-
PDF
- Split View
-
Views
-
Cite
Cite
Nik Arsyad Nik Muhamad Affendi, Sarala Ravindran, Tan Soek Siam, Alex Hwong-Ruey Leow, Ida Hilmi, Jaundice in a Primary Sclerosing Cholangitis Patient: A New Cause in a New Era, Inflammatory Bowel Diseases, Volume 28, Issue 3, March 2022, Pages e29–e30, https://doi.org/10.1093/ibd/izab250
Close - Share Icon Share
To the Editors,
A 63-year-old woman with long-standing ulcerative colitis (UC) and primary sclerosing cholangitis (PSC) presented with a new onset of jaundice and pruritus 2 weeks after her first coronavirus disease 2019 (COVID-19) vaccination (AZD1222). Prior to presentation, she was in clinical remission and her liver function tests were stable. Her medications were 250 mg of ursodeoxycholic acid 3 times daily, 1.5 g of mesalazine twice daily, 50 mg of azathioprine once daily, and 10 mg of atorvastatin once daily. There was no recent change of medication, and she denied taking any complementary medicine. She was jaundiced but otherwise examination was unremarkable.
Investigations showed total bilirubin of 313 umol/L (baseline 29 umol/L), aspartate transaminase of 505 U/L (baseline 81 U/L), alanine transaminase of 354 U/L (baseline 59 U/L), alkaline phosphatase of 299 U/L (baseline 369 U/L), and gamma-glutamyltransferase of 65 U/L (baseline250 U/L). International Normalised Ratio was 1.6 and albumin was 20 g/L (baseline 29 g/L). Cancer antigen 19-9 (CA 19-9) was elevated to 300 U/mL. Immunoglobulin G level was raised to 2030 mg/dL, and antinuclear antibody was positive with titres of 1:320. Viral hepatitis A, B, and C screening were negative. Magnetic resonance cholangiopancreatography showed short segment strictures involving the intrahepatic and extrahepatic ducts suggestive of underlying PSC. There were no interval changes when compared with her last surveillance MRCP. Liver biopsy showed changes consistent with underlying PSC and bridging fibrosis but also revealed interface and lobular hepatitis (Figure 1). The simplified autoimmune hepatitis (AIH) score was 7. In view of the overall clinical picture, a diagnosis of AIH overlap syndrome with underlying PSC was made. She was started on 40 mg of prednisolone once daily. Two weeks later, her liver functions tests showed improvement, and the plan was to taper the prednisolone by 5 mg a week. The second dose of the COVID-19 vaccine was withheld.
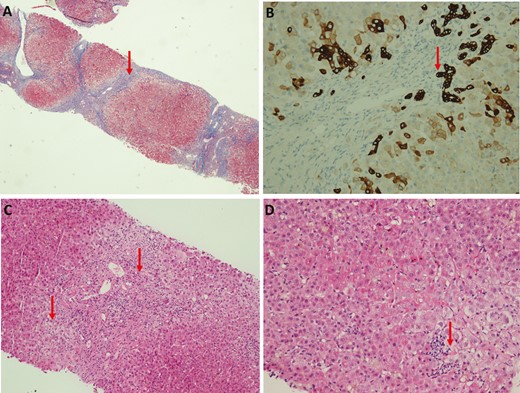
A, Low magnification showed bridging fibrosis. B, CK7 staining showed florid ductal proliferation. C, Medium magnification showed interface hepatitis and ductopenia in portal tract. D, High magnification showed lobular hepatitis.
There are a number of case reports of AIH after COVID-19 vaccination1-3. However, to our knowledge, this is the first case of possible COVID-19 vaccine-induced AIH in inflammatory bowel disease (IBD) and PSC, where our initial concern was the development of a cholangiocarcinoma. Although a true causality will be difficult to confirm, the timing and clinical presentation were certainly highly suggestive.
Patients with IBD have been strongly encouraged to take the COVID-19 vaccination, and data on the safety of these vaccines have been encouraging.4 However, it is important for the IBD physician to be aware of this potential side effect to ensure prompt investigation and management while strongly continuing to advocate vaccination in this vulnerable group of patients.
Funding
None
Conflicts of Interest
No conflicts of interest to declare.
References



