-
PDF
- Split View
-
Views
-
Cite
Cite
Ariela K Holmer, Brigid S Boland, Siddharth Singh, Jennifer Neill, Helen Le, Ara Miralles, Angelina E Collins, William J Sandborn, Parambir S Dulai, A Serum Biomarker Panel Can Accurately Identify Mucosal Ulcers in Patients With Crohn’s Disease, Inflammatory Bowel Diseases, Volume 29, Issue 4, April 2023, Pages 555–562, https://doi.org/10.1093/ibd/izac117
Close - Share Icon Share
Abstract
The Endoscopic Healing Index (EHI) is a serum biomarker panel that can predict endoscopic inflammation in Crohn’s disease (CD).
Paired serum samples with endoscopies from adult patients participating in a prospective biobank (June 2014 to December 2018) were analyzed post hoc. Diagnostic performance for EHI was assessed against the individual parameters of the Simple Endoscopic Score for CD using previously identified cutoffs. Confounders for EHI performance were identified using logistic regression.
A total of 205 CD patients were included (50% male, median age 37 years). An EHI of 20 points was sensitive for ruling out any ulcers (85%; 95% confidence interval [CI], 77%-91%) and large (5-20 mm) or very large (>20 mm) ulcers (93%; 95% CI, 84%-97%). An EHI of 50 points was specific for ruling in any ulcers (86%; 95% CI, 76%-92%) and large or very large ulcers (87%; 95% CI, 79%-92%). After accounting for total extent of inflamed mucosa, strictures, and disease location, each 20-point increase in EHI was associated with a 1.7-fold increased probability for the presence of large or very large ulcers (adjusted odds ratio, 1.7; 95% CI, 1.1-2.6).
The EHI was independently associated with ulcer size and accurately identified large or very large ulcers. A cutoff of 50 points can reliably rule in mucosal ulcers and allow for treatment adjustment. A cutoff of 20 points can reliably rule out mucosal ulcers and signal completion of treatment adjustment algorithms.
What is already known?
The Endoscopic Healing Index (EHI) is a serum-based test that was validated for predicting inflammation in Crohn’s disease (CD).
What is new here?
We performed a post hoc analysis of the EHI for identifying endoscopic mucosal ulcers, a currently recommended treatment target for CD, and found that the EHI is independently and incrementally associated with ulcer size.
How can this study help patient care?
The EHI can be used as a noninvasive test to monitor mucosal ulcers in CD; however, further work in stricturing, penetrating, and isolated ileal disease is warranted.
INTRODUCTION
Crohn’s disease (CD) is a relapsing, chronic inflammatory disorder that may affect any part of the gastrointestinal tract. Classically characterized by skip lesions of mucosal ulceration and transmural inflammation, approximately one-third of patients are diagnosed with severe and complicated disease with deep ulcers, fistulas, and stricture formation. Over time, more than one-half of patients progress to develop an intestinal complication.1 Prevention of disease progression through the achievement of endoscopic remission is a recommended endpoint for disease management.2-4 Specifically, ulcer healing has the greatest association with reduction of disease-related complications and is therefore a primary treatment target in CD.5
Assessment of mucosal ulcer healing after a change in inflammatory bowel disease (IBD)–directed therapy is usually performed with serial endoscopies. Given the limitations of endoscopy including procedural risks, patient burden, and overall cost to society, alternative noninvasive methods of disease surveillance have come into consideration, such as serum C-reactive protein (CRP) and fecal calprotectin (FC).6 In the CALM (Effect of Tight Control Management on Crohn's Disease) trial, treating to a target of FC and CRP in combination with patient-reported symptoms has been demonstrated to result in higher overall endoscopic remission rates compared with symptoms alone, which was associated with a reduced long-term risk of disease-related complications.7,8 The majority of treatment adjustments in this trial were based on elevations in FC, which has a favorable performance as a surrogate for endoscopic inflammation. However, FC is rarely ordered by providers early on after initiating biologic therapy, and patient adherence is variable due to challenges related to collection or storage.9,10 Although patients prefer blood-based tests,6 the performance of CRP is overall poor for detecting endoscopic disease activity, and some patients may never mount a CRP response.11,12 Therefore, there is a need for an alternative blood-based biomarker that can accurately predict endoscopic inflammation and monitor for the presence of mucosal ulcers in patients with CD.
Recently, this gap in the treatment algorithm for CD was addressed with the development and validation of a novel serum-based biomarker named the Endoscopic Healing Index (EHI) (Monitr; Prometheus Biosciences, San Diego, CA, USA).13 The EHI is a panel of 13 biomarkers involved in the pathogenesis and proinflammatory cascade of CD. The EHI was developed using a large multinational training cohort of patients with CD and subsequently validated in 2 independent patient groups including 1 immunosuppressive-naïve cohort and a separate cohort representing routine clinical practice. EHI outperformed CRP for predicting endoscopic inflammation in early CD patients and was comparable to FC across both cohorts.
While promising, the performance of EHI varied across subgroups and patient populations, and the performance characteristics of this biomarker panel were primarily assessed against the full Simple Endoscopic Score for CD (SES-CD) score, without consideration for individual parameters that might be most impactful to disease outcomes. Prior to widespread implementation in routine practice, sources of variability for EHI performance are important to understand, and strength of associations between EHI and endoscopic activity need to be adjusted for after considering these confounders. The aim of this study was to better define the operating characteristics of EHI in a real-world cohort of IBD patients and to identify confounders of EHI performance. Specifically, we aimed to adjust for observed confounders and quantify the performance of EHI for identifying mucosal ulcers, the currently recommended treatment target, and the endoscopic parameter most closely associated with improved disease outcomes.
METHODS
The protocol used a pre-existing IBD biobank. The PRoBE (Prospective specimen collection, Retrospective Blinded Evaluation) study design was followed for evaluating the accuracy of a biomarker used for classification of an outcome (mucosal ulcers).14 The results are reported in accordance with the STARD (Standards for Reporting of Diagnostic Accuracy Studies) guidelines.15 All authors had access to study data and reviewed and approved the final manuscript.
Patient Selection
Adult patients (≥18 years of age) with CD were included in the analysis if they met the following criteria: (1) a confirmed diagnosis of CD based on clinical, endoscopic, and histologic data; and (2) a colonoscopy assessment with available scored disease activity around the time of a collected EHI sample. Patients excluded from this study were those with the presence of an ostomy (ileostomy or colostomy), an ileal pouch–anal anastomosis, or upper gut CD, as there is an unclear application of current endoscopic scoring indices in these patients. Patients formed a convenience sample and were previously enrolled into the University of California San Diego IBD biobank, which prospectively collects serum, stool, and mucosal biopsy samples from patients at regular intervals. Samples included were from June 2014 to December 2018.
Clinical Data Variables
Data were collected on patient characteristics (age, gender, body mass index, smoking status), disease characteristics (disease duration, prior surgeries, presence of ileocecal valve, disease phenotype defined by the Montreal classification, presence of short bowel syndrome), current and prior IBD-directed therapies (biologics and/or immunomodulators, recent treatment adjustment within 1 year of a collected EHI sample), and endoscopic disease activity (defined by the SES-CD).
Endoscopic Data
Endoscopic disease activity was measured using the SES-CD score, which ranges from 0 to 60 and is composed of the following subcomponents: presence and size of ulcers, extent of ulcerated surface area, extent of total surface area affected, and severity of intestinal strictures. Scores were obtained from the endoscopy by the local site investigator. Ulcer size was specifically defined in each segment as the following: small ulcers (<5 mm), large ulcers (5-20 mm), and very large ulcers (>20 mm). Endoscopic remission was defined as SES-CD score ≤2 and no intestinal segment >1. Intestinal segments that were not accessible were documented.
EHI Development
The EHI was developed based on the analytical performance of a subset of biomarkers, in which 13 specific analytes showed the greatest correlation with disease activity. Together, these biomarkers formed the serum panel called the EHI (Monitr), which is currently available for use in routine practice. The panel scores range from 0 to 100, with increasing scores representing increasing severity of CD, and specifically, mucosal inflammation. The EHI was validated in biologic-naïve patients with early disease (cohort 1) and in those with refractory disease and prior biologic exposure (cohort 2). An EHI of 20 points had the greatest sensitivity of 97.1% (95% confidence interval [CI], 93.7%-98.9%) in cohort 1 and 83.2% (95% CI, 75.0%-89.6%) in cohort 2 for identifying endoscopic remission, while an EHI of 50 points had the greatest specificity of 100% (95% CI, 94.6%-100.9%) in cohort 1 and 87.8% (95% CI, 78.7%-94.0%) in cohort 2 for identifying active disease. Further details on the development and validation of EHI can be found in the original validation study.13
Endpoints
Our primary aim was to define the operating characteristics and potential sources of misclassification of EHI and to evaluate its performance for predicting mucosal ulcers. The performance of the EHI for endoscopic disease activity was evaluated using subcomponents of SES-CD scores. EHI performance for predicting mucosal ulcers was evaluated using sensitivity (the proportion of patients with mucosal ulcers and an EHI above 20 points) and specificity (the proportion of patients without mucosal ulcers and an EHI below 50 points). The positive likelihood ratio and negative likelihood ratio were also used to evaluate the performance of EHI for mucosal ulcers.
Sources of Misclassification
Potential confounders of EHI affecting its performance include patient factors or inherent variables that may affect the relative expression of proteins within the EHI, disease characteristics that may influence the association between the EHI and its ability to predict mucosal ulcers, and endoscopic factors such as subscores of the SES-CD that may also influence the primary association of the EHI for mucosal ulcers.
Sample Size
No formal sample size calculation was performed for this study.
Statistical Analyses
Continuous variables were summarized as median and interquartile range (IQR) and compared using the Mann-Whitney test. Categorical variables were reported as number and percentage and compared using the Fisher’s exact test. The 95% CI of the area under the curve (AUC) was calculated. Comparisons of the AUC across patient subgroups were done using parametric and nonparametric approaches.16 Logistic regression was performed to assess the strength of association between the EHI and ulcers (presence, size) after adjusting for potential confounders. Statistical significance was defined as a 2-sided P value <.05. If data were mostly non-normally distributed, the median and IQR were reported instead of the mean. Data analysis were performed using SPSS Version 27 (IBM, Armonk, NY, USA).
Ethical Considerations
Patients provided written informed consent prior to enrollment. The biobank was approved by the Human Research Protections Program (Institutional Review Board 160907, 131487) at the University of California San Diego.
Results
Patient Demographics
A total of 205 unique patients with CD were included in the analysis, with a median age of 37 years, and 50% were female (Table 1). Of these, 162 patients were included in the original validation study.13 The median disease duration was 9 (IQR, 4-18) years, with 54% (n = 110) of patients diagnosed between 17 and 40 years of age. In total, 43% (n = 88) had a prior CD-related bowel resection and 34% (n = 70) had resection of the ileocecum. As for IBD-directed treatment, 60.5% (n = 124) of patients had prior exposure to anti-tumor necrosis factor (TNF) therapy, 10% (n = 20) had prior vedolizumab exposure, and 5% (n = 10) had prior ustekinumab exposure. At the time of EHI sample collection, 41% (n = 84) of patients were receiving anti-TNF therapy and 42% (n = 85) were receiving immunomodulator (IM) therapy.
| Age, y | 37 (27-51) |
| Male (%) | 103 (50%) |
| Current or former smoker | 47 (23%) |
| BMI, kg/m2 | 24.5 (21-28) |
| Disease duration, y | 9 (4-18) |
| Montreal classification | |
| Age | |
| A1 | 51 (25%) |
| A2 | 110 (54%) |
| A3 | 44 (21%) |
| Stricturing or penetrating behavior | |
| B1 | 132 (64%) |
| B2 | 37 (18%) |
| B3 | 36 (18%) |
| Perianal disease | |
| Yes | 39 (19%) |
| Phenotype location | |
| Ileal | 56 (27%) |
| Ileocolonic | 83 (41%) |
| Colonic | 66 (32%) |
| Prior bowel resection | 88 (43%) |
| Ileocecal valve resection | 70 (34%) |
| Short bowel syndrome | 8 (4%) |
| Prior biologic use | |
| Anti-TNF | 124 (60.5%) |
| 1 | 60 |
| 2 | 40 |
| 3 | 23 |
| Vedolizumab | 20 (10%) |
| Ustekinumab | 10 (5%) |
| Current treatment | |
| Anti-TNF | 84 (41%) |
| Vedolizumab | 16 (8%) |
| Ustekinumab | 47 (23%) |
| Current IM use | 85 (42%) |
| Age, y | 37 (27-51) |
| Male (%) | 103 (50%) |
| Current or former smoker | 47 (23%) |
| BMI, kg/m2 | 24.5 (21-28) |
| Disease duration, y | 9 (4-18) |
| Montreal classification | |
| Age | |
| A1 | 51 (25%) |
| A2 | 110 (54%) |
| A3 | 44 (21%) |
| Stricturing or penetrating behavior | |
| B1 | 132 (64%) |
| B2 | 37 (18%) |
| B3 | 36 (18%) |
| Perianal disease | |
| Yes | 39 (19%) |
| Phenotype location | |
| Ileal | 56 (27%) |
| Ileocolonic | 83 (41%) |
| Colonic | 66 (32%) |
| Prior bowel resection | 88 (43%) |
| Ileocecal valve resection | 70 (34%) |
| Short bowel syndrome | 8 (4%) |
| Prior biologic use | |
| Anti-TNF | 124 (60.5%) |
| 1 | 60 |
| 2 | 40 |
| 3 | 23 |
| Vedolizumab | 20 (10%) |
| Ustekinumab | 10 (5%) |
| Current treatment | |
| Anti-TNF | 84 (41%) |
| Vedolizumab | 16 (8%) |
| Ustekinumab | 47 (23%) |
| Current IM use | 85 (42%) |
Values are median (interquartile range), n (%), or n.
Abbreviations: BMI, body mass index; IM, immunomodulator; TNF, tumor necrosis factor.
| Age, y | 37 (27-51) |
| Male (%) | 103 (50%) |
| Current or former smoker | 47 (23%) |
| BMI, kg/m2 | 24.5 (21-28) |
| Disease duration, y | 9 (4-18) |
| Montreal classification | |
| Age | |
| A1 | 51 (25%) |
| A2 | 110 (54%) |
| A3 | 44 (21%) |
| Stricturing or penetrating behavior | |
| B1 | 132 (64%) |
| B2 | 37 (18%) |
| B3 | 36 (18%) |
| Perianal disease | |
| Yes | 39 (19%) |
| Phenotype location | |
| Ileal | 56 (27%) |
| Ileocolonic | 83 (41%) |
| Colonic | 66 (32%) |
| Prior bowel resection | 88 (43%) |
| Ileocecal valve resection | 70 (34%) |
| Short bowel syndrome | 8 (4%) |
| Prior biologic use | |
| Anti-TNF | 124 (60.5%) |
| 1 | 60 |
| 2 | 40 |
| 3 | 23 |
| Vedolizumab | 20 (10%) |
| Ustekinumab | 10 (5%) |
| Current treatment | |
| Anti-TNF | 84 (41%) |
| Vedolizumab | 16 (8%) |
| Ustekinumab | 47 (23%) |
| Current IM use | 85 (42%) |
| Age, y | 37 (27-51) |
| Male (%) | 103 (50%) |
| Current or former smoker | 47 (23%) |
| BMI, kg/m2 | 24.5 (21-28) |
| Disease duration, y | 9 (4-18) |
| Montreal classification | |
| Age | |
| A1 | 51 (25%) |
| A2 | 110 (54%) |
| A3 | 44 (21%) |
| Stricturing or penetrating behavior | |
| B1 | 132 (64%) |
| B2 | 37 (18%) |
| B3 | 36 (18%) |
| Perianal disease | |
| Yes | 39 (19%) |
| Phenotype location | |
| Ileal | 56 (27%) |
| Ileocolonic | 83 (41%) |
| Colonic | 66 (32%) |
| Prior bowel resection | 88 (43%) |
| Ileocecal valve resection | 70 (34%) |
| Short bowel syndrome | 8 (4%) |
| Prior biologic use | |
| Anti-TNF | 124 (60.5%) |
| 1 | 60 |
| 2 | 40 |
| 3 | 23 |
| Vedolizumab | 20 (10%) |
| Ustekinumab | 10 (5%) |
| Current treatment | |
| Anti-TNF | 84 (41%) |
| Vedolizumab | 16 (8%) |
| Ustekinumab | 47 (23%) |
| Current IM use | 85 (42%) |
Values are median (interquartile range), n (%), or n.
Abbreviations: BMI, body mass index; IM, immunomodulator; TNF, tumor necrosis factor.
Overall Diagnostic Accuracy of the EHI
Overall, the AUC of the EHI for the entire cohort was 75% (95% CI, 65%-79%). Of 205 total patients, 74 had an SES-CD score of 0 to 2, defined as endoscopic remission. Of these, 71 patients had all 5 segments evaluated. The AUC of the EHI for accurately distinguishing endoscopic remission based on an SES-CD score of 0 to 2 was 71% (95% CI, 64%-78%). Of 131 patients who had an SES-CD score >2, 14 had no ulcers, 38 had aphthous ulcers, and 79 had large or very large ulcers. The AUC of the EHI for accurately measuring the absence of any ulcer was 68% (95% CI, 61%-75%), and for the absence of large or very large ulcers was 73% (95% CI, 66%-79%).
The sensitivity of the EHI for identifying any ulcer was 85% (95% CI, 77%-91%) and 93% (95% CI, 84%-97%) for identifying large or very large ulcers (Table 2). The specificity for identifying any ulcer was 86% (95% CI, 76%-92%) and for identifying any large or very large ulcer was 87% (95% CI, 79%-92%). Values of EHI significantly increased with increasing ulcer size (P = .001) (Figure 1). The negative likelihood ratio for an EHI of 20 was 0.47 (95% CI, 0.28-0.78) for any ulcer and 0.23 (95% CI, 0.10-0.52) for large or very large ulcers. The positive likelihood ratio for an EHI of 50 was 2.14 (95% CI, 1.21-3.78) for any ulcer and 3.10 (95% CI, 1.85-5.17) for large or very large ulcers.
Sensitivity, Specificity, Positive Likelihood Ratio, Negative Likelihood Ratio of EHI for Identifying Mucosal Ulcers
| . | Any Ulcer(95% CI) . | Large or Very Large Ulcers(95% CI) . |
|---|---|---|
| EHI 20 points | ||
| Sensitivity | 85% (77-91) | 93% (84-97) |
| Specificity | 33% (23-44) | 33% (25-45) |
| PLR | 1.26 (1.07-1.49) | 1.37 (1.19-1.57) |
| NLR | 0.47 (0.28-0.78) | 0.23 (0.10-0.52) |
| EHI 50 points | ||
| Sensitivity | 32% (23-41) | 42% (31-53) |
| Specificity | 85% (76-92) | 87% (79-92) |
| PLR | 2.14 (1.21-3.78) | 3.10 (1.85-5.17) |
| NLR | 0.80 (0.69-0.93) | 0.67 (0.55-0.82) |
| . | Any Ulcer(95% CI) . | Large or Very Large Ulcers(95% CI) . |
|---|---|---|
| EHI 20 points | ||
| Sensitivity | 85% (77-91) | 93% (84-97) |
| Specificity | 33% (23-44) | 33% (25-45) |
| PLR | 1.26 (1.07-1.49) | 1.37 (1.19-1.57) |
| NLR | 0.47 (0.28-0.78) | 0.23 (0.10-0.52) |
| EHI 50 points | ||
| Sensitivity | 32% (23-41) | 42% (31-53) |
| Specificity | 85% (76-92) | 87% (79-92) |
| PLR | 2.14 (1.21-3.78) | 3.10 (1.85-5.17) |
| NLR | 0.80 (0.69-0.93) | 0.67 (0.55-0.82) |
Abbreviations: CI, confidence interval; EHI, Endoscopic Healing Index; NLR, negative likelihood ratio; PLR, positive likelihood ratio.
Sensitivity, Specificity, Positive Likelihood Ratio, Negative Likelihood Ratio of EHI for Identifying Mucosal Ulcers
| . | Any Ulcer(95% CI) . | Large or Very Large Ulcers(95% CI) . |
|---|---|---|
| EHI 20 points | ||
| Sensitivity | 85% (77-91) | 93% (84-97) |
| Specificity | 33% (23-44) | 33% (25-45) |
| PLR | 1.26 (1.07-1.49) | 1.37 (1.19-1.57) |
| NLR | 0.47 (0.28-0.78) | 0.23 (0.10-0.52) |
| EHI 50 points | ||
| Sensitivity | 32% (23-41) | 42% (31-53) |
| Specificity | 85% (76-92) | 87% (79-92) |
| PLR | 2.14 (1.21-3.78) | 3.10 (1.85-5.17) |
| NLR | 0.80 (0.69-0.93) | 0.67 (0.55-0.82) |
| . | Any Ulcer(95% CI) . | Large or Very Large Ulcers(95% CI) . |
|---|---|---|
| EHI 20 points | ||
| Sensitivity | 85% (77-91) | 93% (84-97) |
| Specificity | 33% (23-44) | 33% (25-45) |
| PLR | 1.26 (1.07-1.49) | 1.37 (1.19-1.57) |
| NLR | 0.47 (0.28-0.78) | 0.23 (0.10-0.52) |
| EHI 50 points | ||
| Sensitivity | 32% (23-41) | 42% (31-53) |
| Specificity | 85% (76-92) | 87% (79-92) |
| PLR | 2.14 (1.21-3.78) | 3.10 (1.85-5.17) |
| NLR | 0.80 (0.69-0.93) | 0.67 (0.55-0.82) |
Abbreviations: CI, confidence interval; EHI, Endoscopic Healing Index; NLR, negative likelihood ratio; PLR, positive likelihood ratio.
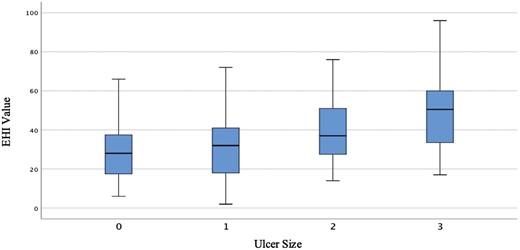
Association between ulcer size (based on the Simple Endoscopic Score for CD) and Endoscopic Healing Index (EHI) value. Box plot of EHI values corresponding to ulcer size, as determined by the Simple Endoscopic Score for CD. EHI values are significantly higher with increasing ulcer size (P = .001).
Patient Factors Influencing EHI Values
Patient factors, or variables that may influence expected normal values for biologic pathways included in the 13-biomarker panel, were evaluated against EHI values using univariate logistic regression analyses (Supplementary Table 1). Of these, the number of inaccessible segments (P = .017), number of segments evaluated (P = .009), gender (P = .061), and current exposure to IM (P = .091) were identified as potential confounders.
When further stratifying by concomitant IM use, stricturing or penetrating behavior (P = .013), prior anti-TNF use (P = .001) and current anti-TNF use (P = .001) were significantly different between patients receiving IM therapy and those not receiving IM therapy (Supplementary Table 2). The SES-CD score was similar among both groups. The distribution of the EHI and SES-CD score were nonuniform between the 2 groups, and therefore nonparametric comparisons were applied for comparison. Overall, the SES-CD score was numerically lower in patients with concomitant IM use; however, this was not statistically significant.
When further stratifying by gender, more women had short bowel syndrome compared with men (P = .035) (Supplementary Table 3). Differences in EHI value between men and women neared significance when including patients with short bowel syndrome (P = .057); however, once patients with short bowel syndrome were excluded (n = 8), the difference in the EHI value was no longer significant (P = .085). Based on this finding, patients with short bowel syndrome were excluded from further analyses, as this subgroup was too small for independent assessments and appeared to be a potential confounder of EHI.
Diagnostic Accuracy of the EHI in Patients With All 5 Segments Evaluated
Given the significant association between number of accessible segments and resected segments with EHI values, a sensitivity analysis was performed to understand true diagnostic performance of the EHI among patients with all 5 segments intact and accessible. Out of 155 patients who had all 5 intestinal segments evaluated, a total of 63 patients had an SES-CD score of 0 to 2, and the AUC of EHI for distinguishing endoscopic remission was 70% (95% CI, 62%-78%). Of 92 patients who had an SES-CD score >2, 7 patients had no ulcers, 28 patients had aphthous ulcers, and 57 patients had large or very large ulcers. The AUC of EHI for identifying the absence of any ulcer was 70% (95% CI, 62%-79%), and the AUC of EHI to identify the absence of large or very large ulcers was 75% (95% CI, 67%-83%).
Factors Influencing EHI Performance (Confounders of EHI Accuracy)
EHI and Prediction of Strictures
The performance of EHI in patient subgroups, after the exclusion of short bowel syndrome patients, was evaluated (Table 3). Median EHI values were significantly higher in patients with presence of a stricture compared with those without a stricture (P = .052; 36 [IQR, 27-44] vs 29 [IQR, 19-41]). When including all patients irrespective of number of segments evaluated or accessed, EHI values were significantly higher with increasing stricture severity, as defined by the SES-CD subscore (P = .040). This was more significant when comparing EHI values between those with a stricture score of 0 to 1 vs 2 to 3 (P = .028). However, the presence or absence of a stricture did not influence the accuracy of EHI (68% vs 67.6%; P = .972).
Patient Factors Influencing Accuracy of EHI (Exclusion of Short Bowel Syndrome Patients)
| Patient Factor . | AUC Difference . | P Value AUC Comparison . |
|---|---|---|
| Gender | 0.038 | .621 |
| Smoking history | 0.027 | .769 |
| Prior bowel resection | 0.028 | .734 |
| Ileocecal resection | 0.126 | .169 |
| Structuring/penetrating behavior | 0.004 | .958 |
| Ileal disease (isolated ileal or ileocolonic) | 0.031 | .692 |
| Perianal disease | 0.108 | .344 |
| Prior anti-TNF use | 0.140 | .057 |
| Prior vedolizumab use | 0.037 | .791 |
| Prior ustekinumab use | 0.138 | .471 |
| Any prior biologic use | 0.130 | .080 |
| Current anti-TNF use | 0.106 | .169 |
| Current biologic use | 0.058 | .472 |
| Concurrent IM | 0.042 | .588 |
| Patient Factor . | AUC Difference . | P Value AUC Comparison . |
|---|---|---|
| Gender | 0.038 | .621 |
| Smoking history | 0.027 | .769 |
| Prior bowel resection | 0.028 | .734 |
| Ileocecal resection | 0.126 | .169 |
| Structuring/penetrating behavior | 0.004 | .958 |
| Ileal disease (isolated ileal or ileocolonic) | 0.031 | .692 |
| Perianal disease | 0.108 | .344 |
| Prior anti-TNF use | 0.140 | .057 |
| Prior vedolizumab use | 0.037 | .791 |
| Prior ustekinumab use | 0.138 | .471 |
| Any prior biologic use | 0.130 | .080 |
| Current anti-TNF use | 0.106 | .169 |
| Current biologic use | 0.058 | .472 |
| Concurrent IM | 0.042 | .588 |
AUC, area under the curve; EHI, Endoscopic Healing Index; IM, immunomodulator; TNF, tumor necrosis factor.
Patient Factors Influencing Accuracy of EHI (Exclusion of Short Bowel Syndrome Patients)
| Patient Factor . | AUC Difference . | P Value AUC Comparison . |
|---|---|---|
| Gender | 0.038 | .621 |
| Smoking history | 0.027 | .769 |
| Prior bowel resection | 0.028 | .734 |
| Ileocecal resection | 0.126 | .169 |
| Structuring/penetrating behavior | 0.004 | .958 |
| Ileal disease (isolated ileal or ileocolonic) | 0.031 | .692 |
| Perianal disease | 0.108 | .344 |
| Prior anti-TNF use | 0.140 | .057 |
| Prior vedolizumab use | 0.037 | .791 |
| Prior ustekinumab use | 0.138 | .471 |
| Any prior biologic use | 0.130 | .080 |
| Current anti-TNF use | 0.106 | .169 |
| Current biologic use | 0.058 | .472 |
| Concurrent IM | 0.042 | .588 |
| Patient Factor . | AUC Difference . | P Value AUC Comparison . |
|---|---|---|
| Gender | 0.038 | .621 |
| Smoking history | 0.027 | .769 |
| Prior bowel resection | 0.028 | .734 |
| Ileocecal resection | 0.126 | .169 |
| Structuring/penetrating behavior | 0.004 | .958 |
| Ileal disease (isolated ileal or ileocolonic) | 0.031 | .692 |
| Perianal disease | 0.108 | .344 |
| Prior anti-TNF use | 0.140 | .057 |
| Prior vedolizumab use | 0.037 | .791 |
| Prior ustekinumab use | 0.138 | .471 |
| Any prior biologic use | 0.130 | .080 |
| Current anti-TNF use | 0.106 | .169 |
| Current biologic use | 0.058 | .472 |
| Concurrent IM | 0.042 | .588 |
AUC, area under the curve; EHI, Endoscopic Healing Index; IM, immunomodulator; TNF, tumor necrosis factor.
EHI and Prediction of Ileal Inflammation
As FC was previously reported to have poor accuracy for identifying inflammation in ileal CD,17 we performed a sensitivity analysis to evaluate EHI performance for disease location and for isolated ileal ulcers, specifically. EHI values did not significantly differ across the range of ileal SES-CD scores (P = .165); however, there was significant variation across the range of colonic SES-CD scores (P = .020). After limiting the analysis to 155 patients with all 5 segments evaluated, EHI values did not differ significantly across the range of ileal SES-CD scores observed on endoscopy (P = .244).
Among patients in whom the ileum was evaluated and scored, irrespective of the number of segments evaluated, the AUC for the EHI was 57% (95% CI, 49%-66%) for identifying the presence of ileal inflammation (SES-CD score ≥1 in the ileum). The AUC for the EHI was 68% (95% CI, 59%-77%) for identifying the presence of colonic inflammation (SES-CD score ≥1 in the colon). Among patients in whom the ileum was evaluated and scored, irrespective of the number of segments evaluated, the AUC of the EHI for identifying endoscopic remission (SES-CD score 0-2) was numerically higher in the absence of ileal inflammation, but this was not statistically significant (77% vs 69%; P = .43). Among patients in whom the ileum was evaluated and scored, irrespective of the number of segments evaluated, the AUC of the EHI for identifying the presence of any ulcers was significantly higher in the absence of ileal inflammation (87% vs 72%; P = .049).
The sensitivity of the EHI for identifying any ulcer specifically in the ileum was 70%, and similarly, the sensitivity for identifying any large or very large ulcer in the ileum was 68%. The specificity of the EHI for identifying any ulcer in the ileum was 79%, and the specificity of identifying any large or very large ulcer in the ileum was 75%. Among patients who had an EHI of 0 to 20 points but had an SES-CD score ≥2 points (false negatives), ileal inflammation was present in 15 patients, whereas colonic inflammation was present in 5 patients. Among the 15 patients with ileal inflammation, but with an EHI of 0 to 20, the ileal SES-CD score was ≥3 in all patients.
Among the 34 patients where all 5 segments were evaluated and who had large or very large ileal ulcers but no colonic inflammation (colonic SES-CD score = 0), the median ileal SES-CD score was 5.5 (IQR, 4-8) and the median EHI value was 36 (IQR, 27-52). While increasing EHI scores showed a steady increase in specificity, approximately 75% of patients with large or very large ulcers in the ileum had EHI scores that would be above the cutoff with the greatest sensitivity to rule out disease (20 points) and below the cutoff with the greatest specificity to rule in disease (50 points).
EHI and Prediction of Affected Surface Area
When evaluating the total extent of surface area affected, the EHI was significantly higher with increased affected surface (P = .012) (Figure 2); however, CIs were wide ranging. This parameter of the SES-CD includes both ulcerated and nonulcerated area, which may be a potential confounder when evaluating the accuracy of EHI for mucosal ulcers, specifically.
![Average surface area affected (based on the Simple Endoscopic Score for CD [SES-CD]) and Endoscopic Healing Index (EHI) value. Box plot of EHI values corresponding to average surface area affected, as determined by the SES-CD. EHI values are significantly higher with increased affected surface area (P = .012).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/ibdjournal/29/4/10.1093_ibd_izac117/1/m_izac117f0002.jpeg?Expires=1749757431&Signature=ZKz6afnyWDhRNoxzIhuPSGPgbMKZTA769feu8GBqVTUFsRpjRTomTIoLBVOiBfBIRj3Dvv4s6AQt~3gwHSEQVRQyIGbPAsWfODjaAz16T54E1Tfn0M8-WJbG~MktjLQu8Z4jpcdWGgOXYrdUxFXgNp0U6oMfSya-mU-UMW74128-JVY4fENQl3vlMaHJAWbJF09k2AAFohaHBcZy5MDfv-4UVcXzvNKIjuxUnRe7w2Xc1qfZDwHipNfduXGUOtuE5C4~qtbusFBgfPGTGvKxSrlaqAahi6C766ZxggzXEl6khTrBRDYsvozmwmvxVUn-mktFXlLvCVq~TOXVSj2OSQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
Average surface area affected (based on the Simple Endoscopic Score for CD [SES-CD]) and Endoscopic Healing Index (EHI) value. Box plot of EHI values corresponding to average surface area affected, as determined by the SES-CD. EHI values are significantly higher with increased affected surface area (P = .012).
EHI and Prediction of Ulcer Size
After adjusting for disease location, total extent of affected surface, and total stricture burden, each 20-point increase in EHI was associated with a 1.7-fold (adjusted odds ratio, 1.70; 95% CI, 1.13-2.55) increased probability for the presence of ulcers >5 mm (Figure 3).
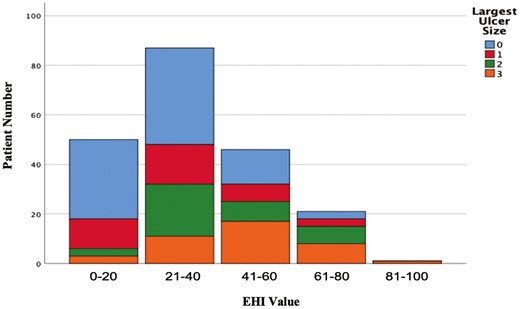
Ulcer size (based on the Simple Endoscopic Score for CD) stratified by Endoscopic Healing Index (EHI) value category. After adjusting for disease location, total extent of affected surface, and total stricture burden, each 20-point increase in EHI is associated with a 1.7-fold (adjusted odds ratio, 1.70; 95% CI, 1.13-2.55) increased probability for the presence of ulcers >5 mm.
EHI and Biologic Use
A consistent difference of >10% in accuracy was observed based on prior or concurrent biologic exposure, specifically for anticytokine biologics (anti-TNFα and ustekinumab); however, these comparisons did not reach statistical significance. Other factors with ≥10% difference in accuracy between groups were prior ileocecal resection and perianal disease history, although these comparisons did not reach statistical significance. When looking specifically at patients who had no prior ileocecal resection, no history of perianal disease, and no prior exposure to anti-TNF or ustekinumab therapy (n = 49), the accuracy of the EHI was 80% (95% CI, 66%-92%).
Discussion
Ulcer healing is the primary treatment target in CD, but it is challenging to perform repeated endoscopic evaluation in routine practice for treat-to-target monitoring. Ideally, providers would have available serum based biomarkers capable of accurately identifying the presence or absence of ulcers to guide treatment adjustments, and these biomarkers would demonstrate a strong association to ulcer size even after accounting for patient or disease confounders. In the current study, we extend on prior work for EHI, a serum-based biomarker of mucosal inflammation in CD, and observed this biomarker to be accurate in determining mucosal ulceration status in CD.
At a predetermined cutoff of 20 points, EHI reliably ruled out the presence of large or very large ulcers with sensitivity of 93% (95% CI, 84%-97%), and at a predetermined cutoff of 50 points, EHI ruled in presence of large or very large ulcers with specificity of 87% (95% CI, 79%-92%). In our cohort, we observed EHI values to be significantly influenced by stricture burden and severity, disease location, and extent of affected surface area. After adjusting for these confounders, a strong incremental association remained for EHI values and ulcer size (adjusted odds ratio, 1.7 per 20-point increase in EHI). These data suggest that the EHI is a valid and accurate biomarker of ulcer size and may be used to guide CD treatment adjustments in routine practice to achieve ulcer healing, our currently recommended treatment target.
A notable observation in our dataset was the variability in performance of EHI for ileal ulcers, specifically. Prior literature has consistently observed that ileal CD patients are at the greatest risk for disease-related complications18 and that symptom-based treat-to-target monitoring is less effective in reducing disease-related complication risk among ileal CD patients compared with colonic CD patients.19 Therefore, ideal biomarkers for treat-to-target monitoring in CD must be able to accurately identify the presence or absence of ulcers in the ileum specifically to be of greatest value in practice for this high-risk population. In our cohort, we observed a lower diagnostic performance of EHI for ileal ulcers, but this does not appear to be consistently observed across all cohorts. A recent study from the University of Miami observed no significant differences in median EHI concentrations between patients with isolated ileal CD (35 [IQR, 24-46]), isolated colonic CD (32 [IQR, 19-40]), or ileocolonic CD (34 [IQR, 23-44]); however, it is unclear if the study adjusted for endoscopic disease activity or ulcer size at the time of assessment specifically when comparing disease locations.20 In our study, among patients with large or very large ileal ulcers but no colonic inflammation, the median EHI value was 36 (IQR, 27-52), suggesting that the cutoff of 50 for ruling in ulcers with the greatest specificity may be too high for this subpopulation. Furthermore, as the ileal SES-CD typically only captures inflammation in the terminal ileum, this may partly explain our observation related to variable ileal performance of the EHI. Had we decided on depth of intubation when scoring the ileum in order to capture more proximal disease, the association of the EHI and ileal inflammation may have been different. Further research is needed to understand the limitations of EHI in ileal CD and whether recalibration of cutoffs for individual biomarkers within the 13-biomarker panel are needed to further optimize performance, or if the addition of small bowel specific biomarkers would be of value.
Limitations to this study include the lack of centrally scored endoscopies, which may have led to variability in the SES-CD based on local endoscopist interpretation. Additionally, data on ethnicity were not collected in this study, which may limit the generalizability of the EHI. Similarly, data on corticosteroid use were not systematically collected; however, this may affect EHI values and alter its accuracy. Future studies evaluating variability in the EHI across different patient ethnicities and understanding its performance with exposure to corticosteroids will be important to determine the role of EHI in clinical practice. Furthermore, values in this cohort were measured at one point in time; however, longitudinal measurements are needed to evaluate EHI variability and how EHI correlates with consecutive endoscopic assessments. Finally, while we do not yet know the expected variance in EHI for healthy control groups, future work on this matter may be important in further understanding EHI performance in patients with CD.
Conclusions
The EHI is significantly associated with various features of disease severity and activity as measured by the SES-CD subcomponents. After accounting for disease location, extent of involvement, and stricture burden, the EHI is independently and incrementally associated with ulcer size. A cutoff of 50 points can reliably rule in the presence of ulcers and allow for treatment adjustment without endoscopy, while a cutoff of 20 points can reliably rule out the presence of ulcers and signal completion of treatment adjustment algorithms. Patients with ileal CD, however, may benefit from the use of a single cutoff of 20 points for treatment adjustment decisions. Future studies are warranted to evaluate the performance of EHI serially over time as compared with repeated endoscopic assessment and in patients with isolated ileal disease.
Author Contribution
A.K.H. and P.S.D. were involved in conception. A.K.H., H.L., and J.N. were involved in data abstraction. A.K.H. and P.S.D. were involved in data analysis and interpretation. A.K.H. and P.S.D. were involved in drafting of the manuscript. A.K.H., B.S.B., S.S., J.N., H.L., A.M., A.E.C., W.J.S., and P.S.D. were involved in critical review of the manuscript. P.S.D. is the guarantor of article. All authors have approved the final version of the manuscript. Prometheus Biosciences ran assays for this study but were not directly involved in data analyses.
Funding
This work was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases–funded San Diego Digestive Diseases Research Center (P30 DK120515). P.S.D. is supported by an American Gastroenterology Association Research Scholar Award. B.S.B. is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant No. K23DK123406 and San Diego Digestive Diseases Research Center Grant No. P30 DK120515. S.S. is supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant No. K23DK117058. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
A.K.H. has received a fee from Pfizer for a fellowship ad board. B.S.B. has received research support from Prometheus Biosciences; and consulting fees from Pfizer, Bristol-Myers Squibb, and Takeda. S.S. has received research grants from AbbVie and Janssen; and personal fees from Pfizer for ad hoc grant review. J.N., H.L., and A.M. have no conflicts of interest. A.C. has received consulting and speaking fees from AbbVie, Janssen, and Takeda. W.S. has received research grants from AbbVie, Abivax, Arena Pharmaceuticals, Boehringer Ingelheim, Bristol-Myers Squibb, Genentech, Gilead Sciences, GlaxoSmithKline, Janssen, Lilly, Pfizer, Prometheus Biosciences, Seres Therapeutics, Shire, Takeda, Theravance Biopharma; consulting fees from AbbVie, Abivax, Alfasigma, Alimentiv (previously Robarts Clinical Trials, owned by Alimentiv Health Trust), Allakos, Amgen, Arena Pharmaceuticals, AstraZeneca, Atlantic Pharmaceuticals, Beigene, Boehringer Ingelheim, Bristol-Myers Squibb, Celltrion, Clostrabio, Forbion, Galapagos, Genentech (Roche), GlaxoSmithKline, Gossamer Bio, Index Pharmaceuticals, Iota Biosciences, Janssen, Lilly, Morphic Therapeutics, Novartis, Oppilan Pharma (now Ventyx Biosciences), Pfizer, Pharm Olam, Polpharm, Progenity, Prometheus Biosciences, Protagonist Therapeutics, PTM Therapeutics, Seres Therapeutics, Shoreline Biosciences, Sublimity Therapeutics, Surrozen, Takeda, Theravance Biopharma, Vedanta Biosciences, Ventyx Biosciences, Vimalan Biosciences, Vivreon Gastrosciences, Xencor, and Zealand Pharmaceuticals; owns stock or stock options from Allakos, BeiGene, Gossamer Bio, Oppilan Pharma (now Ventyx Biosciences), Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonists Therapeutics, Shoreline Biosciences, Ventyx Biosciences, Vimalan Biosciences, and Vivreon Gastrosciences; is employee of Shoreline Biosciences; and has a spouse who has served as a consultant for Iveric Bio and Prometheus Laboratories; owns stock in Progenity, Oppilan Pharma (now Ventyx Biosciences), Prometheus Biosciences, Prometheus Laboratories, Ventyx Biosciences, and Vimalan Biosciences; owns stock options in Iveric Bio, Prometheus Biosciences, Prometheus Laboratories, Ventyx Biosciences, and Vimalan Biosciences; and is an employee of Prometheus Biosciences. P.S.D. has received research support and/or consulting fees from Takeda, Janssen, Pfizer, AbbVie, Gilead, Lily, BMS, Novartis; owns stock options and has served as a board member for DigbiHealth; and owns licensing royalties from Precidiag.



