-
PDF
- Split View
-
Views
-
Cite
Cite
Francesco Graziano, Fabio Salvatore Macaluso, Nicola Cassata, Michele Citrano, Ambrogio Orlando, Pyoderma Gangrenosum in An Ulcerative Colitis Pediatric Patient During Vedolizumab Therapy Successfully Treated With Oral Cyclosporine, Inflammatory Bowel Diseases, Volume 27, Issue 9, September 2021, Pages e110–e111, https://doi.org/10.1093/ibd/izab106
Close - Share Icon Share
To the Editors,
It was with great interest that we read the letter by Dos Santos and Koga1 about the challenge of treating pyoderma gangrenosum (PG) in adult patients with inflammatory bowel disease (IBD). We agree with the authors that PG can be extremely challenging, especially because of the lack of well-designed studies to determine the best therapeutic option.
We would like to share our experience of a teenage girl with ulcerative colitis (UC) complicated by PG, successfully treated with oral cyclosporine A (CsA) during intensive dose optimization of vedolizumab (VDZ). The patient was diagnosed with UC (endoscopic Mayo score 2) when she was 12 years old. After developing steroid dependence, the patient started treatment with azathioprine, but this was ceased after 12 months due to lack of efficacy. Infliximab (IFX) was commenced in a standard dosage regimen, leading to clinical, biochemical, endoscopic, and histologic remission after 12 months. However, due to repeated infusion reactions, IFX was also discontinued and the patient was switched to adalimumab (ADA). As clinical remission was not achieved with ADA, VDZ was proposed as an off-label treatment. The patient was 16 years old when VDZ was started. After a successful induction, VDZ was administered every 8 weeks as maintenance treatment. Clinical remission was maintained for the following 12 months.
Then due to severe steroid refractory UC relapses, VDZ was administered every 4 weeks, enabling a rapid wean of corticosteroids and clinical and endoscopic remission.
In January 2021, about 7 days after the last administration of VDZ, the patient was referred to our pediatric department with a painful skin ulcer on the right tibia 2.5 × 1 cm in diameter (Fig. 1A). No intestinal symptoms were referred, and inflammation markers were normal. The diagnosis of PG was confirmed after dermatological consultation. Bacterial cultures of the ulcer were negative. Immunosuppressive treatment was started with oral CsA (5 mg/kg) based on the diagnosis of PG, previous intolerance to IFX, and lack of response to ADA. The patient continued CsA, an the cyclosporinemia was monitored every 2 weeks. At the first outpatient visit after 1 week of therapy, the lesion had improved (Fig. 1B).
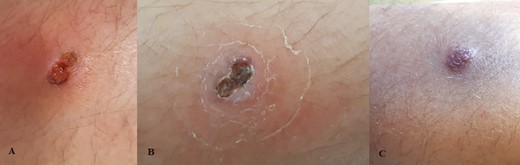
A, Pyoderma gangrenosum of the right tibia before treatment with oral cyclosporin. B, Lesion after 1 week of treatment with oral cyclosporin. C, Lesion after 5 weeks of treatment.
The dosage of CsA was optimal. Creatinine, glucose, electrolytes (including magnesium), lipid levels, and blood pressure were normal. No adverse events occurred. At the last outpatient visit in March 2021, the patient was in good clinical and nutritional status, and the dermatological lesion (Fig. 1C) was in remission.
The worldwide incidence of PG is estimated to be 3 to 10 cases per 1 million inhabitants per year—and 4% are in children. In pediatric IBD patients, PG is diagnosed most frequently in association with Crohn’s disease (CD), but it is also reported in UC. It is more common in female patients and in those with active colitis. The occurrence and the severity of PG lesions may not be directly correlated to IBD clinical activity.2, 3
A recent review by Vaidy et al reported 12 studies in which 19 pediatric patients with IBD- associated PG are described (13 patients with CD and 6 with UC). Eight of these patients achieved complete PG resolution with an anti TNF-α on monotherapy; only 1 patient achieved complete remission after CsA on monotherapy.2 To the best of our knowledge, this is the first pediatric case of IBD-associated PG onset during VDZ therapy and treated with CsA.
Cyclosporine A alone or in combination with systemic corticosteroids is recommended among pediatric IBD patients as a low cost first line treatment for IBD-associated PG because of its important role in inhibiting T-cell activation, inflammatory cell proliferation, and pro-inflammatory cytokine production. It may be administered orally, without requiring hospitalization; its adverse events are generally mild and transient; and its scheduled doses for treatment are standardized.4
The management of pediatric PG is mainly based on clinical experience. Due to small case series and the lack of large randomized prospective trials, current practice guidelines have not established the standard of care for this debilitating skin disease. Our report emphasizes that in children we should not forget CsA as a treatment option for PG, even if it is refractory to biologics.
Data Availability Statements
No new data were generated or analysed in support of this research.
Author Contribution: FG, MC, NC, and AO were involved in the diagnosis and care of the patient. FG wrote the manuscript. FSM and AO reviewed and revised the manuscript. All authors read and approved the final manuscript.
Conflicts of Interest: AO served as an advisory board member for AbbVie, MSD, Janssen, Pfizer, and Takeda Pharmaceuticals and received lecture grants from AbbVie, MSD, Sofar, Chiesi, Janssen, Pfizer, and Takeda Pharmaceuticals. FBM served as an advisory board member and/or received lecture grants from AbbVie, Biogen, MSD, Pfizer, Samsung Bioepis, and Takeda. All other authors have nothing to disclose.
References



