may consider fecal microbiota transplantation for patients with inflammatory bowel disease and recurrent CDI.23
A limitation of these analyses is that the population of patients in the tofacitinib UC program, which excluded patients with a positive test for C. difficile toxin at study screening, may not be fully representative of the global population with UC. Moreover, the limited patient-years of study drug exposure from a small number of patients are further limitations.
Conclusions
CDIs were infrequent in the tofacitinib UC clinical program, and two-thirds of the patients who experienced a CDI had no interruption to their tofacitinib treatment.
Acknowledgments
This study was sponsored by Pfizer. The authors thank the patients, investigators, and study teams involved in the tofacitinib UC clinical program. Medical writing support, under the guidance of the authors, was provided by Caitlin Duncan, PhD, CMC Connect, McCann Health Medical Communications and was funded by Pfizer (New York, NY, USA) in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med. 2015;163:461-464). Hyejin Jo contributed to the statistical analysis within this study in her role as an employee of Syneos Health, which was a paid contractor to Pfizer in the development of this manuscript and related statistical analysis.
Author Contributions
S.B.C., L.S., CS, K.K.K., and J.C.W.: study design. E.V.L., D.C.B., K.G., J.A.K., S.B.C., L.S., C.S., K.K.K., J.C.W., and A.A.: data analysis and interpretation. K.K.K.: statistical analysis. E.V.L., D.C.B., K.G., J.A.K., S.B.C., L.S., C.S., K.K.K., J.C.W., and A.A.: drafted the manuscript and approved the final version of the article. All authors approved the final version of the article, including the authorship list.
Funding
This study was sponsored by Pfizer. Medical writing support was funded by Pfizer Inc.
Conflicts of Interest
E.V.L. has received consultancy fees from AbbVie, Amgen, Arena, Boehringer Ingelheim, Bristol-Myers Squibb, CALIBR, Celgene, Eli Lilly, Gilead Sciences, Genentech, Gossamer Bio, Iterative Scopes, Janssen, Morphic Therapeutic, Ono Pharma, Pfizer Inc, Protagonist Therapeutics, Scipher Medicine, Sun Pharma, Surrozen, Takeda, and UCB; and research support from AbbVie, Bristol-Myers Squibb, Celgene, Genentech, Gilead Sciences, Gossamer Bio, Janssen, Pfizer Inc, Receptos, Robarts Clinical Trials, Takeda, Theravance, and UCB. D.C.B. has received institutional unrestricted grants, consulting fees, or nonpersonal financial support (ie, travel support or unrestricted institutional research grants) from 4D Pharma, AbbVie, Allergan, Amgen, Applied Medical, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Centocor, CSL Behring, Dr. Falk Pharma, Elan, Ferring Pharmaceuticals, Forward Pharma, Genentech, Hitachi, Janssen-Cilag, Karl Storz, Merck, Merz, MSD, Nestlé, Ocera, Ogilvy, Otsuka, PDL, Pfizer Inc, Recordati, Roche, Shield Therapeutics, Shire, Takeda, TiGenix, Tillotts, UCB, and Vifor. K.G. has received research support from Celltrion, Galapagos, and Pfizer Inc; and speaker honoraria and/or consultancy fees from AbbVie, Arena Pharmaceuticals, Celltrion, Ferring Pharmaceuticals, Galapagos, Gilead Sciences, Immunic Therapeutics, Janssen, Novartis, Pfizer Inc, Samsung Bioepis, Takeda, and Tillotts. J.A.K. has served on advisory boards for Pfizer Inc. S.B.C., L.S., C.S., K.K.K., and J.C.W. are employees and stockholders of Pfizer Inc. A.A. has received consultancy fees from AbbVie, Allergan, Amgen, Arena, Biogen, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Celltrion, Eli Lilly, Ferring Pharmaceuticals, Galapagos, Gilead Sciences, Janssen, MSD, Mylan, Pfizer Inc, Protagonist Therapeutics, Roche, Samsung Bioepis, Sandoz, and Takeda; lecture fees from AbbVie, Amgen, Arena, Biogen, Bristol-Myers Squibb, Ferring Pharmaceuticals, Galapagos, Gilead Sciences, Janssen, MSD, Novartis, Pfizer Inc, Roche, Samsung Bioepis, Sandoz, Takeda, and TiGenix; and research support from Biogen, MSD, Takeda, and Pfizer Inc.
Data Availability
Upon request, and subject to review, Pfizer will provide the data that support the findings of this study. Subject to certain criteria, conditions, and exceptions, Pfizer may also provide access to the related individual de-identified participant data. See https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information.



