-
PDF
- Split View
-
Views
-
Cite
Cite
Kaitlin Whaley, Vivian Xiong, Rebekah Karns, Jeffrey Hyams, Subra Kugathasan, Brendan Boyle, Tom Walters, Judith Kelsen, Neal LeLeiko, Jason Shapiro, Amanda Waddell, Sejal Fox, Yael Haberman, Phillip Minar, Lee Denson, Geert D’Haens, Alexander Vinks, Michael Rosen, ANTI-TNF PHARMACOKINETICS AND RESPONSE TO THERAPY IN PEDIATRIC ACUTE SEVERE ULCERATIVE COLITIS (THE ARCH STUDY), Inflammatory Bowel Diseases, Volume 27, Issue Supplement_1, January 2021, Pages S55–S56, https://doi.org/10.1093/ibd/izaa347.132
Close - Share Icon Share
Abstract
25% of children hospitalized with acute severe ulcerative colitis (ASUC) rescued with infliximab (IFX) at labeled dosing undergo a colectomy prior to discharge. Our aim was to determine whether IFX pharmacokinetics (PK) are associated with treatment response in pediatric ASUC.
We prospectively enrolled hospitalized pediatric patients initiating IFX for ASUC or IBD-U (PUCAI ≥65) at 7 North American centers and followed for 26 weeks in this pilot and feasibility cohort study. Serial IFX levels (Prometheus Biosciences, Inc.) were obtained and individual PK parameter estimates, such as volume of distribution, clearance (CL), elimination half-life (T1/2) and IFX exposure (area under the concentration-time curve) were estimated using Bayesian methodology. The primary outcome was Day 7 clinical response (CR-D7, PUCAI <35). Key secondary outcomes were Week 8 clinical remission (CR-W8, PUCAI <10) and Week 26 corticosteroid-free clinical remission (CFR-W26).
38 participants (mean age 14.5 years, 50% female, 95% UC, 87% extensive/pancolitis) were treated with IFX at a mean higher than labeled dosing of 9.9 [9.3,10.3] mg/kg, and 16% received an early second dose 4–6 days after the first infusion. CR-D7, CR-W8, and CFR-W26 were achieved in 71%, 55%, and 41%, respectively. Only one participant (2.7%) underwent colectomy by week 26. Using 304 IFX level measurements, we developed a novel pediatric population PK model for IFX in ASUC that incorporated albumin, antibodies to IFX, CRP, and height to characterize the PK profile for participants. The median IFX T1/2 was 5.8 [4.2,7.0] days at Day 7 and lengthened to 7.4 [5.4,8.4] days by Week 26 (P=0.014), but notably remained below the median 10.8 [8.6, 15.4] days reported in a randomized controlled trial of IFX for moderate to severe pediatric UC (Fig. 1). IFX exposure was not associated with CR-D7, CR-W8, or CSR-W26. More rapid IFX CL at Week 26 was significantly associated with inability to achieve CFR-W26 (P=.013). This finding was in line with a higher, but not statistically significant, median trough IFX level nearest Week 26 in those with (19.5 [13.6, 30.3]) versus without (14.2 [6.0, 21.3] μg/ml) CFR-W26 (P=.13) (Fig. 2).
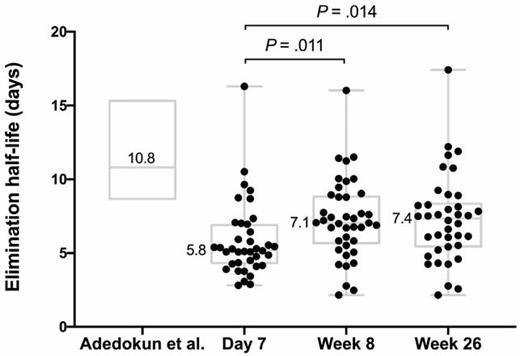
Infliximab elimination half-life in pediatric ASUC at Day 7, Week 8, and Week 26 from start of treatment. The elimination half-life reported in the pediatric randomized control trial for moderate to severe UC is provided as a reference comparison.
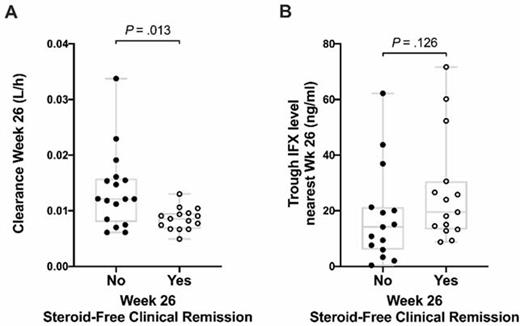
Infliximab clearance (A) and trough levels (B) at Week 26 compared between participants achieving and not achieving Week 26 steroid-free clinical remission.
At the higher than standard IFX dosing used to treated children with ASUC in this observational study, we observed a lower colectomy rate compared to prior studies but did not observe a positive association between IFX exposure and clinical outcomes. Albumin, CRP, height, and ATI were associated with IFX PK and incorporated into a new pediatric ASUC PK model. Initial 10 mg/kg IFX dosing may be sufficient to optimize early outcomes in pediatric ASUC. Additional studies are needed to determine if sustained intensification of maintenance IFX regimens can overcome persistent rapid clearance and improve later outcomes in pediatric ASUC.
- tumor necrosis factors
- drug distribution volume
- half-life
- albumins
- adrenal corticosteroids
- colectomy
- inflammatory bowel disease
- ulcerative colitis
- drug clearance
- glucocorticoids
- child
- pediatrics
- steroids
- antibodies
- mineralocorticoids
- pharmacokinetics
- treatment outcome
- irritable bowel syndrome
- persistence
- infliximab
- trough concentration
- pancolitis
- infusion procedures
- disease remission
- primary outcome measure



