-
PDF
- Split View
-
Views
-
Cite
Cite
Christopher J Schmoyer, Jakob Saidman, Jaime L Bohl, Claire L Bierly, John F Kuemmerle, Stephen J Bickston, The Pathogenesis and Clinical Management of Stricturing Crohn Disease, Inflammatory Bowel Diseases, Volume 27, Issue 11, November 2021, Pages 1839–1852, https://doi.org/10.1093/ibd/izab038
Close - Share Icon Share
Abstract
Stricturing of the gastrointestinal tract is a common complication in Crohn disease and is a significant cause of morbidity and mortality among this population. The inflammatory process initiates fibrosis, leading to aberrant wound healing and excess deposition of extracellular matrix proteins. Our understanding of this process has grown and encompasses cellular mechanisms, epigenetic modifications, and inherent genetic predisposition toward fibrosis. Although medications can improve inflammation, there is still no drug to attenuate scar formation. As such, management of stricturing disease requires a multidisciplinary and individualized approach including medical management, therapeutic endoscopy, and surgery. This review details the current understanding regarding the pathogenesis, detection, and management of stricturing Crohn disease.
Crohn disease (CD) complicated by stricturing significantly increases morbidity and mortality in this population.1 The fibrotic phenotype is also associated with considerable financial costs to both the patient and society.2 Although uncomplicated inflammatory disease is the most common presentation of CD, approximately 30% of patients have stricturing at diagnosis.3 In addition, up to 50% of patients will eventually develop stricture formation.4, 5 The prevalence of fibrotic disease is similar between the adult and pediatric populations.6 Whereas either the upper or lower gastrointestinal tract can develop fibrostenotic lesions, the most common site is the terminal ileum and ileocecal valve.3 Higher rates of anorectal strictures are seen in the pediatric population.6
Stricturing is a transmural process. Its pathogenesis relates to an abnormal regulatory response to wound healing in the gastrointestinal tract in response to chronic inflammation. Strictures are characterized by the expansion of the mesenchymal cell component including fibroblasts, myofibroblasts, and smooth muscle cells with excessive deposition of extracellular matrix (ECM) proteins.7 The fibroblast population within the mesenchyme is further expanded by the processes of epithelial-to-mesenchymal transition (EMT), endothelial-to-mesenchymal transition (EndoMT), and invasion of bone marrow–derived circulating fibrocytes into the intestine. Early strictures are composed of both an intense inflammatory infiltrate and scar formation. As fibrosis becomes more established, the stricture contains extensive mesenchymal cells and ECM proteins.8
The ability to detect stricturing disease, both endoscopically and through noninvasive imaging techniques, is quite sensitive. Accurately discerning the inflammatory and fibrotic components of the stricture with these tests is a key step in gauging the likelihood of response to therapy. This distinction between inflammation and fibrosis is key in that currently available medical therapies for CD improve inflammation but do not attenuate or reverse fibrosis.9 Although antifibrotic medications have been investigated experimentally, endoscopic and surgical techniques are the principal strategies for managing chronic stricturing disease. This review summarizes the current understanding of the pathogenesis, diagnosis, and management of stricturing CD.
PATHOGENESIS OF FIBROSIS
The inflammatory insult to the gastrointestinal tract in CD is characterized by tight junction disassociation with the loss of epithelial barrier function, inflammatory cell infiltration with granuloma formation, and apoptosis and necrosis of cellular structures.10 Both the innate and adaptive immune systems are integral to this inflammatory response and the development of fibrosis. In CD, the entry of microorganisms and microbial products through the disrupted epithelial barrier exposes cells of the innate immune system to various pathogen-associated molecular patterns such as the bacterial lipopolysaccharides muramyl dipeptide and flagellin, fungal β-glucan, and viral oligonucleotides (Fig. 1). Injured epithelial cells release damage-associated molecular patterns including interleukin (IL)-1a, IL-33, S100 proteins, calprotectin, and lactoferrin. Both damage-associated molecular patterns and pathogen-associated molecular patterns are taken up by resident tissue macrophages and dendritic cells through toll-like receptors, nucleotide-binding oligomerization domain receptors, and the inflammasome. This process leads to antigen presentation and the secretion of multiple cytokines and chemokines such as Il-1β, IL-6, Il-12, Il-23, and tumor necrosis factor (TNF)-α initiating the inflammatory cascade.11 Cellular damage incited by this inflammation is seen grossly as the characteristic appearance of mucosal erythema, ulceration, and luminal narrowing.
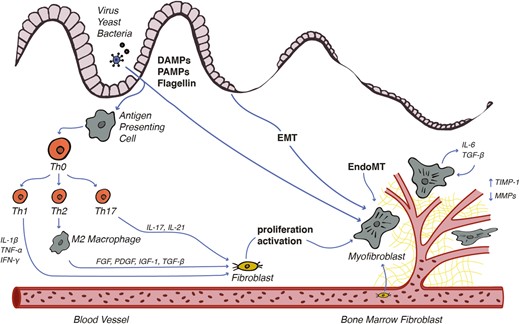
Fibrostenosis in CD is dependent on immune activation of Th1, Th2, and Th17 cells in response to microbial antigens. Cytokine stimulation induces mesenchymal cell transdifferentiation, proliferation, and activation with subsequent dysregulated production of ECM proteins.
Adaptive immunity in CD is governed by the regulatory activity of T cells, which tailor the host response toward an immunologic challenge. Naïve helper T cells (Th0) are activated by antigen presentation and inflammatory cytokines, causing differentiation into 1 of 4 subtypes. Broadly, Th1 cells dominate the response to intracellular bacteria and protozoa, Th2 cells respond to extracellular pathogens and allergy, Th17 cells work against fungal infections and in autoimmunity, and regulatory T cells contribute to immune suppression and regulation.
The interplay between these 4 classes is dynamic and dictates the type and intensity of the immune response. The initial phase of acute inflammation in CD features a predominance of Th1 cells. The Th1 response involves IL-1β, interferon-γ, and TNF-α, leading to robust inflammation and subsequent tissue damage. The activation of Th1 drives inflammation in CD but also plays a role in intestinal fibrosis by upregulating the mesenchymal cell production of collagen, fibronectin, and matrix metalloproteinase-1 (MMP-1).12 As immune activation persists, the response becomes chronic, involving a larger portion of Th2 and Th17 subtypes. These classes are key to the development of intestinal fibrosis. Although the Th2 release of IL-4 and IL-13 promotes intestinal inflammation, these cytokines also cause macrophage differentiation to the M2 subtype. The M2 macrophages produce a number of key profibrotic mediators, including platelet-derived growth factor, fibroblast growth factor, insulin-like growth factor (IGF), and transforming growth factor β1 (TGF-β1). Research has recognized TGF-β1 as the most potent driver of intestinal fibrosis through the regulation of mesenchymal cell proliferation and activity. Furthermore, an excessive Th17 response is seen in a number of other autoimmune diseases and is characterized by the production of IL-17 and IL-21. The signaling of IL-17 potentiates the Th1 response but also leads to fibroblast proliferation and ECM production.13, 14
The interplay of these cytokines regulates and sustains wound healing, the physiologic response to tissue injury. In this response, damaged cells are replaced followed by the deposition of an immature layer of ECM containing collagen I, III, and V; elastin; fibronectin; and proteoglycans. The cellular basis for fibrosis involves mesenchymal cells including fibroblasts, myofibroblasts, smooth muscle cells, and transdifferentiated cells. Histologically, disordered arrays of mesenchymal cells make up the bulk of CD fibrostenosis.15 Mesenchymal cell hyperplasia is driven by TGF-β1, IGF-1, and platelet-derived growth factor, and mesenchymal cell hypertrophy is driven by an IGF-1 splice variant, mechanogrowth factor. These mediators are released by neighboring immune cells and damaged parenchymal cells and via autocrine signaling.16 Mesenchymal activation causes the adoption of a synthetic phenotype with ECM production and deposition.17 All subtypes of mesenchymal cells play a role in fibrosis because they contain robust machinery capable of ECM production in addition to contractile properties most appreciated in smooth muscle cells. This allows the specialized deposition of ECM, posttranslational modification of the scar, and contraction of the fibrotic layer.
Wound healing is typically a self-limited process. In CD, mesenchymal expansion and ECM deposition continue in a dysfunctional self-sustaining fashion even in the absence of mucosal inflammation. It is theorized that mesenchymal cellular adhesion molecules may drive this process. Integrins are heterodimeric intracellular proteins involved in cell-cell and cell-ECM binding. Binding of the αVβ3 integrin on smooth muscle to the ECM proteins fibronectin and vitronectin augments the profibrotic response to IGF-1.18 Another class of cell adhesion molecules, cadherins, is upregulated in response to TGF-β1. The expression of N-cadherin is augmented in CD strictures, enhancing fibroblast migration.19 Cadherin-11 expression is also upregulated in CD fibroblasts and other diseases associated with fibrosis.20 Mesenchymal cells respond to the mechanical stiffness of their surrounding tissue through adhesion molecule–ECM interactions. When exposed to stiff fibrotic/edematous tissue, CD fibroblasts increase the proliferation and production of intracellular contractile machinery.21
Mesenchymal cells accumulate at the site of intestinal fibrosis through proliferation, local migration, and transdifferentiation of other cell types. The transformation of EMT and EndoMT cells to mesenchymal cells is crucial to CD fibrosis and constitutes >30% of the mesenchymal cell population.22 During EMT, epithelial cells lose polarity and adopt myofibroblast-like properties, including contractile machinery with the expression of α-smooth muscle actin and vimentin and enhanced ECM synthesis. This process is mediated by IL-13 and TGF-β1 signaling with subsequent upregulation of the transcription factor SLUG, believed to be the key regulator in EMT.23 The roles of additional cell types in the development of fibrostenosis including vascular endothelial pericytes, bone marrow–derived fibrocytes, and mesenteric adipocytes are areas of active investigation. Increasing evidence supports a bidirectional interplay between visceral adipocytes and preadipocytes with mesenchymal cells supporting chronic intestinal inflammation and the development of fibrosis.24
The maturation and turnover of ECM are also aberrant in CD fibrosis. The basement membrane and interstitial matrix undergo a constant dynamic process of breakdown and deposition, mediated by the interplay of catabolic MMPs, neutrophil elastase, and meprins with regulatory factors including tissue inhibitors of metalloproteinases (TIMPs). The production of MMP-1, MMP-2, MMP-3, and MMP-9 by intestinal fibroblasts is increased by synergistic IL-21 and TNF-α signaling in inflammatory CD, resulting in tissue breakdown and mucosal injury.25 However, lower expression of MMP-3 and MMP-12 is noted in intestinal strictures and results in decreased ECM catabolism.26 This reduction in catabolic activity in intestinal fibrosis is controlled by a marked increase in the inhibitory glycoprotein TIMP-1. The TGF-β isotypes 1 and 2 are strong inducers of TIMP-1 expression by myofibroblasts.26, 27 Fibroblast-activating protein is also upregulated in areas of CD fibrosis, leading to increased TIMP-1 and collagen I expression.28 In addition, there may be a genetic determinant favoring stricture formation. A single nucleotide polymorphism (SNP-1613 5T/6T) in MMP-3 increases the risk of stenosis.29
Many unanswered questions remain for intestinal fibrosis in CD. One major challenge is the complexity of the immune response and limitations in the scientific methods to study it. Immune research relies on identifying populations of cells based on common markers using flow cytometry, immunohistochemistry, or tissue-based analysis of protein and gene expression. Although these techniques are informative, they have a limited ability to study individual cells or heterogeneous groups within the population that may drive disease processes.30 Single-cell analysis is a recent development to address this issue. Individual cells are isolated using laser microdissection, dielectrophoretic sorting, or fluorescence-activated cell sorting. Cellular functions are identified by cellular RNA sequencing (transcriptomics), epigenomics, or DNA sequencing. This technique has been used in CD to identify aberrant immune signatures responsible for TNF-α nonresponse.31 Single-cell analysis of immune or mesenchymal cell subgroups could elucidate the processes that drive intestinal fibrosis.
GENETIC ABERRATIONS IN FIBROSTENOSIS
Research has shown that CD is a complex polygenetic disease. Genome-wide association studies have identified >200 risk alleles for CD and ulcerative colitis, or as shared variants. The most highly associated risk loci, NOD2, ATG16L1, IRGM, and IL23R, are indicative of the combined effects of the environment, gut dysbiosis, and alterations in the innate and adaptive immune responses that contribute to intestinal inflammation. Characteristic genetic mutations, epigenetic modifications, and alterations in immune function are present in patients predisposed toward the fibrotic phenotype. Genetic susceptibility may explain why a subset of patients progresses rapidly to stenosis. Evidence for this hypothesis originated with the discovery of the NOD2/CARD15 gene, coding for an intracellular pattern recognition receptor integral to the identification of bacterial peptidoglycans, notably muramyl dipeptide. Mutations in the NOD2 gene cause alterations in intestinal immune sampling, increased nuclear factor-kappa B signaling, and a heightened immune response.32 Multiple mutations of this gene have been identified and are present in 30% to 50% of White patients with CD. These mutations predispose individuals to a stenotic phenotype, earlier need for surgical intervention, and increased rates of recurrent surgery.33, 34
Additional genetic factors associated with the development of fibrostenosis have since been identified, including the fractalkine receptor CX3CR-1. This receptor, expressed on cells of the intestinal epithelia and innate immune system, is upregulated in response to inflammatory cytokines including TNF-α. The CX3CR-1 expressing macrophages are key regulators of mucosal immunity through IL-22 and the Th17 response. Mutations in this gene predispose toward colitis, fibrostenosis, and higher frequency of ileal disease.35, 36
Furthermore, a large genome-wide association study, the European IBDChip,34 revealed the rs10758669 mutation of the tyrosine kinase signaling molecule Janus-activated kinase 2 (JAK2), which activates the signal transducer and activator of transcription-3 (STAT3). This protein stimulates TGF-β1-driving collagen transcription, conferring increased susceptibility toward stenosis.
Polymorphisms in the genes that normally regulate the JAK-STAT pathway have also been identified. There is increased fibrostenosis with the minor allele of PTPN2 (rs7234029).37 Genetic mutations are likely just 1 factor leading to the development of stenotic CD. The proportion of patients with fibrotic disease harboring particular risk variant(s) remains unknown.
EPIGENETICS OF FIBROSTENOSIS
The role of the epigenome in the development of fibrostenosis is also evolving. The regulation of gene expression by the epigenome involves 4 main mechanisms: (1) promoter methylation of CpG-rich islands that governs the accessibility of chromatin to transcription factors, (2) DNA-associated histone modifications that activate or repress gene transcription, (3) adenosine triphosphate–dependent remodeling complexes that regulate nucleosome positioning and help compact chromatin, and (4) interfering noncoding RNA including microRNA (miRNA) and long noncoding RNA.
Genome-wide mapping of differentially methylated regions in fibrotic human intestinal fibroblasts has identified the presence of a fibrosis-specific DNA methylome that revealed new gene networks and epigenetic states through which one can understand the mechanisms of gene expression that lead to fibrosis.38 Specific examples of promoter hypermethylation that results in gene silencing in the setting of myofibroblasts include the silencing of SOCS3, resulting in the loss of the normal suppression of JAK-STAT signaling, and the silencing of Smad7 that results in the loss of the inhibition of TGF-β signaling.
Furthermore, the role of miRNA segments in promoting intestinal fibrosis in CD has been elucidated. Research has shown that miRNAs are derived from hairpin loops present in the noncoding regions of RNA. After transcription and processing, these small nucleotide segments are incorporated into a multiprotein complex called the miRNA-induced silencing complex, where they bind complementary mRNA sequences. This process leads to the inhibition of protein translation and the degradation of the miRNA. The expression of miRNA in CD can be regulated by inflammatory cytokines. Three distinct miRNAs are known to have profibrotic properties in CD. One family of miRNA, miR-29, is involved in the downregulation of miRNA coding for collagen I and III in intestinal fibroblasts. Levels of miR-29 are diminished in areas of fibrostenosis, leading to excessive collagen production.39 Conversely, increased levels of miR-19,40 miR-21, and miR-200b41 are correlated with the development of fibrostenotic disease. Further research is needed to determine whether these miRNAs can be utilized as biomarkers or therapeutic targets.
DIAGNOSIS OF STRICTURING DISEASE
Stenotic disease in the absence of obstruction presents either asymptomatically or with symptoms difficult to distinguish from uncomplicated CD. When intestinal narrowing reaches a critical diameter, causing partial or complete obstruction, crampy abdominal pain, nausea, vomiting, absence of flatus, and obstipation can occur. Because symptoms alone are unreliable to diagnose a stricture, endoscopy or imaging studies are required. Despite advancements in these techniques, a precise definition for stricturing CD remains controversial. An expert panel recently published a consensus definition for strictures in the absence of a surgical anastomosis. This definition involves luminal narrowing of at least 50%, bowel wall thickening that is 25% greater than the neighboring unaffected bowel, and dilation proximal to the stricture of at least 3 cm on radiologic imaging. The inability to pass an adult colonoscope through the narrowing is also grounds for diagnosis.42 An alternate consensus statement broadly defines radiographically identified strictures as an area of thickened intestine with proximal dilation of >3 cm. Areas of thickening without dilation would favor uncomplicated inflammatory disease.43 The most conclusive method of diagnosing a fibrostenosis in CD is by histologic assessment of intestinal tissue after surgical excision. Although it is routinely performed, forceps biopsy during endoscopy may be too superficial to detect the true extent of transmural fibrosis.
The histologic features of CD strictures include thickening of the muscularis mucosa, smooth muscle hyperplasia, submucosal accumulation of ECM proteins, adipose hyperplasia, and edema. Multiple histologic scoring systems are available to characterize a stricture, but a consensus definition is lacking. Common markers of inflammation in those systems include the depth of neutrophilic infiltration, lymphoplasmacytic infiltration, ulceration, fissures, cryptitis, crypt abscess, and edema. Scoring systems of fibrosis typically include the presence and extent of fibrosis, muscular hypertrophy, muscular hyperplasia, and degree of architectural distortion.44 Once the stricture is identified, new radiographic technologies can distinguish its inflammatory or fibrotic makeup. Although medications to reduce inflammation may be helpful at all stages of stricturing, they are most beneficial when initiated before significant scar formation. The ability to detect strictures early and to differentiate the inflammatory or fibrotic makeup of the stenosis is critical (Table 1).
| . | Inflammation . | Fibrosis . |
|---|---|---|
| Symptoms | Unable to differentiate | |
| Endoscopy | Edematous mucosa, erythema friability, ulceration, soft to touch | Pale atrophic mucosa, lack of friability or ulceration, firm to touch |
| Histology | Neutrophilic and lymphoplasmacytic infiltration, ulceration, crypt abscess, granuloma, edema | Muscular hypertrophy and hyperplasia, architectural distortion, collagen deposition (abnormal Masson trichrome staining) |
| Ultrasound | Hypervascularity, contrast enhancement, submucosal echogenicity indicating edema, loss of stratification | Thickened bowel with lack of vascularity or contrast enhancement, stratification present, possibly increased elastographic parameters |
| CT | Mucosal enhancement, mesenteric stranding and hypervascularity, nodular luminal thickening | Minimal enhancement, fatty submucosal infiltration, no mesenteric inflammation, smooth luminal thickening |
| Magnetic resonance | Early layered or homogeneous mucosal enhancement, mesenteric stranding and hypervascularity, T2 hyperintensity of bowel wall compared to muscle, nodular luminal thickening | Lack of or heterogeneous enhancement, no mesenteric inflammation, T1 and T2 isointensity or hypointensity, smooth luminal thickening, elevated magnetization transfer ratio |
| . | Inflammation . | Fibrosis . |
|---|---|---|
| Symptoms | Unable to differentiate | |
| Endoscopy | Edematous mucosa, erythema friability, ulceration, soft to touch | Pale atrophic mucosa, lack of friability or ulceration, firm to touch |
| Histology | Neutrophilic and lymphoplasmacytic infiltration, ulceration, crypt abscess, granuloma, edema | Muscular hypertrophy and hyperplasia, architectural distortion, collagen deposition (abnormal Masson trichrome staining) |
| Ultrasound | Hypervascularity, contrast enhancement, submucosal echogenicity indicating edema, loss of stratification | Thickened bowel with lack of vascularity or contrast enhancement, stratification present, possibly increased elastographic parameters |
| CT | Mucosal enhancement, mesenteric stranding and hypervascularity, nodular luminal thickening | Minimal enhancement, fatty submucosal infiltration, no mesenteric inflammation, smooth luminal thickening |
| Magnetic resonance | Early layered or homogeneous mucosal enhancement, mesenteric stranding and hypervascularity, T2 hyperintensity of bowel wall compared to muscle, nodular luminal thickening | Lack of or heterogeneous enhancement, no mesenteric inflammation, T1 and T2 isointensity or hypointensity, smooth luminal thickening, elevated magnetization transfer ratio |
| . | Inflammation . | Fibrosis . |
|---|---|---|
| Symptoms | Unable to differentiate | |
| Endoscopy | Edematous mucosa, erythema friability, ulceration, soft to touch | Pale atrophic mucosa, lack of friability or ulceration, firm to touch |
| Histology | Neutrophilic and lymphoplasmacytic infiltration, ulceration, crypt abscess, granuloma, edema | Muscular hypertrophy and hyperplasia, architectural distortion, collagen deposition (abnormal Masson trichrome staining) |
| Ultrasound | Hypervascularity, contrast enhancement, submucosal echogenicity indicating edema, loss of stratification | Thickened bowel with lack of vascularity or contrast enhancement, stratification present, possibly increased elastographic parameters |
| CT | Mucosal enhancement, mesenteric stranding and hypervascularity, nodular luminal thickening | Minimal enhancement, fatty submucosal infiltration, no mesenteric inflammation, smooth luminal thickening |
| Magnetic resonance | Early layered or homogeneous mucosal enhancement, mesenteric stranding and hypervascularity, T2 hyperintensity of bowel wall compared to muscle, nodular luminal thickening | Lack of or heterogeneous enhancement, no mesenteric inflammation, T1 and T2 isointensity or hypointensity, smooth luminal thickening, elevated magnetization transfer ratio |
| . | Inflammation . | Fibrosis . |
|---|---|---|
| Symptoms | Unable to differentiate | |
| Endoscopy | Edematous mucosa, erythema friability, ulceration, soft to touch | Pale atrophic mucosa, lack of friability or ulceration, firm to touch |
| Histology | Neutrophilic and lymphoplasmacytic infiltration, ulceration, crypt abscess, granuloma, edema | Muscular hypertrophy and hyperplasia, architectural distortion, collagen deposition (abnormal Masson trichrome staining) |
| Ultrasound | Hypervascularity, contrast enhancement, submucosal echogenicity indicating edema, loss of stratification | Thickened bowel with lack of vascularity or contrast enhancement, stratification present, possibly increased elastographic parameters |
| CT | Mucosal enhancement, mesenteric stranding and hypervascularity, nodular luminal thickening | Minimal enhancement, fatty submucosal infiltration, no mesenteric inflammation, smooth luminal thickening |
| Magnetic resonance | Early layered or homogeneous mucosal enhancement, mesenteric stranding and hypervascularity, T2 hyperintensity of bowel wall compared to muscle, nodular luminal thickening | Lack of or heterogeneous enhancement, no mesenteric inflammation, T1 and T2 isointensity or hypointensity, smooth luminal thickening, elevated magnetization transfer ratio |
Diagnostic imaging is the primary method for detecting strictures in CD. Multiple techniques are used, including computerized tomography (CT), magnetic resonance imaging (MRI), positron emission tomography (PET), and ultrasonography. The majority of these techniques are highly accurate in detecting stricturing CD (Table 2). Ultrasonography has emerged as a safe and cost-effective modality to detect CD strictures. Traditional B-mode ultrasonography is accurate to identify strictures >4 cm in diameter (sensitivity 85%, specificity 98%).45 However, the detection rate greatly diminishes for smaller strictures and those located deep within the abdominal cavity. Ultrasound also requires a skilled operator for optimal detection rates.
| . | Pros . | Cons . |
|---|---|---|
| Ultrasonography | Cost-effective and widely available | Deep strictures (jejunum, proximal ileum, rectum) are difficult to assess |
| High specificity | Can miss subtle areas of fibrosis | |
| Detection increased with contrast enhancement | Limited evaluation of multiple or overly long strictures | |
| Potential in-office use for quick re-evaluation | Limited by patient’s body habitus | |
| High intraobserver variability | ||
| CT enterography | Highly sensitive and specific | High radiation exposure |
| Allows discrimination between inflammation and fibrosis | ||
| Widely available | ||
| Magnetic resonance enterography | Highly sensitive and specific | Expensive |
| Allows discrimination between inflammation and fibrosis | Lengthy test | |
| Widely available | Requires patient cooperation to prevent motion artifact | |
| Low risk to patient | ||
| 18FDG PET/CT | Good correlation with strictures found on explant histology | Unable to differentiate between fibrosis, inflammation, and muscular hypertrophy |
| Expensive, poor insurance coverage | ||
| High radiation exposure |
| . | Pros . | Cons . |
|---|---|---|
| Ultrasonography | Cost-effective and widely available | Deep strictures (jejunum, proximal ileum, rectum) are difficult to assess |
| High specificity | Can miss subtle areas of fibrosis | |
| Detection increased with contrast enhancement | Limited evaluation of multiple or overly long strictures | |
| Potential in-office use for quick re-evaluation | Limited by patient’s body habitus | |
| High intraobserver variability | ||
| CT enterography | Highly sensitive and specific | High radiation exposure |
| Allows discrimination between inflammation and fibrosis | ||
| Widely available | ||
| Magnetic resonance enterography | Highly sensitive and specific | Expensive |
| Allows discrimination between inflammation and fibrosis | Lengthy test | |
| Widely available | Requires patient cooperation to prevent motion artifact | |
| Low risk to patient | ||
| 18FDG PET/CT | Good correlation with strictures found on explant histology | Unable to differentiate between fibrosis, inflammation, and muscular hypertrophy |
| Expensive, poor insurance coverage | ||
| High radiation exposure |
| . | Pros . | Cons . |
|---|---|---|
| Ultrasonography | Cost-effective and widely available | Deep strictures (jejunum, proximal ileum, rectum) are difficult to assess |
| High specificity | Can miss subtle areas of fibrosis | |
| Detection increased with contrast enhancement | Limited evaluation of multiple or overly long strictures | |
| Potential in-office use for quick re-evaluation | Limited by patient’s body habitus | |
| High intraobserver variability | ||
| CT enterography | Highly sensitive and specific | High radiation exposure |
| Allows discrimination between inflammation and fibrosis | ||
| Widely available | ||
| Magnetic resonance enterography | Highly sensitive and specific | Expensive |
| Allows discrimination between inflammation and fibrosis | Lengthy test | |
| Widely available | Requires patient cooperation to prevent motion artifact | |
| Low risk to patient | ||
| 18FDG PET/CT | Good correlation with strictures found on explant histology | Unable to differentiate between fibrosis, inflammation, and muscular hypertrophy |
| Expensive, poor insurance coverage | ||
| High radiation exposure |
| . | Pros . | Cons . |
|---|---|---|
| Ultrasonography | Cost-effective and widely available | Deep strictures (jejunum, proximal ileum, rectum) are difficult to assess |
| High specificity | Can miss subtle areas of fibrosis | |
| Detection increased with contrast enhancement | Limited evaluation of multiple or overly long strictures | |
| Potential in-office use for quick re-evaluation | Limited by patient’s body habitus | |
| High intraobserver variability | ||
| CT enterography | Highly sensitive and specific | High radiation exposure |
| Allows discrimination between inflammation and fibrosis | ||
| Widely available | ||
| Magnetic resonance enterography | Highly sensitive and specific | Expensive |
| Allows discrimination between inflammation and fibrosis | Lengthy test | |
| Widely available | Requires patient cooperation to prevent motion artifact | |
| Low risk to patient | ||
| 18FDG PET/CT | Good correlation with strictures found on explant histology | Unable to differentiate between fibrosis, inflammation, and muscular hypertrophy |
| Expensive, poor insurance coverage | ||
| High radiation exposure |
Newer ultrasound technologies can define stricture composition. One technique, contrast enhanced ultrasound (CEUS), utilizes intravenous contrast media to highlight strictures with increased vascularity. Because angiogenesis is a feature of early inflammatory strictures, the degree of contrast enhancement can identify lesions with a greater inflammation that may benefit from medical therapy.46 A study comparing CEUS to histology obtained upon surgical resection found that CEUS correctly identified inflammatory or fibrotic strictures in 23 of 28 patients.47 A more recent study conversely found no correlation between CEUS and explant histology identified by validating scoring systems.48 Ultrasound has also been explored to identify fibrosis in strictures. Ultrasound elasticity imaging detects fibrosis by measuring strain, the degree of deformation of an object when force is applied. In ultrasound elasticity imaging, the ultrasound probe compresses the abdomen to a set depth and colonic stiffness is determined qualitatively by the degree of strain. This technique has shown promise in identifying intestinal fibrosis.49, 50 In addition, this principle was used to develop shear wave elastography, where the speed of acoustic waves traveling through intestinal tissue is used to calculate stiffness quantitatively using the Young’s modulus. Elastographic shear wave speed correlated well with intestinal fibrosis but not inflammation in the 2,4,6-trinitrobenzene sulfonic acid (TNBS) rat colitis model.51 The same group then went on to evaluate this technology in samples of resected human CD strictures. Elastography could differentiate between strictures with low and high fibrotic composition, but the researchers did not explore the ability to distinguish inflammation from fibrosis.52 These techniques, although promising, require validation in the clinical setting.
Because they are ubiquitous and can detect both small and large bowel disease, CT and MRI are widely used for diagnosis. Detection rates are increased with the use of contrast both intravenously and within the bowel lumen. To better define the internal bowel anatomy, neutral or low-density contrast is either given orally (enterography) or instilled through a nasoenteric tube (enteroclysis). Of these, enteroclysis is more accurate but enterography is commonly used given ease of use and patient satisfaction.53 Both CT enterography (sensitivity 85%, specificity 100%) and magnetic resonance enterography (sensitivity 92%, specificity 90%) are accurate in diagnosing CD strictures.54 In general, MRI is preferred for patients aged ≤35 years because of greater rates of radiation-induced malignancy among young patients with CT exposure.55
Advanced MRI techniques can distinguish fibrotic from inflammatory strictures. Both a homogeneous pattern of enhancement with intravenous gadolinium contrast and the intensity of enhancement after 7 minutes of exposure can be used to differentiate inflammatory strictures with no fibrosis from those with advanced fibrosis (P < 0.01). Strictures with intermediate fibrosis have been less accurately characterized.56 In addition, magnetization transfer can be used for this purpose. In this process, tissue intensity is determined by differences in the magnetization of hydrogen atoms bound to larger macromolecules such as collagen and ECM proteins with those bound to mobile atoms including free water. The magnetization transfer ratio between these media accurately discriminates intestinal fibrosis from normal small bowel (sensitivity 95%, specificity 90%). The ratio has not been useful in distinguishing normal colon or acute inflammatory strictures.57
18F-fludeoxyglucose (18FDG) PET/CT has also been investigated for diagnosing strictures. The radioactive 18FDG tracer localizes to tissues with high metabolic activity. The 18FDG tracer in murine CD highlights areas of intestinal inflammation and the bone marrow given the increased production of inflammatory precursors.58 In a small study of patients with stricturing disease, explant histology was compared to 18FDG PET/CT obtained before resection. Uptake of 18FDG was increased in strictures but could not differentiate inflammatory from fibrotic tissue.59 In general, PET/CT is not a viable tool for this purpose and offers little advantage over conventional CT or magnetic resonance enterography.
Endoscopy is a useful tool for the diagnosis and characterization of CD strictures. Once identified, strictures should be defined in a standardized method such as that defined by Paine and Shen.60 Lesions should be described by their etiology as either primary or anastomotic and either benign or malignant. The number of strictures; their length, shape, location, and degree of obstruction; and any associated complications such as angulation, ulceration, and fistula should be recorded. Once identified, biopsy of the stenotic region may be performed to screen for malignancy or dysplasia. Although the incidence of colorectal cancer is similar between uncomplicated CD and the average population, patients with stricturing harbor a 4.5% 10-year risk.61 Resected strictures in CD contain dysplasia in 1.6% and malignancy in 0.8% of patients.62 Regardless, the routine biopsy of stenoses is controversial because of the perceived risk of perforation and scar formation that may complicate future surgery. These claims have not been formally investigated. Routine biopsy should be considered in all CD strictures.
PUTATIVE BIOMARKERS
Various serological biomarkers have been proposed as noninvasive means to detect the presence of stricturing disease and to identify those at risk for its development. One potential biomarker is cathelicidin. This protein is released by innate immune cells to permeabilize bacterial cell membranes. It is also believed to have antifibrotic properties. Low levels of circulating cathelicidin were associated with increased stricture formation in CD in 1 study, albeit rather inaccurately (43% sensitivity, 76% specificity).63 Another serum protein significantly elevated in CD strictures is the putative growth factor for mesenchymal cells, YKL-40. Its use as a biomarker has undergone only preliminary evaluation.64 An additional avenue of biomarker research involves antimicrobial antibodies. These antibodies, including anti-Saccharomyces cerevisiae, anti-outer membrane protein C, anti-Pseudomonas fluorescens-associated sequence I2, and anti-bacterial flagellin have all been implicated in CD pathogenesis.65 A meta-analysis found that these antibodies were associated with complicated disease, including both stricturing and penetrating phenotypes, and the need for surgery. Positivity for ≥2 of these antibodies correlated with complicated disease including stricturing (odds ratio, 2.93).66 Recently, isoforms of the anti-GP2 antibody against a glycoprotein on pancreatic acinar cells and M cells of the Peyer’s patch were associated with stenotic CD.67 The α isoform also predicted the need for surgery. Noninvasive biomarkers have the potential to identify those at risk for stricturing. Although testing for antimicrobial antibodies is commercially available, routine use is not recommended because of its low sensitivity and the lack of robust validation.
MEDICAL MANAGEMENT
Asymptomatic strictures can often be managed in the outpatient setting, but patients with obstruction warrant hospitalization. Conservative measures including intravenous fluids, bowel rest, and nasogastric drainage are often required.68 The efficacy of medical management depends on stricture composition. Whereas lesions with a larger inflammatory component are more amenable to medical therapy, fibrotic strictures often require other modalities including endoscopic or surgical intervention. Most strictures harbor a mixed morphology and may benefit from a trial of anti-inflammatory medications.
High-quality clinical trials investigating the use of specific medications for stricturing disease are lacking. In general, corticosteroids can be used to reduce the degree of inflammation. Biologic therapy, especially using TNF-α inhibitors, may also be initiated in the acute setting. Posthoc analysis of the ACCENT-1 trial indicated that the frequency of stricturing disease and obstruction was decreased with infliximab use.69 A small prospective trial of TNF-α inhibitors in stricturing disease showed improvement in symptoms and stricture severity, but 39.2% of patients still required surgery after 37.9 months of follow-up.70 Recently, the prospective multicenter CREOLE study71 proved the success of adalimumab for treating symptomatic strictures. Success, defined as symptom improvement without steroids, endoscopic, or surgical intervention, occurred in 64% of patients at 24 weeks. The likelihood of improvement was greater in those with shorter symptom duration, stricture length <12 cm, proximal dilation <30 mm, and no fistula formation. After 4 years of surveillance, 45.7% of patients still required surgery for symptomatic strictures.
Conversely, long-term surveillance of pediatric patients treated with early anti-TNF therapy has shown significant reduction in penetrating disease but no difference in the incidence of stricture formation.72 Inhibition of TNF-α improves Th1-mediated inflammation, leading to a reduction in subsequent fibrosis. There are also minor direct antifibrotic effects of a TNF-α blockade. The activation of TNF receptor 2 on myofibroblasts induces TIMP-1 production with the inhibition of ECM degradation.73 The TNF-α signaling is also involved in myofibroblast migration through p38 mitogen activated protein kinase (MAPK) signaling.74
There have been no studies on the outcomes of vedolizumab in the management of stricturing disease. It is unclear how a blockade of the α4β7 integrin on lymphocytes, preventing diapedesis to intestinal tissue, would have any direct antifibrotic effects aside from improving local inflammation. A recent case series showed a short-term endoscopic improvement of CD strictures in 2 patients treated with ustekinumab.75 This biologic is unique in that it downregulates both Th1 (anti-IL-12) and Th17 (anti-IL-23) responses with the potential to mitigate downstream profibrotic effects. Immunomodulators including azathioprine, 6-mercaptopurine, and methotrexate do reduce the incidence of surgery for all indications in CD (hazard ratio [HR], 0.59; 95% confidence interval, 0.48-0.73).76 In the CREOLE study, higher rates of treatment success for TNF-α inhibitors were seen in those already on immunomodulator therapy.71
Although these drugs may be useful as adjunctive therapy, their slow onset likely provides little benefit in the acute setting, and long-term data are lacking. One small study investigated exclusive enteral nutrition in adults with symptomatic inflammatory strictures. Enteral nutrition alone achieved complete symptomatic remission in 6/10 patients.77
Although there are benefits to anti-inflammatory therapies for CD strictures, no medications are available to improve intestinal fibrosis. Historically, fibrosis was considered an irreversible process. Expanding knowledge regarding the pathogenesis of scarring has challenged this tenet and led to the identification of multiple potential drug targets. The majority of these act directly or indirectly on TGF-β signaling, which is crucial in wound healing, mesenchymal cell proliferation and activity, ECM production, and scar formation.
Because the process of fibrosis is believed to be similar among different diseases, medications under investigation for other conditions including pulmonary, renal, dermatologic, and hepatic fibrosis may also be effective for CD fibrosis. One such therapy, currently approved for the treatment of idiopathic pulmonary fibrosis, is pirfenidone. This oral medication has anti-inflammatory, antifibrotic, and antioxidant properties. In pulmonary tissue, it prevents TGF-β-induced collagen production and reduces fibroblast proliferation. A study of pirfenidone in fibroblasts harvested from patients with stricturing CD showed similar inhibitory actions toward intestinal fibroblasts and collagen production in vitro.78 However, there have been no clinical trials involving this medication in CD.
In addition, the oral arginine-glycine-aspartic acid mimetic cilengitide, active against the αVβ3 integrin, was investigated in the murine TNBS colitis model.9 Cilengitide ameliorated intestinal collagen production via the downregulation of TGF-β activation and resulted in decreased SMAD2/3 activation. This pathway is integral in TGF-β myofibroblast activation and subsequent profibrotic activities.
An additional class of signaling molecules downstream from TGF-β, the rho kinases, was also investigated for possible antifibrotic potential.80 A selective rho kinase 1 and 2 inhibitor with local effects on intestinal tissue, AMA0825, induced myofibroblast autophagy, reduced the progression of fibrosis, and even reversed existing fibrosis in the murine TNBS colitis model.
Drug targets outside the TGF-β pathway have shown promise as well. The activity of peroxisome-proliferator activated receptor-γ (PPARγ), a transcription factor with pleiotropic effects, is downregulated in inflammatory bowel disease and correlates with disease activity. Treatment with 5-aminosalicylates increases the activity of PPARγ, but no direct antifibrotic effect has been seen with these medications. However, the 5-aminosalicylate analog GED-0507-34 Levo activates PPARγ 100 to 150 times more potently than the original compound. It led to the reduced expression of collagen type 1–3, TGF-β, Il-13 and others in the murine TNBS model. These factors were also reduced in cultured human intestinal fibroblasts. Finally, GED-0507-34 Levo prevented myofibroblast differentiation and EMT as well.81
Still, none of these medications have undergone clinical trials in humans. It is important to clarify appropriate endpoints to ensure rigorous clinical trials. An expert panel recently analyzed studies involving antifibrotic medications in CD and defined critical endpoints.82 These endpoints included symptom-based measures such as the absence of occlusive symptoms, lack of disability, symptom-free survival, and normal quality of life. Objective measures were identified with stricture regression, radiologic remission with decreased bowel wall thickness and upstream dilation, and clinical remission as identified by lack of steroid use, Harvey-Bradshaw Index score, or fecal calprotectin level. Treatment failure, defined as medication discontinuation or surgery, was also deemed a critical endpoint. As antifibrotic drugs are developed, caution should be taken to consider potential adverse effects. There is a theoretical concern that the modulation of ECM deposition could create weakness within the bowel wall with transition to the penetrating phenotype.83
ENDOSCOPIC MANAGEMENT
Advances in therapeutic endoscopy have produced minimally invasive techniques for the management of CD strictures. Endoscopy can be a bridge to surgery or the primary therapeutic option, especially in those with significant risk factors or who defer surgical procedures. The most common technique is through scope balloon dilation, during which a colonoscope is passed through the narrowing to visualize stricture anatomy and determine length. Tight strictures can be traversed using a pediatric colonoscope or an upper endoscope. Once the extent of the stricture and the proximal anatomy are identified, the balloon is deployed in a cephalad-to-caudad fashion. For especially narrow or angulated strictures preventing the passage of the entire scope, the balloon is advanced through the stricture typically with the assistance of a guidewire and fluoroscopy. Of these techniques, dilation after direct visualization is preferred because of the reduced possibility for complication.60
The maximum diameter of the balloon inflation is important. Inflation to 15 mm greatly reduces the need for future surgery, whereas 16 to 18 mm leads to longer symptom-free intervals.84 Overall, the consensus is to dilate to 18to 20 mm for most patients. The optimal inflation time has not been studied, but 1 to 3 minutes is generally accepted. Dilation can be performed on structures up to 5 cm in length, but longer strictures are associated with greater failure rates and the need for subsequent surgery.85 Both anastomotic and de novo strictures, regardless of their inflammatory or fibrotic makeup, can be dilated. Mucosal ulceration of the stricture is not a contraindication to proceed. Balloon dilation of upper gastrointestinal strictures has also been performed successfully in select patients.86, 87
Despite the widespread acceptance of balloon dilation for managing CD strictures, the heterogeneity of studies regarding stricture length, balloon diameter, and length of follow-up make evaluating this technique difficult. A few systematic reviews and meta-analyses are available regarding the efficacy of balloon dilation.88-91 These studies showed a technical success rate of 86% to 90.6% with short-term clinical success, defined as improvement in obstructive symptoms, achieved in 63% to 80.8% of patients. However, long-term improvement is difficult to achieve because ≥1 repeat dilations were required in 31.6% to 56% of patients. Surgical intervention because of technical or clinical failure increased over the length of the follow-up. At 1 year postdilation, 21.5% to 30.1% of patients required surgery. This rate increased to 40.2% to 42.9% after 2 years. Dilation in these studies was more efficacious in anastomotic strictures than in de novo disease. Adverse events occurred in 6.4% of patients, whereas major events including bleeding and perforation were less common (2.4% to 3%). Interestingly, fecal markers of inflammation, including lactoferrin and calprotectin, may be useful to predict the need for repeat dilation. Elevated calprotectin (95.5% sensitivity; 69.2% specificity; negative predictive value, 94.7) was more useful for this purpose than lactoferrin (77.3% sensitivity; 69.2% specificity; negative predicted value, 78.4).92
Small studies have investigated combining balloon dilation with intralesional steroid injections. In a pilot study, balloon dilation with steroid injection led to worse outcomes with greater need of repeat dilation and reduced symptom-free intervals compared with placebo.93 A subsequent small randomized controlled trial in the pediatric population did show improved outcomes with steroid injection.94 Alternatively, 3 patients treated with an intralesional injection of infliximab alone had symptomatic improvement, albeit with a 2-week follow-up.95 These techniques cannot currently be recommended for routine practice.
After early case reports,96 the placement of endoluminal stents to mechanically expand strictures has gained acceptance for both benign and malignant disease. Although there are no stents specifically designed for CD, self-expanding metal stents (SEMS), lumen-apposing metal stents, and biodegradable stents have all been studied for this purpose. The SEMS are commercially available in sizes >5 cm and offer a therapeutic option for longer strictures where balloon dilation would be inadequate. Uncovered stents are available, but stents fully or partially covered with a plastic or silicone polymer are preferred in CD because they allow for stent removal. Given the propensity for stent migration when deployed in CD, removal after 4 weeks is suggested.97
A study of SEMS for CD strictures <8 cm in length98 showed a 92% technical success rate with symptomatic resolution in 64.7% of patients. There was a 43.7% recurrence in long-term follow-up requiring further endoscopic or surgical intervention. Adverse events included stent impaction in 16% of patients and rare proximal stent migration. No intestinal perforation was seen in this study. Distal stent migration, largely because of improvement in stricture diameter, occurred in 52% of patients.
The use of lumen-apposing metal stents has also been explored.99 These stents are an option for short strictures given their length of 1 cm.
Biodegradable stents are theoretically advantageous for CD strictures. In the absence of complication, stent removal is unnecessary because they tend to dissolve over a period of 4 months. Small studies have shown comparable technical success and long-term outcomes to metal stents.100, 101 However, the use of biodegradable stents requires further validation in the CD population, and these stents are not commercially available in the United States.
Although balloon dilation and stent placement act mechanically to disrupt the dense fibrotic scar and expand the stricture, endoscopic stricturotomy has been utilized in select centers to incise stenotic intraluminal tissue. This procedure was first explored using lasers to vaporize the obstructing tissue with the addition of linear cuts to increase bowel surface area.102 Laser stricturotomy was then followed by balloon dilation with promising results. More recently, needle knife electroincision and electrocautery have been used to cut away stenotic tissue.103 These techniques improve symptoms in 72.7% of patients with CD with comparable recurrence rates to balloon dilation. Needle knife stricturotomy has a 3.7% complication rate, including occasionally severe postprocedure bleeding and rare perforation.104 Although this technique may be an option for recurrent stricturing, it requires an expert hand for optimal success and safety. It is best reserved to teams that include a colorectal surgeon.
SURGICAL MANAGEMENT
Although the surgical management of strictures is an important and often necessary tool, modern medical management and endoscopic techniques have reduced the need for surgery. The risk of surgery in CD since 2000 (1-year risk, 12.6%; 5-year risk, 24.2%) has considerably decreased compared to the 1970s (1-year risk, 14.8%; 5-year risk, 31.2%; 10-year risk, 43.4%).105 Nevertheless, both resection and strictureplasty remain viable options for fibrostenosis. A multidisciplinary approach for all patients with stricturing CD is required with early involvement of colorectal surgeons. The European Crohn’s and Colitis Organisation guideline suggests that nonsurgical management should be attempted first for those with a symptomatic stricture. Patients refractory to conservative therapy, those presenting with complete obstruction or bowel ischemia, and those with isolated obstruction at the ileocecal valve without evidence of inflammation will require an operation.106 There is evidence that an initial operative approach confers longer clinical remission and reduced reoperation rates compared to up-front medical therapy.107
For most patients, it is difficult to predict who will respond poorly to conservative therapy and require surgery. A potentially useful predictive tool was developed by Stidham, Guentner, et al.108 Among patients hospitalized for stricturing small bowel and ileocecal disease, those with small bowel dilation >35 mm on imaging (HR, 2.92) and a platelet:albumin ratio of >125 (HR, 2.13) required surgery within 2 years.108
When surgery is indicated, perioperative nutritional support through enteral or parenteral means is indicated for patients who are malnourished. These patients may benefit from a staged procedure given their increased surgical risk. Operative complications are also increased by perioperative steroid use. The use of biologics and immunomodulators around the time of surgery for CD is controversial. In some studies, TNF-α inhibitors are associated with a small but significant risk of infectious complications, anastomotic leak, and need for readmission after surgery in CD.109 Still, no firm guidelines beyond enhanced recovery after surgery protocols exist regarding perioperative medical management.
Complication rates are lower using laparoscopic surgery for strictures compared to an open technique. Laparoscopy is associated with a shorter recovery and length of hospital stay, reduced bowel obstruction and herniation, lower cost, and improved cosmesis with similar success rates.110 After surgery, ileocolonoscopy is generally performed within 6 to 12 months to assess for recurrent inflammation, stricture, or complication. A standardized scoring system such as the Rutgeerts score is suggested for postoperative assessment.111 The optimal time to institute medical therapy after surgery remains unclear.
Stricture resection followed by anastomosis is the most common surgical procedure in CD. Resection is ideal for patients with a solitary short stricture. Resection of multiple strictures or a particularly long stricture results in decreased bowel length, potentially causing malabsorption or diarrhea. Most patients experience symptomatic relief and improved quality of life after resection. However, surgery is not curative. Before the advent of biologics, recurrent anastomotic stricturing was found in approximately 15% of patients at 1 year, 45% at 5 years, and 75% at 7 years after surgery.112 Recent data suggest that recurrent surgery is required in approximately 20% of patients at 5 years and 40% at 10 years after initial surgery.113 Increased recurrence has been seen among tobacco users114 and those with positive margins on explant histology. Preoperative disease duration and resection of multiple strictures had no effect on recurrence, whereas initiation of medical therapy <4 weeks after surgery produced longer disease-free intervals.115
Outcomes may also be influenced by the type of anastomosis. End-to-end anastomosis involves joining the 2 ends of the bowel en face and can be either hand-sewn or stapled. End-to-end ileocolic anastomoses are technically challenging given the difference in bowel diameter, resulting in longer operative time. A number of studies have investigated clinical outcomes bases on anastomotic type. Two recent meta-analyses showed a modest benefit of stapled side-to-side and functional end-to-end anastomoses compared to hand-sewn anastomoses especially in the end-to-end configuration for patients with ileocolonic CD.116, 117 Although outcomes in 1 meta-analysis showed a decrease in anastomotic leak, this difference was lost when only patients without cancer were analyzed.116 In comparison, the second meta-analysis, which included only patients with CD with ileocolonic anastomoses, showed a decrease in anastomotic leak rate, disease recurrence, and surgical treatment for recurrences.117
Differences in clinical outcomes between anastomotic configurations should be balanced with other potential consequences. Theoretically, maintaining the normal bowel orientation and peristaltic wave may prevent stasis and bacterial overgrowth. Antiperistaltic side-to-side anastomoses creates a small defunctionalized pouch in which luminal contents can pool and contribute to fecal stasis. Animal models have shown a short-term difference in myoelectrical function and a trend toward bacterial overgrowth.118, 119 However, these differences were not seen at longer postoperative intervals, and no clinical studies have confirmed these findings. In comparison to side-to-side linear stapled anastomoses, an end-to-end configuration provides easier endoscopic visualization and therapeutic access.120 Overall, stapled linear anastomoses may be easier to fashion, with a slight benefit in outcomes compared to the end-to-end hand-sewn technique.
Strictureplasty is an alternative option to relieve obstructive symptoms from fibrotic strictures in CD. Because it preserves bowel length, strictureplasty is ideal in patients with disease localized to the small bowel, multiple strictures, or a history of previous resection to prevent short bowel complications. Colonic strictureplasty is technically feasible but often unnecessary because resection will not affect nutrient absorption. Traditionally, short strictures <10 cm in length were amenable to this procedure. Newer strictureplasty techniques can treat longer bowel segments. The presence of fistula, abscess, significant inflammation, or concern for underlying malignancy are contraindications to strictureplasty.
Multiple techniques exist, the most common of which is the Heineke-Mikulicz technique, constituting >80% of all surgeries121 (Figs. 2A, B122). In this technique, the antimesenteric side of the stricture is incised longitudinally 1 to 2 cm past the strictured segment, creating an enterotomy. The bowel is then sutured transversely in a 2-layer fashion. Strictures 10 to 20 cm in length can be managed with the Finney technique (Figs. 2C, D). A stay suture is placed in the middle of the diseased segment, arranging the bowel in a U-shaped configuration. The antimesenteric border is then incised, followed by the creation of a stapled or hand-sewn side-to-side anastomosis. Strictures >20 cm can be managed using the Michelassi side-to-side antiperistaltic technique (Figs. 2E, F). Here, the mesentery and stenotic bowel are divided at the midpoint and arranged side by side. An enterotomy is made down the length of both segments, and the bowel is sutured together in a 2-layer fashion.123 The Michelassi technique can also be performed successfully over the ileocecal valve.124 Variations of these techniques, including the double Heineke-Mikulicz and the combined Heineke-Mikulicz/Finney strictureplasties, allow for successful bowel-preserving surgery in the majority of patients.
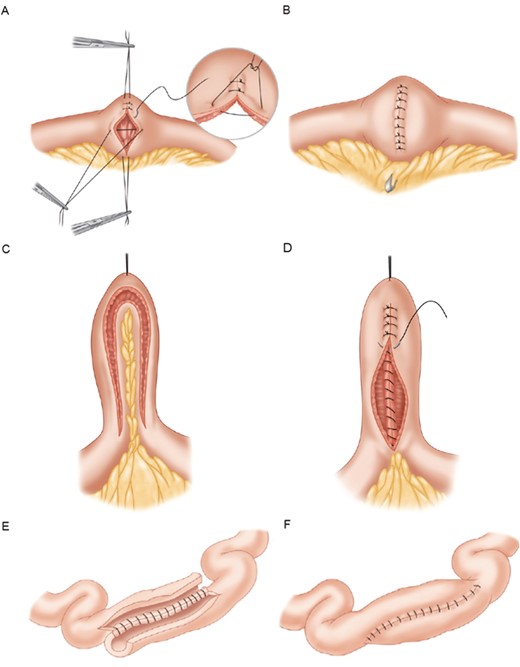
A, Heineke-Mickulicz strictureplasty. Longitudinal enterotomy followed by transverse apposition and suture closure. B, Completed Heineke-Mickulicz strictureplasty. C, Finney strictureplasty. Bowel arranged in U-shape with incision of the antimesenteric border. D, Creation of side-to-side anastomosis. E, Michelassi strictureplasty. Midpoint division of the bowel with longitudinal enterotomy. F, Adjacent loops of bowel joined in 2-layer fashion. Figure adapted with permission from Elsevier: Scott A. Strong, Chapter 23. Surgery for Crohn Disease. In: H.R. Bailey, et al. Colorectal Surgery, Elsevier: Saunders, 2013, pages 375-377.
Strictureplasty leads to clinical success in >90% of patients, with site-specific recurrence occurring in 26% of patients at 5 years and 29% of patients at 10 years.125 The frequency of postoperative complications varies depending on the site of the strictureplasty. Disease localized to the small bowel shows a 13% complication rate, and the ileocolic strictureplasty rate is slightly higher at 17% to 23%. The highest rates of postoperative morbidity are seen with septic complications, defined as anastomotic leak, fistula, and abscess, and occur in 4% of patients. Other common complications include wound infection, significant bleeding, and ileus/obstruction, seen in 2%, 4%, and 3% of patients, respectively.121 Similar success and complication rates have been seen between the Heineke-Mikulicz and Finney techniques compared with the nonconventional strictureplasty techniques, with the exception of decreased bleeding events and less-recurrent obstructive symptoms in the nonconventional group.126 In addition, outcomes of strictureplasty compare similarly to those of resection. In 1 meta-analysis, there was a trend toward less complications with strictureplasty (12.7% vs 19.1%; P = 0.09), but patients with resection had a slightly longer recurrence-free survival (HR, 1.08, 95% confidence interval, 1.02-1.15; P = 0.01).127 Strictureplasty is an increasingly uncommon operation in CD for unclear reasons, with rates dropping from 5.1% in 2005 to 1.7% in 2012.128
CONCLUSIONS
The management of stricturing CD remains a challenge. Although the immediate success of endoscopic and surgical techniques are well established, the benefits are often transient because many patients develop recurrent stricturing in long-term follow-up. In the absence of complete obstruction or other clear surgical indications, an initial trial of anti-inflammatory medication is typically prescribed. However, there have been few studies regarding the outcomes of medical management and scant evidence to guide medication choice. The success of medical therapy for CD strictures depends on the degree of inflammation within the lesion. Although radiologic techniques to discriminate inflammation from fibrosis are improving, they remain inaccurate for most strictures because they typically exhibit a mixed morphology. These shortcomings eclipse the progress made in understanding the cellular pathogenesis of stricturing disease. There is clearly a call for further research.
REFERENCES



